Abstract
This paper investigates desensitization in vitro, e.g. the inhibition of the transfer of contact sensitivity to picryl chloride by incubation of the passive transfer population with picrylated spleen cells. It asks whether desensitization is based on the same T-suppressor circuit which is responsible for the inhibition of passive transfer by antigen-specific T-suppressor factor (TsF). In this circuit, the T-suppressor cell which acts at the efferent stage (Ts-eff) makes TsF. This TsF depresses contact sensitivity indirectly by arming a T-acceptor cell (Tacc). The armed Tacc, when exposed to antigen (picrylated spleen cells), liberates a non-specific inhibitor which blocks the transfer of contact sensitivity.
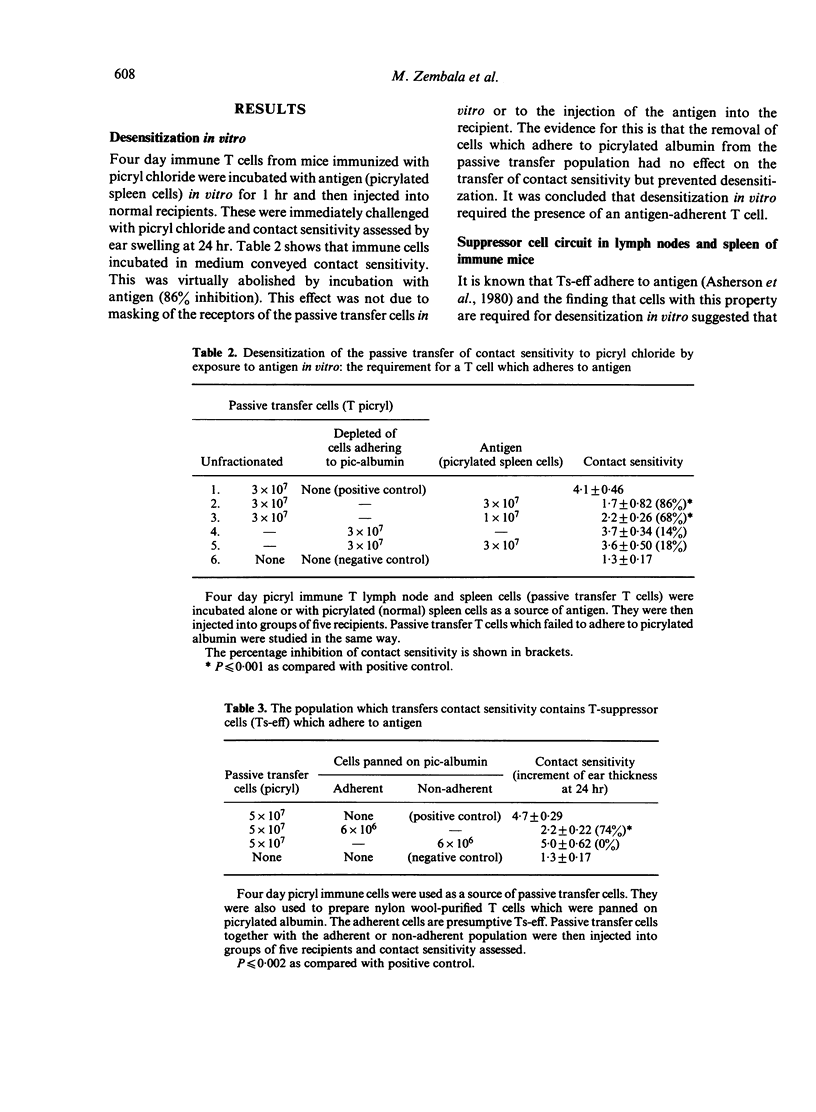
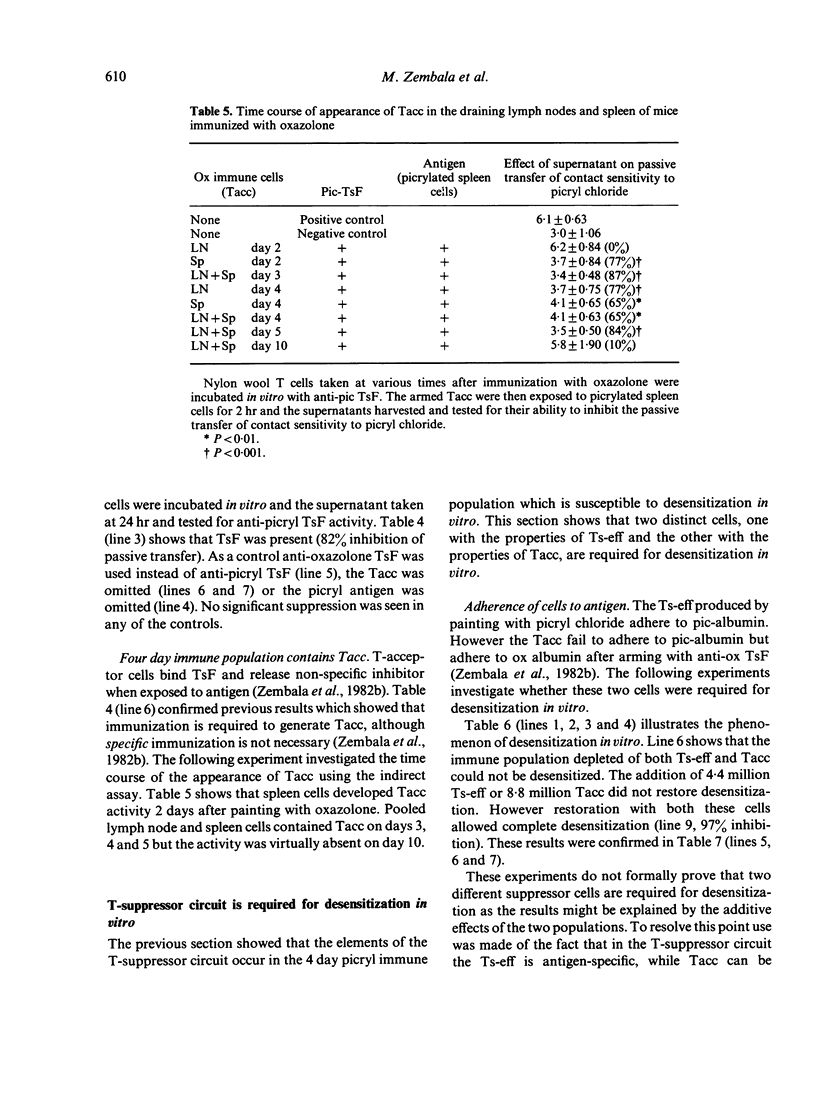
The three elements of this T-suppressor circuit occur in nylon wool-purified T cells prepared from the lymph nodes and spleens of mice four days after immunization with picryl chloride. This population transfers contact sensitivity and can be desensitized in vitro. It contains Ts-eff which can be isolated by panning (adherence) on picrylated albumin and detected by their ability to inhibit passive transfer. The 24 hr supernatant of cultures of these cells contains TsF. Finally the population contains Tacc which appear in the spleen 2 days after immunization and virtually disappear by 10 days.
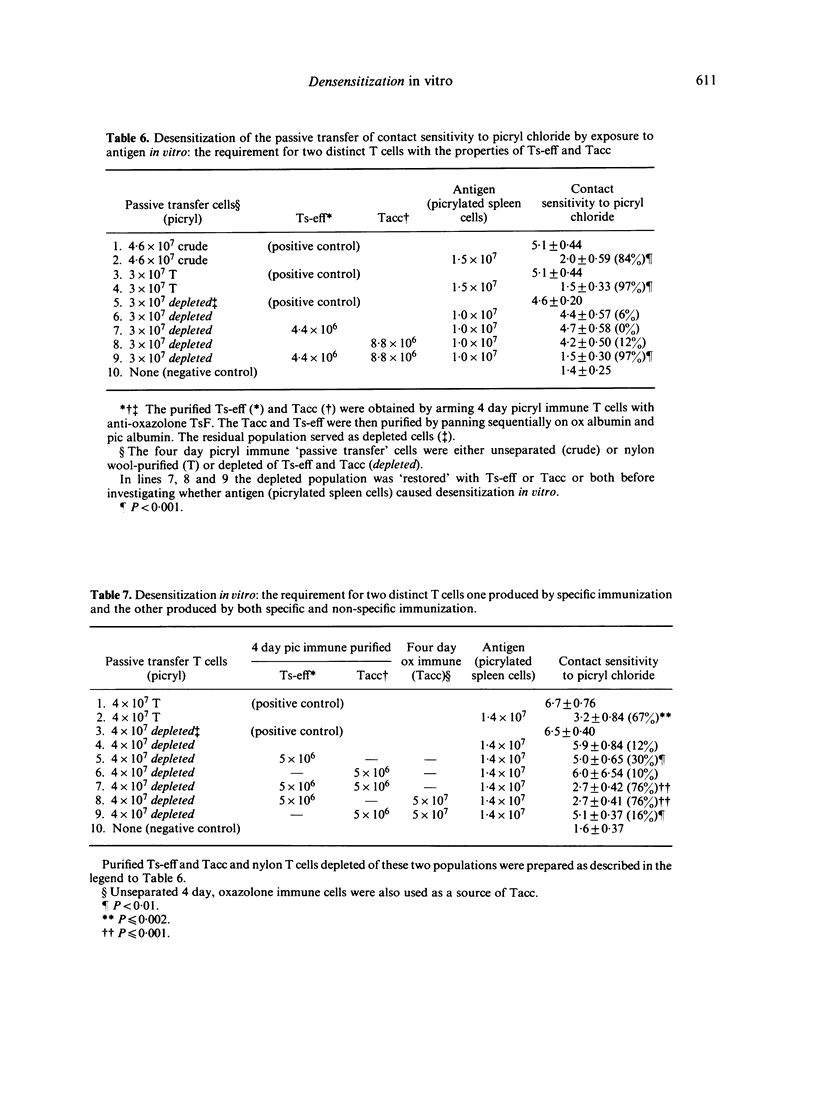
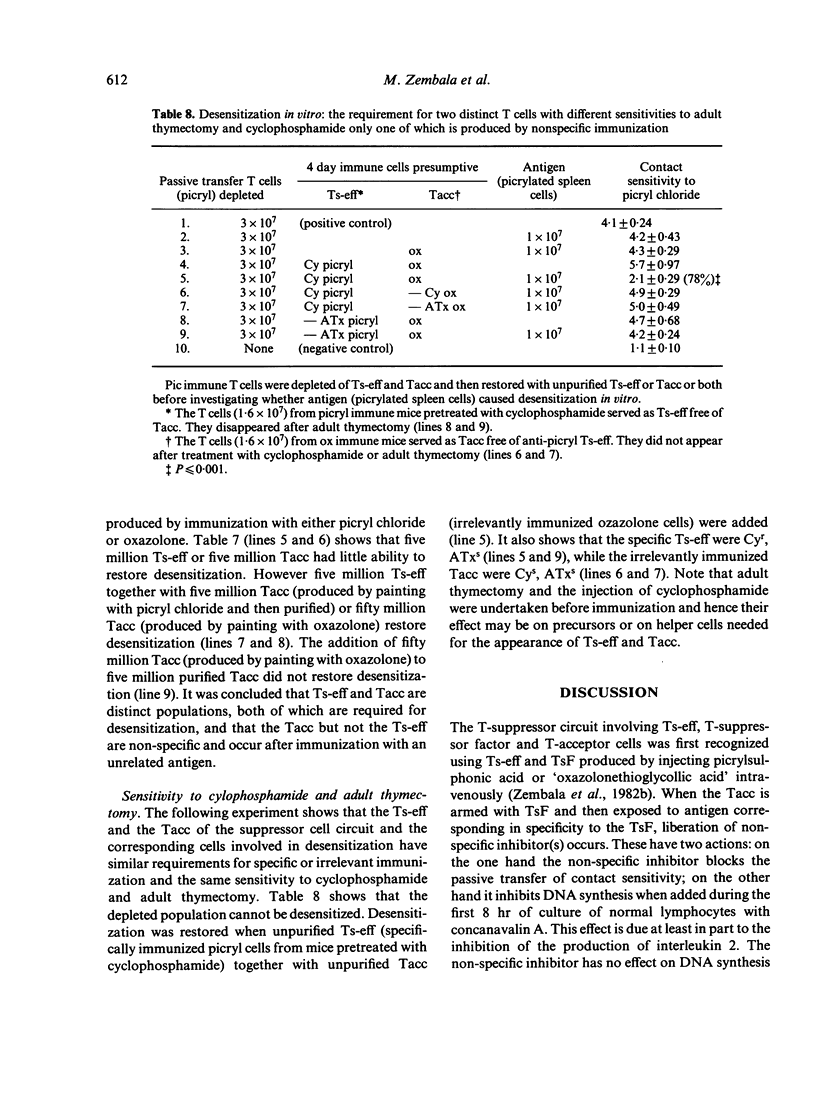
Further experiments demonstrated that the Ts-eff and the Tacc were not merely present but actually required for desensitization in vitro. Immune cells depleted of both Ts-eff (by panning on picrylated albumin) and Tacc (by arming with anti-oxazolone TsF and panning on oxazolonated albumin) cannot be desensitized. To restore desensitization both Ts-eff and Tacc must be added back. The Ts-eff were characterized as cyclophosphamide resistant, adult thymectomy sensitive cells (Cyr, ATxs), which adhered to antigen and were produced only by specific immunization. The Tacc were characterized as Cys, ATxs cells which adhered to antigen only after arming with antigen-specific T-suppressor factor and were produced after immunization with an unrelated contact sensitizer, `oxazolone'. It was concluded that desensitization in vitro was due to the interaction of two distinct T cells: the T-suppressor cell which acts at the efferent stage of the contact sensitivity reaction and the T-acceptor cell which becomes armed with the specific T-suppressor factor produced by the Ts-eff.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Stone S. H. Desensitization in vitro--the specific inhibition, by antigen, of the passive transfer of delayed hypersensitivity by peritoneal exudate cells. Immunology. 1967 Nov;13(5):469–475. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M. T suppressor cells and suppressor factor which act at the efferent stage of the contact sensitivity skin reaction: their production by mice injected with water-soluble, chemically reactive derivatives of oxazolone and picryl chloride. Immunology. 1980 Dec;41(4):1005–1013. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M. The role of the T acceptor cell in suppressor systems. Antigen-specific T suppressor factor acts via a T acceptor cell; this releases a nonspecific inhibitor of the transfer of contact sensitivity when exposed to antigen in the context of I-J. Ann N Y Acad Sci. 1982;392:71–89. doi: 10.1111/j.1749-6632.1982.tb36099.x. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Thomas W. R., Perera M. A. Suppressor cells and the handling of antigen. Immunol Rev. 1980;50:3–45. doi: 10.1111/j.1600-065x.1980.tb00306.x. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Desensitization of delayed-type hypersensitivity by antigen and specific antibody. Cell Immunol. 1975 Sep;19(1):129–136. doi: 10.1016/0008-8749(75)90297-x. [DOI] [PubMed] [Google Scholar]
- Maguire H. C., Jr, Faris L., Weidanz W. Cyclophosphamide intensifies the acquisition of allergic contact dermatitis in mice rendered B-cell deficient by heterologous anti-IgM antisera. Immunology. 1979 Jun;37(2):367–372. [PMC free article] [PubMed] [Google Scholar]
- Papermaster V., Yoshida T., Cohen S. Desensitization. II. Passive Transfer of the desensitized state by serum from desensitized animals. Cell Immunol. 1978 Feb;35(2):378–391. doi: 10.1016/0008-8749(78)90157-0. [DOI] [PubMed] [Google Scholar]
- Parker D., Dwyer J. M., Turk J. L. The effect of cyclophosphamide and role suppressor cells in the desensitization of delayed hypersensitivity. Immunology. 1981 May;43(1):191–196. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Asherson G. L., Marcinkiewicz J. Inhibition of contact sensitivity by macrophages. Int Arch Allergy Appl Immunol. 1981;65(2):121–128. doi: 10.1159/000232747. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Claman H. N. Immune suppression with supraoptimal doses of antigen in contact sensitivity. I. Demonstration of suppressor cells and their sensitivity to cyclophosphamide. J Immunol. 1977 Jul;119(1):240–244. [PubMed] [Google Scholar]
- Sy M. S., Miller S. D., Moorhead J. W., Claman H. N. Active suppression of 1-fluoro-2,4-dinitrobenzene-immune T cells. Requirement of an auxiliary T cell induced by antigen. J Exp Med. 1979 May 1;149(5):1197–1207. doi: 10.1084/jem.149.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thestrup-Pedersen K., Dwyer J. M., Askenase P. W. Studies on the role of the thymus and T cells in the in vivo suppression of delayed hypersensitivity (desensitization): radiosensitivity of the mechanism inducing nonspecific anergy. J Immunol. 1977 May;118(5):1665–1671. [PubMed] [Google Scholar]
- UHR J. W., PAPPENHEIMER A. M., Jr Delayed hypersensitivity. III. Specific desensitization of guinea pigs sensitized to protein antigens. J Exp Med. 1958 Dec 1;108(6):891–904. doi: 10.1084/jem.108.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Cohen S. Lymphokine activity in vivo in relation to circulating monocyte levels and delayed skin reactivity. J Immunol. 1974 Apr;112(4):1540–1547. [PubMed] [Google Scholar]


