Abstract
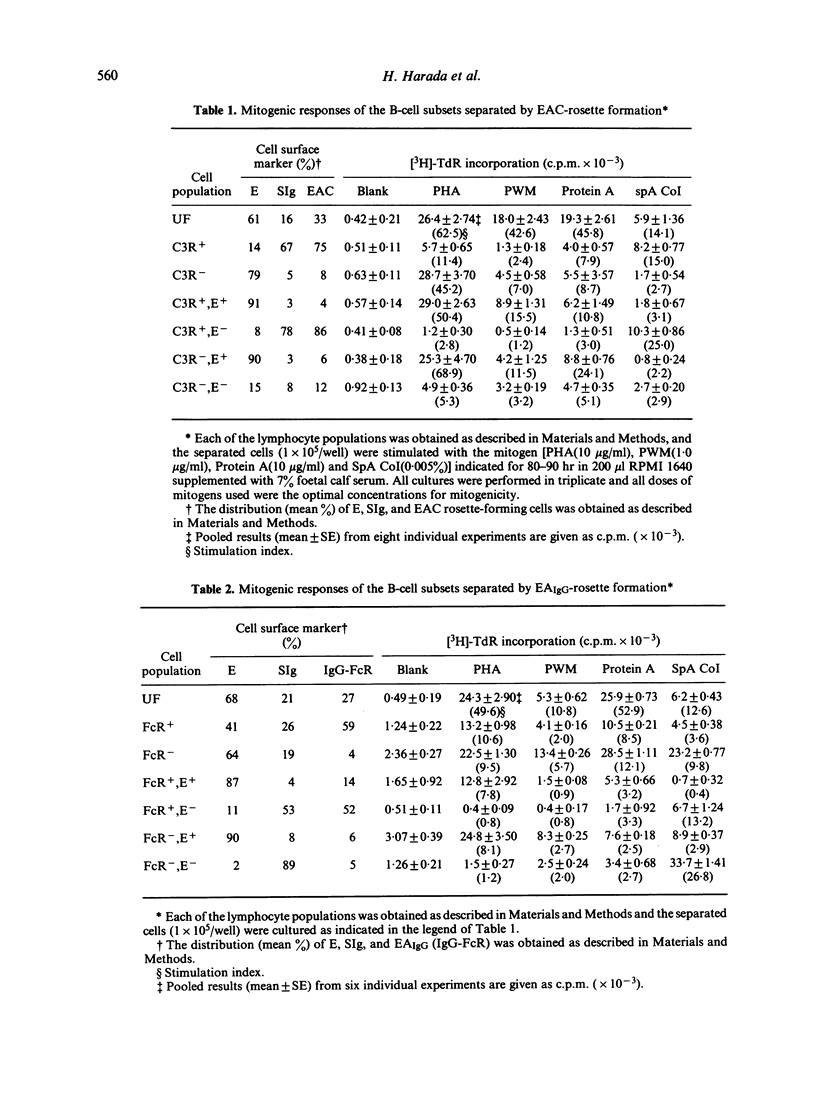
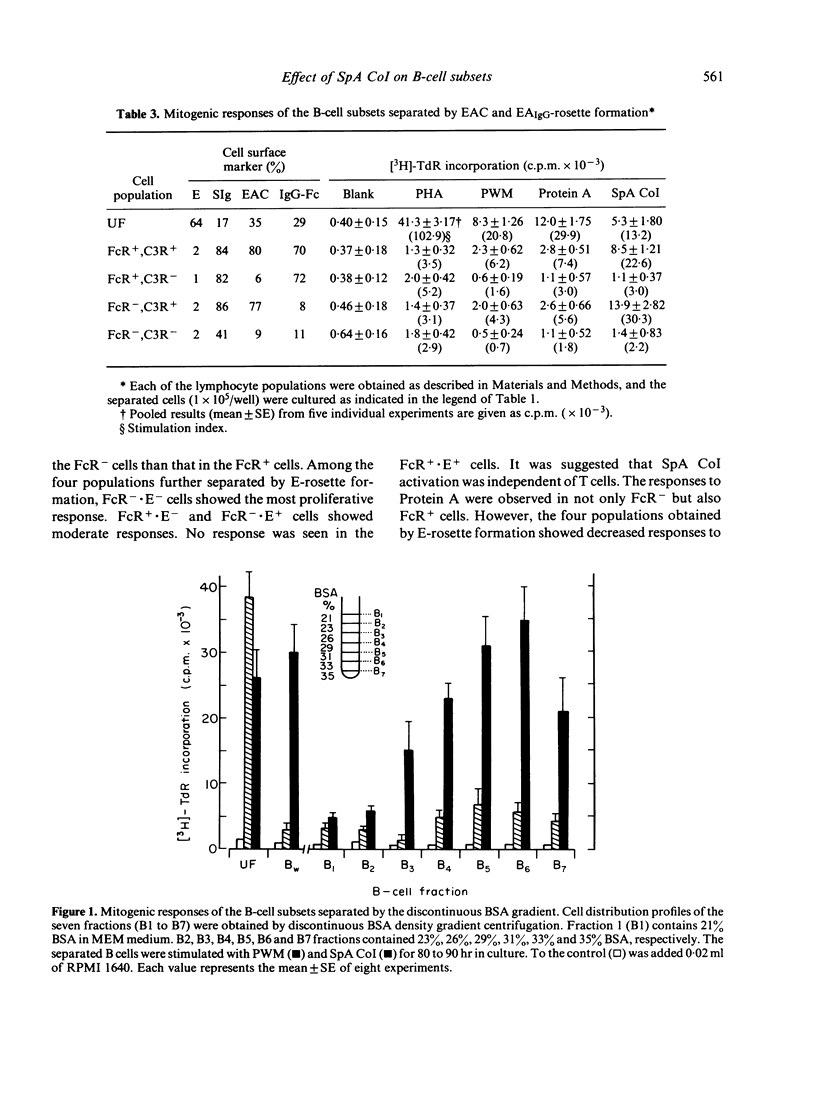
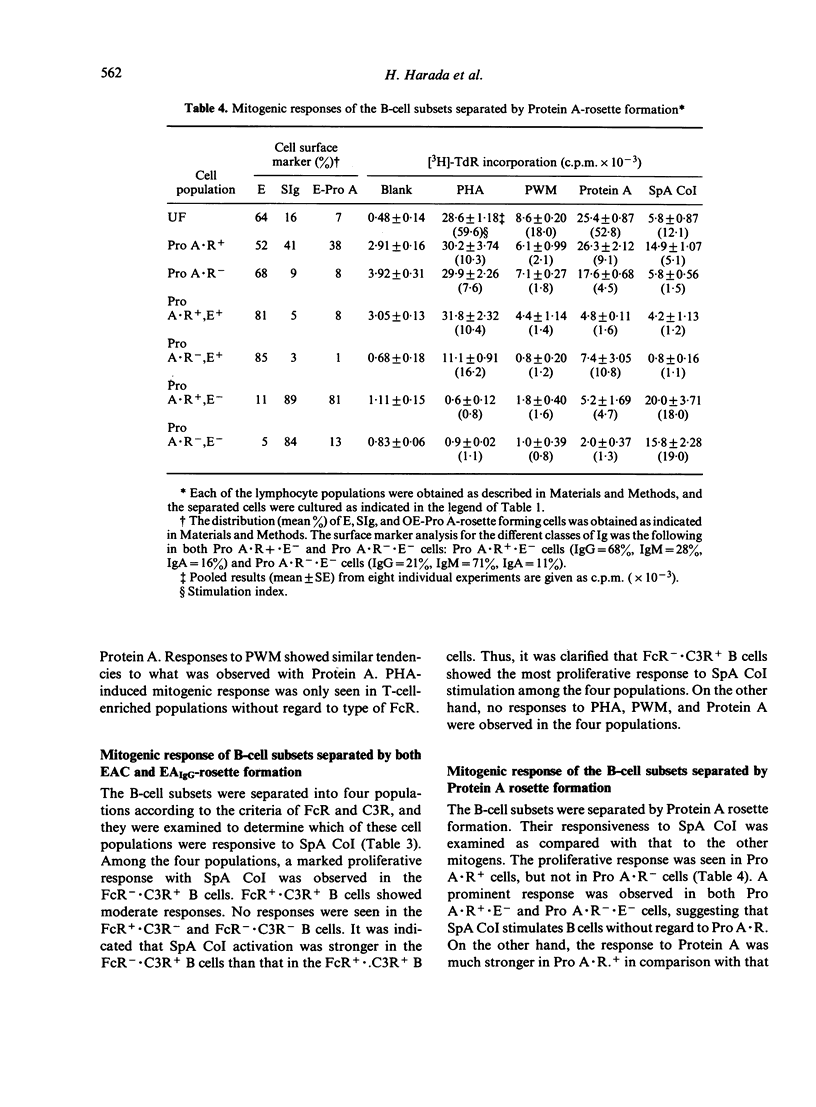
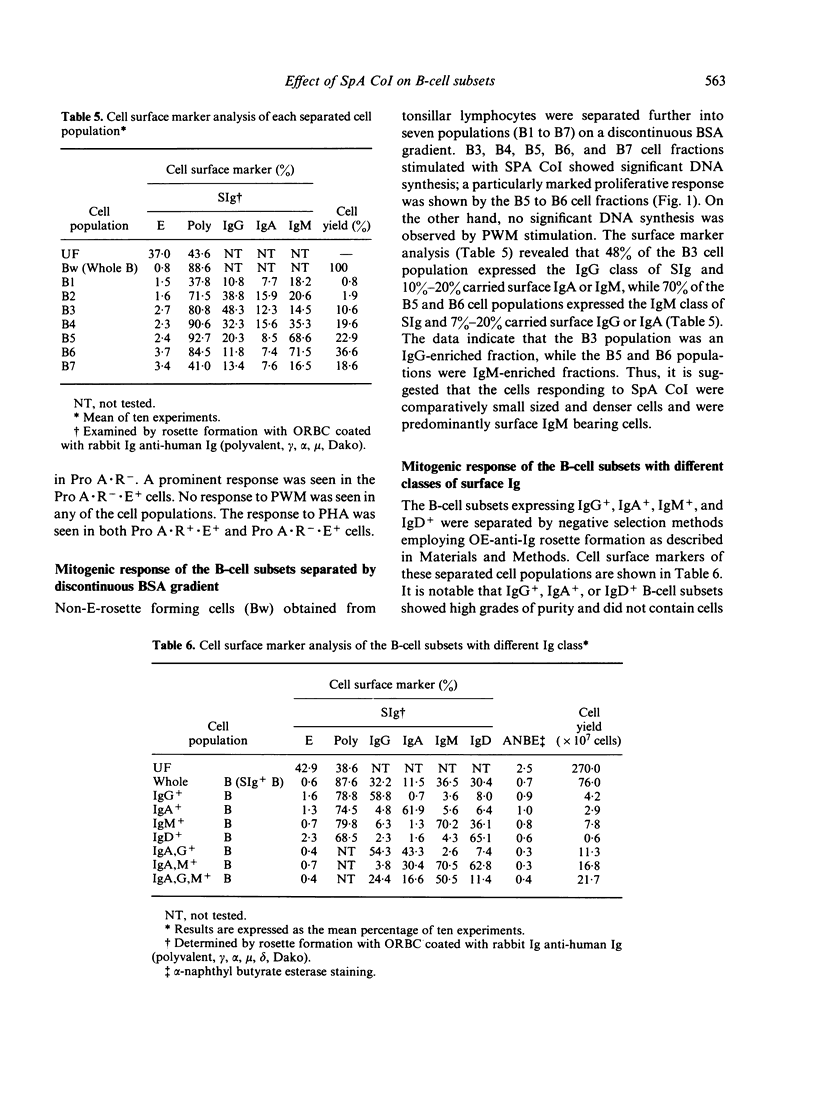
We have made a detailed investigation to determine which of the B-cell subsets could be stimulated by Staphylococcus aureus Cowan I bacterium (SpA CoI). B-cell subsets were separated from peripheral blood and tonsil lymphocytes by means of rosette formation with E, EAIgG, anti-immunoglobulin (Ig) conjugated OE (OE-Pro A) or by separation on a bovine serum albumin (BSA) discontinuous density gradient. The cells responding to SpA CoI included E receptor negative (E-), C3 receptor positive (C3R+), and surface Ig positive (SIg+) B-cell subsets. Among these B-cell subsets, FcR-n cells were more responsive than FcR+ cells. These B-cell subsets responded alone to SpA CoI and significantly proliferated, although, they failed to respond alone to pokeweed mitogen (PWM) and Protein A of S. aureus (Protein A). Among the SIg+ B-cell subsets stimulated with SpA CoI, IgM+ and IgG+ B cells showed much less response. Both Protein A receptor positive (Pro A . R+) and negative (Pro A . R-) cells responded well to SpA CoI. Fractionation of B cells on a BSA gradient revealed that comparatively small sized and denser B-cell subsets responded well to SpA CoI. From these criteria, it is suggested that B cells responding to SpA CoI are capable of stimulating not only mature B cells, but can also stimulate immature B cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. G., Britton S. A live human B-cell activator operating in isolation of other cellular influences. Scand J Immunol. 1979;9(6):507–510. doi: 10.1111/j.1365-3083.1979.tb03278.x. [DOI] [PubMed] [Google Scholar]
- Bona C., Broder S., Dimitriu A., Waldmann T. A. Polyclonal activation of human B lymphocytes by Nocardia water soluble mitogen (NWSM). Immunol Rev. 1979;45:69–92. doi: 10.1111/j.1600-065x.1979.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Delespesse G., Duchateau J., Gausset P., Govaerts A. In vitro response of subpopulations of human tonsil lymphocytes. I. Cellular collaboration in the proliferative response to PHA and Con A. J Immunol. 1976 Feb;116(2):437–445. [PubMed] [Google Scholar]
- Forsgren A., Sjöquist J. "Protein A" from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966 Dec;97(6):822–827. [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Gold E. R., Fudenberg H. H. Chromic chloride: a coupling reagent for passive hemagglutination reactions. J Immunol. 1967 Nov;99(5):859–866. [PubMed] [Google Scholar]
- Greaves M., Janossy G., Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974 Jul 1;140(1):1–18. doi: 10.1084/jem.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Chin A. F., Abbott J. Ontogeny of murine B lymphocytes: sequence of B-cell differentiation from surface-immunoglobulin-negative precursors to plasma cells. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2008–2012. doi: 10.1073/pnas.73.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. Functional analysis of murine and human B lymphocyte subsets. Transplant Rev. 1975;24:177–236. doi: 10.1111/j.1600-065x.1975.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Harada H., Shioiri-Nakano K., Imai M., Sano T. Potentiation of natural killer activity of human lymphocytes by Staphylococcus aureus bacteria and its protein A. Immunology. 1981 Feb;42(2):175–183. [PMC free article] [PubMed] [Google Scholar]
- Kasahara T., Kin K., Itoh Y., Kawai T., Morita M., Shioiri-Nakano K. Cellular cooperation in lymphocyte activation. I. Cooperative and noncooperative responses of human T and B lymphocytes to various mitogens. Int Arch Allergy Appl Immunol. 1979;58(3):260–273. [PubMed] [Google Scholar]
- Kasahara T., Kin K., Itoh Y., Kawai T., Morita M., Shioiri-Nakano K. Cellular cooperation in lymphocyte activation. IV. Requirement of cell-to-cell interaction for the activation of human T and B lymphocytes by protein A. Cell Immunol. 1980 Jan;49(1):142–153. doi: 10.1016/0008-8749(80)90064-7. [DOI] [PubMed] [Google Scholar]
- Kim Y. T., Schwartz A., Blum J., Weksler M. E. The plaque-forming cell response of human blood lymphocytes. I. PFC response of lymphocytes to formalin-treated staphylocci. Cell Immunol. 1979 Dec;48(2):308–317. doi: 10.1016/0008-8749(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Lind I., Harboe M., Folling I. Protein A reactivity of two distinct groups of human monoclonal IgM. Scand J Immunol. 1975;4(8):843–848. doi: 10.1111/j.1365-3083.1975.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Mellstedt H. In vitro activation of human T and B lymphocytes by pokeweed mitogen. Clin Exp Immunol. 1975 Jan;19(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Möller G., Landwall P. The polyclonal B-cell-activating property of protein A is not due to its interaction with the FC part of immunoglobulin receptors. Scand J Immunol. 1977;6(4):357–366. doi: 10.1111/j.1365-3083.1977.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Pryjma J., Muñoz J., Galbraith R. M., Fudenberg H. H., Virella G. Induction and suppression of immunoglobulin synthesis in cultures of human lymphocytes: effects of pokeweed mitogen and Staphylococcus aureus Cowan I. J Immunol. 1980 Feb;124(2):656–661. [PubMed] [Google Scholar]
- Ringdén O., Rynnel-Dagö B., Waterfield E. M., Möller E., Möller G. Polyclonal antibody secretion in human lymphocytes induced by killed staphylococcal bacteria and by lipopolysaccharide. Scand J Immunol. 1977;6(11):1159–1169. doi: 10.1111/j.1365-3083.1977.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Amadori A., Giudizi M. G., Biagiotti R., Maggi E., Ricci M. Different mitogenic activity of soluble and insoluble staphylococcal protein A (SPA). Immunology. 1978 Sep;35(3):471–478. [PMC free article] [PubMed] [Google Scholar]
- Romagnani S., Giudizi G. M., Almerigogna F., Nicoletti P. L., Ricci M. Protein A of Staphylococcus aureus is mitogenic for IgG-bearing, but also for a subpopulation of IgM- and/or IgD-bearing human lymphocytes. Immunology. 1980 Mar;39(3):417–425. [PMC free article] [PubMed] [Google Scholar]
- Ruuskanen O., Pittard W. B., 3rd, Miller K., Pierce G., Sorensen R. U., Polmar S. H. Staphylococcus aureus Cowan I-induced immunoglobulin production in human cord blood lymphocytes. J Immunol. 1980 Jul;125(1):411–413. [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Saltvedt E., Harboe M. Binding of IgA to protein-A-containing staphylococci: relationship to subclasses. Scand J Immunol. 1976;5(10):1103–1108. doi: 10.1111/j.1365-3083.1976.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Dosch H. M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980 Aug;125(2):820–826. [PubMed] [Google Scholar]
- Strelkauskas A. J., Teodorescu M., Dray S. Enumeration and isolation of human T and B lymphocytes by rosette formation with antibody-coated erythrocytes. Clin Exp Immunol. 1975 Oct;22(1):62–71. [PMC free article] [PubMed] [Google Scholar]


