Abstract
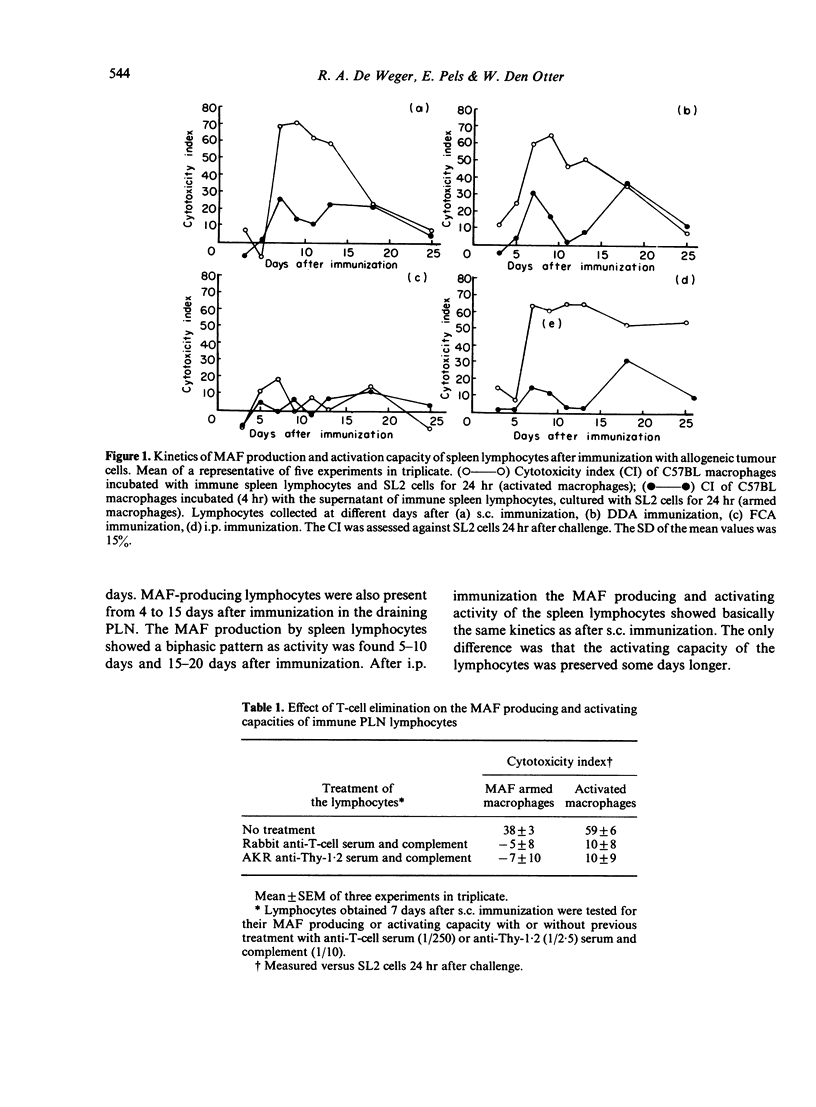
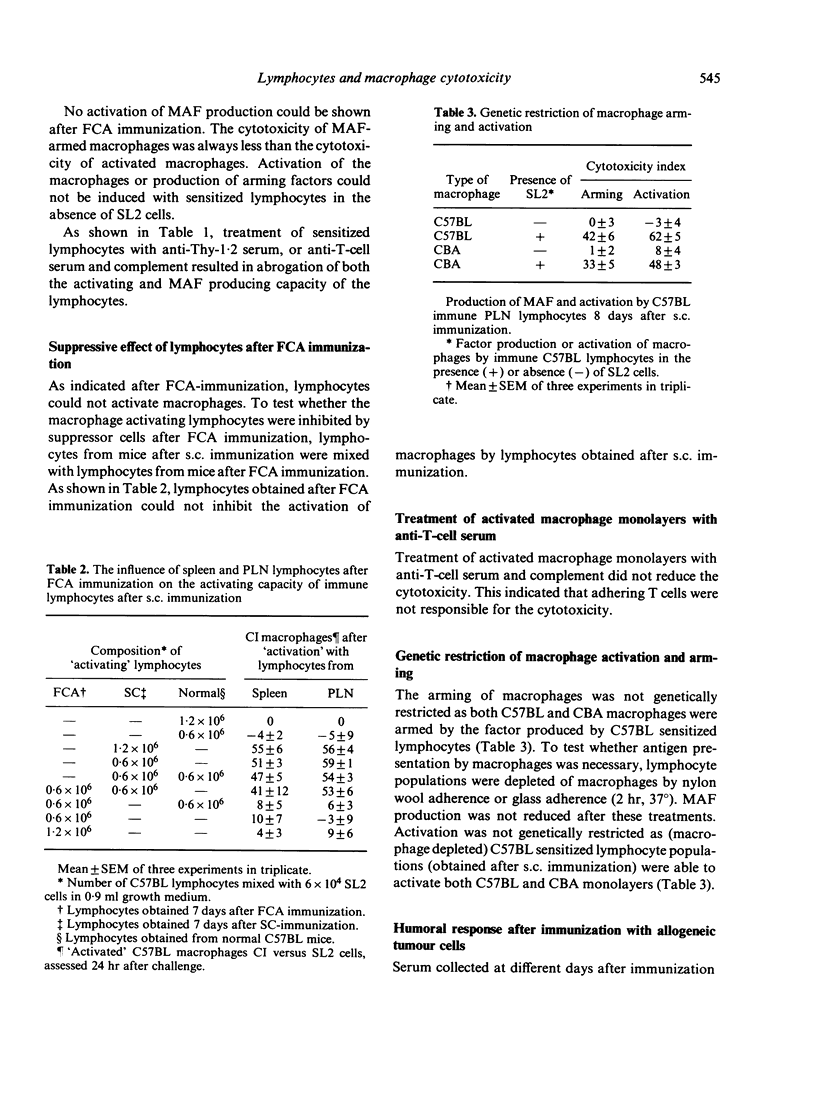
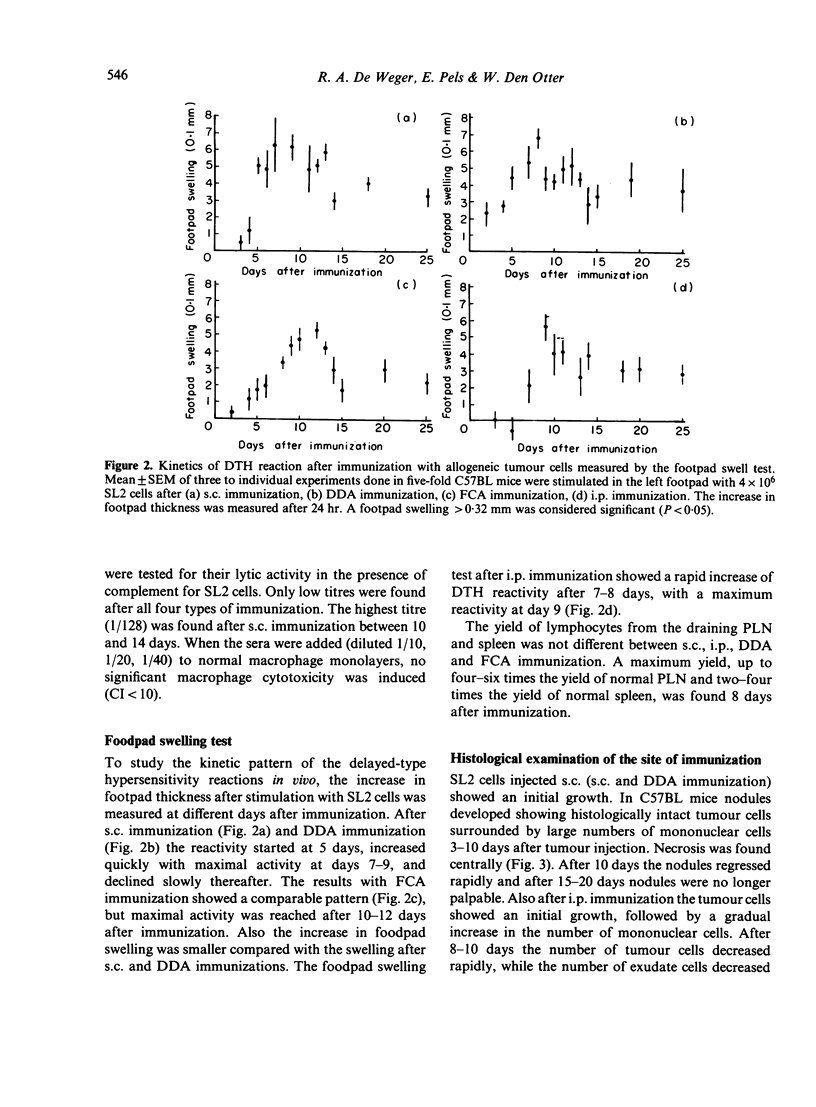
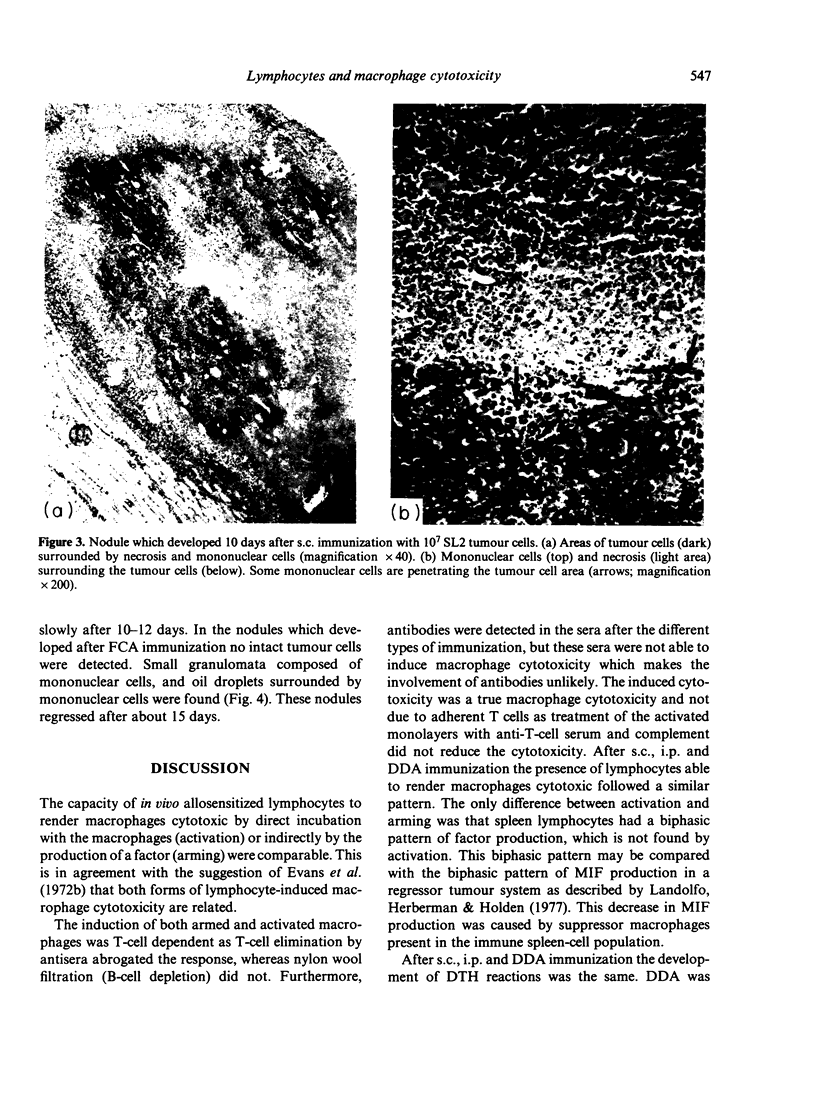
Sensitized spleen and peripheral lymph node lymphocytes were tested after different types of immunization with allogeneic tumour cells for their capacity to induce macrophage cytotoxicity in vitro. The macrophages were rendered cytotoxic either by direct contact with lymphocytes and tumour cells (activation of macrophages) or by a factor (macrophage arming factor, MAF), released by the sensitized lymphocytes incubated with tumour cells (arming of macrophages). Both types of reactions are T-cell dependent. Macrophage activation is a more sensitive way to detect lymphocytes with the capacity to render macrophages cytotoxic than arming of macrophages. The route of immunization subcutaneously (s.c.) or intraperitoneally (i.p.) with allogeneic cells did not influence the induction of lymphocytes with the capacity to render macrophages cytotoxic. However, the tumour cells had to be intact as disrupted cells (suspended in Freund's complete adjuvant, FCA) did not induce macrophages activating lymphocytes. The adjuvant dimethyl dioctadecyl ammonium bromide (DDA) did not increase the lymphocyte response. Intact allogeneic tumour cells were needed in vitro when used for secondary antigenic stimulation. This secondary stimulation was independent of antigen presentation by macrophages. This suggests that also in vivo the primary response is independent of macrophage antigen presentation. Delayed-type hypersensitivity and antibody responses against the allogeneic tumour cells were comparable after s.c. and i.p. immunization and after immunization with FCA and DDA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crowle A. J. Delayed hypersensitivity in the mouse. Adv Immunol. 1975;20:197–264. doi: 10.1016/s0065-2776(08)60209-6. [DOI] [PubMed] [Google Scholar]
- Den Otter W., Evans R., Alexander P. Differentiation of immunologically specific cytotoxic macrophages into two types on the basis of radiosensitivity. Transplantation. 1974 Nov;18(5):421–428. doi: 10.1097/00007890-197411000-00006. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Mechanism of immunologically specific killing of tumour cells by macrophages. Nature. 1972 Mar 24;236(5343):168–170. doi: 10.1038/236168a0. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Role of macrophages in tumour immunity. I. Co-operation between macrophages and lymphoid cells in syngeneic tumour immunity. Immunology. 1972 Oct;23(4):615–626. [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Darnell J. H., Budmen M. B. In vitro activation of mouse macrophages by rat lymphocyte mediators. J Immunol. 1976 Aug;117(2):666–673. [PubMed] [Google Scholar]
- Hofhuis F. M., Van der Meer C., Kersten M. C., Rutten V. P., Willers J. M. Effects of dimethyldioctadecylammonium bromide on phagocytosis and digestion of Listeria monocytogenes by mouse peritoneal macrophages. Immunology. 1981 Jul;43(3):425–431. [PMC free article] [PubMed] [Google Scholar]
- Kerckhaert J. A., Van den Berg G. J., Willers J. M. Influence of cyclophosphamide on the delayed hypersensitivity of the mouse. Ann Immunol (Paris) 1974 Mar-Apr;125(3):415–426. [PubMed] [Google Scholar]
- Landolfo S., Herberman R. B., Holden H. T. Cellular immunity to murine sarcoma virus-induced tumors as measured by macrophage migration inhibition assays. J Natl Cancer Inst. 1977 Dec;59(6):1675–1683. doi: 10.1093/jnci/59.6.1675. [DOI] [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Ziegler F. G., Fischer H. Macrophage cytotoxicity factor. A product of in vitro sensitized thymus-dependent cells. Eur J Immunol. 1973 Jan;3(1):56–58. doi: 10.1002/eji.1830030112. [DOI] [PubMed] [Google Scholar]
- Mottram P. L., Miller J. F. Delayed-type hypersensitivity induced by antigen-pulsed, bone marrow-derived macrophages. Eur J Immunol. 1980 Mar;10(3):165–170. doi: 10.1002/eji.1830100302. [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pels E., Den Otter W. Natural cytotoxic macrophages in the peritoneal cavity of mice. Br J Cancer. 1979 Dec;40(6):856–861. doi: 10.1038/bjc.1979.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pels E., den Otter W. The role of a cytophilic factor from challenged immune peritoneal lymphocytes in specific macrophage cytotoxicity. Cancer Res. 1974 Nov;34(11):3089–3094. [PubMed] [Google Scholar]
- Smith F. I., Miller J. F. Delayed-type hypersensitivity to allogeneic cells in mice. III. Sensitivity to cell-surface antigens coded by the major histocompatibility complex and by other genes. J Exp Med. 1979 Oct 1;150(4):965–976. doi: 10.1084/jem.150.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippe H., Belder M., Willers J. M. Dimethyl diotadecyl ammonium bromide as adjuvant for delayed hypersensitivity in mice. Immunology. 1977 Dec;33(6):931–936. [PMC free article] [PubMed] [Google Scholar]




