Abstract
Physiological evidence indicates that the resting tremor of Parkinson’s disease originates in oscillatory neural activity in the forebrain, but it is unknown whether that activity is globally synchronized or consists of parallel, independently oscillating circuits. In the present study, we used dual microelectrodes to record tremor-related neuronal activity from eight sites in the internal segment of the globus pallidus (GPi) from an awake Parkinson’s disease patient undergoing stereotaxic pallidotomy. We utilized spectral analysis to evaluate the temporal correlations between multiunit activity at spatially separated sites and between neural and limb electromyographic activity. We observed that some GPi neural pairs oscillated synchronously at the tremor frequency, whereas other neural pairs oscillated independently. Additionally, we found that GPi tremor-related activity at a given site could fluctuate between states of synchronization and independence with respect to upper limb tremor. Consistent with this finding, some paired recording sites within GPi showed periods of transient synchronization. These observations support the hypothesis of independent tremor-generating circuits whose coupling can fluctuate over time.
The motor disorders of Parkinson’s disease (PD) and their relief by pallidotomy implicate the basal ganglia in the control of movement. However, neither the nature of the basal ganglia’s role in movement control nor the neural mechanisms that underlie this role or the deficits in PD are understood. Most neurophysiological investigations of basal ganglia function and PD symptoms have focused on the mean firing rates of neurons as a descriptive parameter of neural activity. However, this approach limits the assessment of neuronal interactions and their temporal properties. A more thorough understanding of basal ganglia activity and the dynamic symptoms of PD, such as tremor and drug-induced dyskinesia, requires a finer analysis of the temporal structure of neuronal and neuromuscular interactions. This approach has recently been successfully applied to the study of pallidal activity in a primate model of PD (1). Here, we have applied spectral analysis to study the temporal properties of tremor-related activity in the globus pallidus in a single patient undergoing stereotactic pallidotomy.
Parkinsonian tremor consists of a rhythmic (4- to 8-Hz) activation of muscles, more prominent in distal musculature, that is manifested under resting conditions and in some cases when maintaining posture. This symptom is one of the cardinal features of PD and occurs in about 75% of the cases (2, 3). “Tremor-related activity” is defined as rhythmic neural activity at a frequency in the range of parkinsonian tremor; it does not imply that the neural activity is phase-locked to the tremor. Although tremor-related activity in the basal ganglia and motor thalamus has been reported by many authors, in only a few cases has the temporal relationship between the neural and muscle activity been rigorously examined (4, 5).
It has been proposed that parkinsonian tremor is generated by a central oscillator that entrains the corticothalamic system (6–9) and the basal ganglia (10, 11). Several lines of evidence support this hypothesis. First, tremor-related neural activity has been observed in the motor thalamus (4, 12–14) and the posteroventral globus pallidus (11). Second, tremor is effectively ameliorated by making a lesion in the motor thalamus. Third, limb tremor persists even when proprioceptive feedback is interrupted by dorsal rhizotomy (15, 16), suggesting a central mechanism that does not require sensory feedback. Although these observations support a central origin for parkinsonian tremor, they are not informative as to whether tremor in different muscle groups is generated, on each side of the brain, by a single central oscillator or by multiple independent oscillators.
Alberts et al. (16) proposed that tremor might be generated by independent pacemakers, not requiring a global synchronizing mechanism. The hypothesis of independent tremor-generating circuits gives rise to three predictions: First, simultaneously recorded tremor-related activity may or may not be correlated, depending on whether the cells belong to the same or to different functional groups. Second, tremor-related activity in a given population is likely to be correlated only to the limbs that are functionally linked to that population and not to other limbs. Third, tremor is not likely to be synchronized between limbs, because each limb is, in general, controlled by anatomically and functionally distinct central circuits.
As a preliminary test of these predictions, we analyzed the temporal correlations between simultaneously recorded tremor-related cells in the internal segment of the globus pallidus (GPi) in one PD patient who had undergone stereotactic pallidotomy. Paired recordings were obtained with dual microelectrodes separated by 3 mm. To test the prediction that tremor-related activity is linked to specific muscle groups, we also analyzed the correlations between neural activity in GPi and biceps tremor activity. The data collected from this patient were chosen for analysis because they formed the most complete set with which to address the questions posed here.
METHODS
Data Collection.
Electrophysiological recordings were obtained from a 56-year-old right-handed woman undergoing left pallidotomy for PD. The patient had classical parkinsonian symptoms that developed over a period of 23 years. The initial symptoms were left upper extremity tremor and bradykinesia, and progressed into bilateral bradykinesia, rigidity, severe ON and OFF symptoms, and levodopa-induced dyskinesia.
The base of the Leksell stereotactic frame (Elekta Instruments, Atlanta) was mounted on the patient’s head, parallel to the intercommissural (IC) line, and tightly secured with pins. A high-resolution computerized axial tomography (CAT) scan was performed in the area of the intended lesion such that the anterior and posterior commissures, globus pallidus, internal capsule, and putamen were clearly distinguishable. The target for the lesion was the left ventroposterior GPi, as defined by the Schaltenbrand–Bailey atlas (17), 3 mm below the IC line, 2 mm anterior to the midpoint, and 21 mm lateral to the IC line.
A burr hole was made anterior to the left coronal suture under local anesthesia. The patient’s medication (levodopa/carbidopa) was withheld the morning of surgery. During pallidotomy, the patient was under mild sedation to manage pain and anxiety, but she was fully awake and responsive, with prominent tremor in her arms.
The micromanipulator was mounted on a Leksell xyz stereotactic arc support, and the appropriate target coordinate was selected. The two recording electrodes were advanced in parallel. Three recording trajectories were required to determine the borders of GPi confidently, as well as to identify neighboring structures that should be avoided, mainly the optic tract and the internal capsule. One recording trajectory was obtained with a single electrode and the other two with the dual electrode drive. GPi localization was based on the presence of continuous discharge at a higher rate than neighboring structures, kinesthetic responses, and tremor-related activity. Kinesthetic responses were determined by passively flexing and extending at the contralateral joints, including fingers, wrist, elbow, shoulder, hip, knee, and ankle.
The recordings of neural activity were obtained with 50-μm, beveled, stainless steel electrodes (≈200 kΩ of impedance at 1 kHz). The electrodes were advanced independently through two guide cannulae by using a dual electrode manipulator mounted on the stereotactic frame. The electrodes were 3 mm apart, enabling simultaneous recordings at separate sites. Signals were amplified (10,000×) and bandpass filtered between 600 Hz and 6 kHz. All of the recordings were of multiunit activity in which the action potential amplitudes were at least 3 times the level of the background noise. The neural data were digitized at 30 kHz with a Data Translation digitizing board, using a PC running custom acquisition software. From each recorded site, data were typically acquired for 60 s. Action potentials were threshold extracted from the continuous signal, and the waveforms around the threshold crossing were stored as 32 data points (1.1-ms duration). Extracted waveforms were further analyzed by using a spike-sorting program, allowing identification of spikes from different units based on waveform parameters (18). Spikes that remained unclassified were combined to yield multiunit spike trains. As a result of this procedure, the original raw data were split into single and multiunit signals at 1-kHz resolution.
Electromyograms (EMGs) were recorded from the right biceps and triceps muscles with Grass disk electrodes, band pass filtered (10–300 Hz), amplified (1,000×), and then digitized at 1 kHz, using a PC-based LabView (National Instruments) data acquisition program. Data records for a given recording site were acquired in register with the neural data.
Spectral Analysis.
We used spectral analysis methods (19) to determine the presence and statistical properties of oscillatory activity. For each individual record, we estimated the power spectrum by computing the fast Fourier transform (FFT). The data were separated into 60 nonoverlapping epochs of 1-s duration. The FFT of individual epochs was squared and then averaged, yielding the power spectral estimate. We employed a simple statistical criterion to detect significant peaks in the power spectrum. In this scheme, we tested the null hypothesis that the spike trains were generated from a stationary, Poisson process with rate, λ spikes per second, equal to the mean firing rate of the test data. To determine a significance limit, we took advantage of the fact that the statistical power spectrum of a stationary random process follows a χ2 distribution with 2N degrees of freedom:
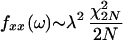 |
for all ω ≠ 0 mod(2π), where fxx is the power, N is the number of sampling windows, and ω is the frequency in Hz. From this distribution, we obtained a 99.99% confidence limit. Peak values exceeding the limit were classified as significant, and neural data yielding significant peaks were considered as oscillatory recordings. One important drawback of this method is that the multiunit spike trains may contain oscillatory cells coexisting with randomly firing cells. This can result in a clear peak below the significance line. A good example of this is the multiunit power spectrum shown in Fig. 4B.
Figure 4.
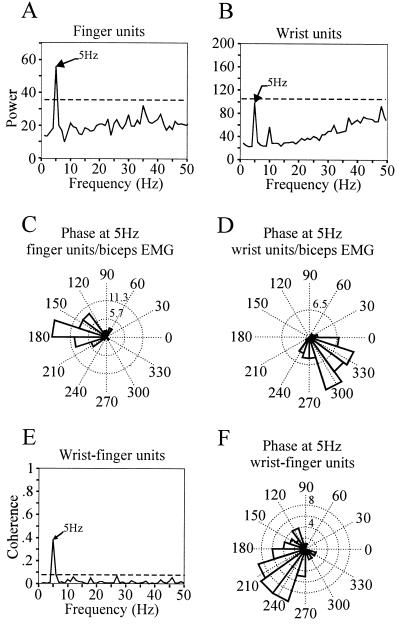
Synchronous tremor-related activity recorded between two sites within GPi separated by about 3 mm. (A and B) Power spectra of the GPi multiunit activity recorded at the two sites. The cells in A showed kinesthetic responses to flexion and extension of the fingers, while the cells in B responded to flexion of the wrist. (C and D) Phase difference histograms at 5 Hz between the biceps EMG and finger units (C) and wrist units (D). Phase differences are calculated for each of the 60 FFTs and are plotted in degrees on a polar axis. The clustering of phase values is indicative of a relatively constant phase lag between the signals at 5 Hz. (E) Coherence spectrum between the finger and wrist units shows significant coupling at 5 Hz. (F) Phase difference histogram between wrist and finger units at the tremor frequency (5 Hz).
To compute the power spectrum of the EMGs, we applied the same method, but the EMGs were rectified prior to FFT computation. To reject nonsignificant peaks in EMG power spectra, we compared the data to a white noise signal with the same power in the 0- to 30-Hz range as the test data. Similar to the spike train, the statistical power spectrum follows a χ2 distribution from which a 99.99% significance limit can be computed.
We used the magnitude squared coherence spectral estimator to quantify the amount of temporal correlation between neural pairs and neural–EMG pairs (20). The coherence spectrum provides a correlation index between 0 and 1 for each analyzed frequency. It is analogous to the correlation coefficient of linear regression analysis, differing in that the data points for each frequency are represented in the complex plane to capture amplitude and phase correlations. For example, a coherence value of 0.9 at 4 Hz indicates an almost constant phase relation of the 4-Hz components of both signals. The data were divided into nonoverlapping windows of 1-s duration, FFTs were computed for each window, and the coherence estimator was computed from the individual paired FFTs. To evaluate the significance of estimated coherence, we tested our data pairs against the null hypothesis of independence. For linearly independent processes, the coherence is zero. However, the coherence estimator is biased, and the bias decreases with the number of nonoverlapping windows used in its computation. Thus, to assess the significance level of the estimated coherence spectrum, we used the distribution in ref. 20:
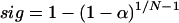 |
where α is the probability for a pair of independent processes of exceeding the coherence value, sig is the confidence level, and N is the number of windows used in the computation. Peaks were considered significant when they exceeded the 99% confidence level.
RESULTS
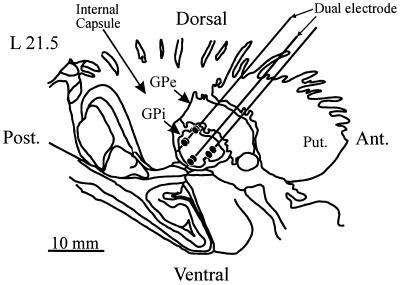
In this patient, we recorded neural activity from 20 sites in GPi. Of these, 8 sites showed tremor-related activity, and these were selected for further analysis. At 7 sites, we sampled multiunit activity and at the remaining site we recorded multiunit activity and a well isolated single unit. Fig. 1 shows a parasagittal section through the basal ganglia with the recording trajectories and the sites at which neuronal activity was recorded. The trajectories were adjusted by using a best-fit criterion according to the established firing properties of putamen, GPe, and GPi (21). Optic tract location was determined by the presence of visually evoked responses.
Figure 1.
Parasagittal section through the basal ganglia showing the reconstructed recording trajectories superimposed on a section from the Schaltenbrand and Bailey stereotactic atlas at 21.5 mm lateral. The parallel lines represent the recording trajectories and the filled ellipses indicate the recording sites. Ant., anterior; Post., posterior; Put., putamen; GPe, globus pallidus external segment.
All of the analyzed sites had oscillatory activity in the range of 4–6 Hz. One site did not show statistically significant oscillations, but consisted of a multiunit recording with a clearly oscillatory component that did not reach significance (Fig. 4B). This can occur when a multiunit recording consists of oscillatory units together with irregularly firing units.
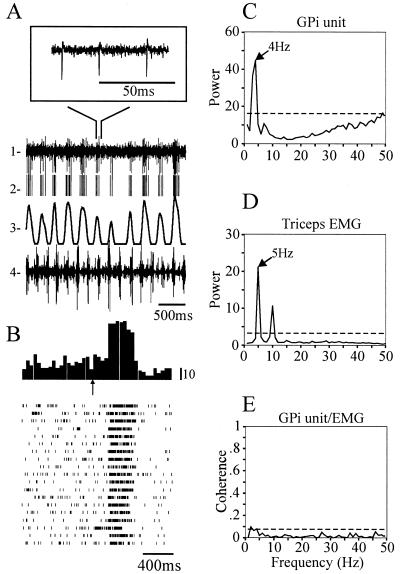
Of the 8 GPi sites, 6 were tested for kinesthetic responses, and all showed clear activation by passive movement of the contralateral upper or lower limbs. Three sites showed multiple joint responses (spanning different combinations of the upper and lower limbs), one responded to the fingers, one to the elbow, and one to the wrist (Table 1). A tremor-related oscillatory cell with kinesthetic activity is shown in Fig. 2. This cell displayed a vigorous sensory response to flexion of the elbow. In response to an auditory cue, the elbow was passively flexed and then extended for 19 trials. This movement evoked an increase of firing on flexion, and inhibition on extension, an example of directional selectivity (Fig. 2B). When the patient had tremor in the arm, involving the flexors and extensors of the elbow, the cell showed oscillatory activity within the tremor frequency range (Fig. 2C); interestingly, the neuronal activity was not significantly correlated to the limb tremor, but instead occurred at a different frequency (Fig. 2 C and D). In accordance, the coherence spectrum between the cell and triceps EMG shows very low coupling at the tremor frequency (Fig. 2E).
Table 1.
Recording sessions and tremor-related activity
| Recording site | Single or multiunit | Kinesthetic response* | No. of data samples† | Tremor-related | Biceps/triceps tremor | No. of units coherent with triceps‡ | Coherence values |
|---|---|---|---|---|---|---|---|
| 1 | m | Thumb, wrist, elbow, shoulder | 1 | Yes | Yes | 0/1 | — |
| 2 | m | Elbow, shoulder, ankle, knee | 3 | Yes | Yes | 2/3 | 0.190, 0.288 |
| 3 | s + m | Elbow | 5 | Yes | Yes | 3/5 | 0.098, 0.222, 0.106 |
| 4 | m | Wrist | 2 | Yes | Yes | 1/2 | 0.601 |
| 5 | m | Not tested | 2 | Yes | Yes | 2/2 | 0.481, 0.662 |
| 6 | m | Not tested | 3 | Yes | Yes | 3/3 | 0.182, 0.090, 0.227 |
| 7 | m | Ankle, knee, hip | 1 | Yes | Yes | 0/1 | — |
| 8 | m | Fingers | 7 | Yes | Yes | 3/7 | 0.613, 0.313, 0.091 |
Units respond to flexion/extension around the specified joints.
Number of recordings performed at location.
Number of coherent recordings/total recordings at that site.
Figure 2.
Tremor-related activity in GPi is not synchronized with tremor in the triceps EMG. (A) Examples of raw and processed data collected during a tremor episode. Trace 1 shows raw data collected from a single site in GPi, with the Inset showing a portion of the raw data on an expanded time base. Trace 2 shows the activity of a single unit, extracted from the raw data in trace 1. Trace 3 represents the spike density, obtained by convolving the spike train in trace 2 with a 100-ms hamming window. Trace 4 shows the EMG activity recorded simultaneously from the triceps muscle. (B) Peri-stimulus-time histogram and raster plots of the activity evoked in the GPi unit by passive flexion and extension of the elbow. The arrow indicates the onset of a tone used to signal the start of the joint movement. (C and D) Power spectra of the GPi unit activity and triceps EMG, respectively, during a tremor episode. Broken lines represent the 99.99% confidence limits computed from an equivalent random signal (see Methods). (E) Coherence spectrum between the GPi unit and triceps EMG activities during the same tremor episode as shown in C and D.
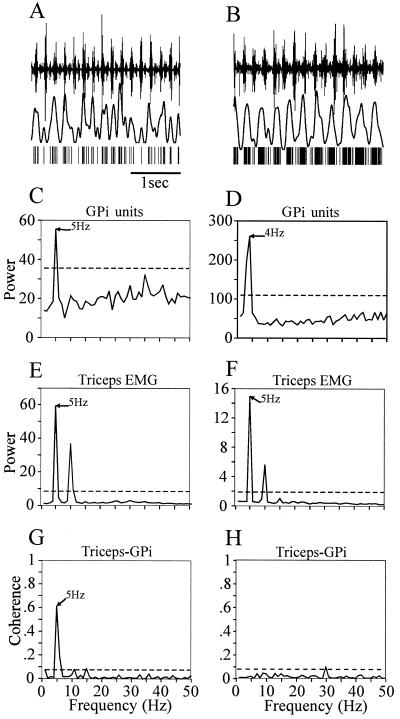
When analyzing longer periods of neural activity, we noticed that tremor-related firing could become transiently synchronized to the limb tremor. Synchronized activity between GPi cells and limb EMG could be present for a period of time, and break down later, giving rise to independent oscillations. An example of this behavior is illustrated in Fig. 3. The figure shows a cell cluster that responded to passive flexion of the fingers. For a recording period of 60 s the multiunit activity was significantly correlated to the triceps tremor, as is visible in the coherence spectrum (Fig. 3G). Later, recording from the same cluster of cells revealed complete independence of its tremor-related activity to the triceps EMG (Fig. 3H). This observation demonstrates that the coupling of neural tremor-related activity to specific skeletal muscles is a dynamic process. Although this recording was very stable over time, the possibility exists that the multiunit recording contained different units at different times.
Figure 3.
Synchronization between multiunit activity in GPi and the triceps EMG is transient in nature. The plots shown on Left and Right illustrate the raw signals (A and B) and the corresponding spectral analyses (C–H) of two epochs of data sampled 5 min apart. (A and B) Top trace, triceps EMG; middle trace, spike density function; bottom trace, extracted spike train. (see Fig. 2A). Note that in A the peaks in the spike density function coincide with the EMG bursts, whereas in B the oscillations in the spike density function occur at a lower frequency than the EMG. (C–F) Power spectra of unit activity and EMG signals, respectively, in plots C and E for the first episode, and D and F for the second episode, occurring 5 min later. In each case, the power spectrum was computed from 1 min of spontaneous tremor (see Methods). Note the change in the neuronal oscillation frequency in GPi from 5 Hz to 4 Hz (compare C and D), while the tremor frequency in the triceps muscle remains constant (compare E and F). The secondary peaks in the spectra at 10 Hz are harmonics of the fundamental frequency. (G and H) Coherence spectra between the GPi unit activity and the triceps EMG for the two recording periods.
Taking into account that tremor-related activity may occur transiently, six of the eight GPi sites showing tremor-related activity displayed significant coherence with the triceps EMG during tremor for at least a 1-min period (Table 1). All the sites that displayed coherence with the contralateral upper limb for at least a 1-min period showed kinesthetic responses to stimulation of the same limb, suggesting a functional link between tremor-related activity and sensory responses. Of the sites that did not show coherence with the EMG tremor, one responded to elbow flexion and the other to ankle flexion. However, in these two cases recordings were carried out only for a 1-min period.
Using dual electrodes, we recorded multiunit activity simultaneously from four pairs of GPi sites that displayed concurrent tremor-related activity (Table 2). Only one of them (site 2) showed clear statistical coupling between the two sites. Of the other three pairs, one was uncorrelated (site 1) and the other two (sites 3 and 4) showed coupling that just reached statistical significance (P = 0.01).
Table 2.
GPi paired recordings
| Paired recording sites | Electrode 1 kinesthetic response* | Electrode 2 kinesthetic response* | No. of recordings† | Significant coherence‡ | Coherence values |
|---|---|---|---|---|---|
| 1 | Elbow | Ankle | 1 | 0/1 | — |
| 2 | Wrist | Fingers | 2 | 2/2 | 0.125, 0.382 |
| 3 | Not tested | Fingers | 2 | 1/2 | 0.140 |
| 4 | Not tested | Fingers | 1 | 1/1 | 0.200 |
Units respond to flexion/extension around the specified joint.
Number of total recording epochs (1 min) performed for each pair.
Number of significantly coherent recording epochs/total recording epochs for each pair.
The pair that showed clear coherent firing consisted of one cluster responsive to passive movement of the wrist and the other responsive to passive movements of the fingers. Both cell groups fired at 5 Hz and showed significant coherence at that frequency (Fig. 4). However, the clusters fired at different phases, with an average relative phase shift of 225°, indicating that their respective peaks tended to occur with ≈80-ms time lag on average. The distribution of phase differences for 60 recording epochs of 1-s duration is tightly clustered around that value, as is shown in Fig. 4F. In addition, both clusters were correlated to the tremor recorded in the triceps EMG, although neither of them responded to passive movement of the elbow. This relation would be expected if tremor activity in the triceps muscle was coupled to the extensors and flexors of the fingers and wrist.
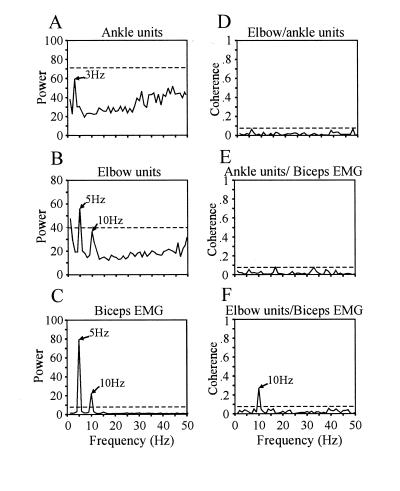
In another case, two tremor-related clusters oscillated with complete independence. Fig. 5 shows an example of two multiunit recordings, ≈3 mm apart, oscillating at different frequencies during a tremor episode (Fig. 5 A and B). One cluster showed a strong kinesthetic response to flexion and extension of the ankle, and a weaker response to the knee and hip, and the other units responded to the elbow. The latter cluster was synchronized with the oscillation in the biceps EMG (Fig. 5F), while the former oscillated at a lower frequency, independently of the other, as is evident from the coherence spectrum (Fig. 5D). Moreover, the cluster showing a passive response to the elbow was coherent with the biceps EMG, at a frequency harmonic of the tremor frequency, whereas the ankle responding cluster showed no correlation to that muscle. This observation and that illustrated in Fig. 4 are consistent with the hypothesis that tremor-related activity is correlated between units responding to movement in the same limb and independent between distant joint units.
Figure 5.
Independent firing among spatially separated GPi tremor-related activities. (A and B) Power spectra of multiunit activity that displayed kinesthetic responses to flexion and extension of the ankle (A) and elbow (B). The spectral peak at 3 Hz in A is not statistically significant. (C) Power spectrum of the biceps EMG. (D) Coherence spectrum between the ankle and elbow units. (E) Coherence spectrum between the ankle units and the biceps EMG. (F) Coherence spectrum between the elbow units and the biceps EMG.
The other two pairs (at recording sites 3 and 4) showed low yet significant coherence (P = 0.01) at 5 Hz during one recording period (1 min). A second recording in one of them showed completely independent activity. The low coherence value may be due to transient synchronization of the signals during a short period of time, which is captured as a low peak by the coherence spectrum.
DISCUSSION
Tremor-related activity has been reported previously in the human GPi (11, 22, 23), yet no study has properly addressed the dynamical relationship between limb tremor and GPi neural activity. Hutchinson and colleagues (11) demonstrated a linear correlation between the peak frequencies of limb tremor and simultaneously occurring GPi tremor-related activity. However, their analysis did not provide information about the phase relationship between the two signals. Signals oscillating within a similar frequency range may do so in complete independence because statistical variation in the power and phase components of the spectrum of two signals can be uncorrelated, even when they share a peak frequency value. Consequently, a different method is necessary to quantify the amount of dynamic coupling. The coherence spectrum is an effective alternative that estimates the amount of power and phase correlation from independent samples of the complex spectrum, resulting in an index between 0 and 1 for each frequency.
When this method was used to analyze data from this single case, some previously unreported properties of tremor-related activity in GPi were observed: First, tremor-related oscillatory activity in GPi can be either synchronized or desynchronized to tremor in a particular limb. Second, spatially separated neural pairs in GPi oscillating simultaneously can do so either in a phase-locked fashion or independently of each other. And third, synchronization between GPi oscillations and limb tremor, when present, may occur transiently: during a tremor episode, GPi oscillations can lock in phase to the limb tremor for a period of time and later become independent yet still oscillatory. This latter conclusion should be taken with some caution, however, because our data sample is small and consisted largely of multiunit recordings.
From these observations, we hypothesize that tremor-related activity in the central nervous system can be generated and propagated independently by several parallel pathways. Given the apparent anatomical and functional segregation of upper and lower limb representations in various movement-related neural structures, and the wide variations in the severity of tremor across different limbs in most parkinsonian patients, it is plausible that tremor in different limbs is generated by separate, independent circuits. This hypothesis implies that tremor in different limbs in PD patients is statistically not synchronized. Current work in our laboratory with multichannel EMGs in patients with tremor supports this conjecture (24).
Various neural mechanisms can account for the presence of synchronous as well as independent tremor-related activity in GPi. One possibility is that tremor-related activity is generated in a globally synchronous manner by the motor thalamus (8, 10) and is transmitted to the globus pallidus. The plausibility of a thalamic origin is supported by the presence of tremor-related activity in the motor thalamus (4, 12–14). This activity has been proposed to result from a low-threshold calcium current in thalamocortical neurons that can sustain an oscillation in membrane potential and can be triggered by hyperpolarizing input from the globus pallidus (8, 10). This thalamic oscillatory activity can be transmitted back to the globus pallidus by two possible pathways, cortical–striatal or corticosubthalamic. There is little support for tremor-related activity in the striatum. Transmission through the corticosubthalamic pathway, however, is supported by the presence of oscillations in the subthalamic nucleus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys (25) and in field potential recordings in the human motor cortex (26). Upon reaching the pallidum the activity could be desynchronized by populations of pallidal neurons, acting as independent nonlinear filters. In other words, different populations of pallidal cells could perform independent operations on the oscillatory input, resulting in noncorrelated outputs. A second possibility is that the thalamic generator itself consists of independent oscillatory neuronal populations.
These mechanisms are complicated by the existence of transiently synchronized limb–GPi oscillations, as reported here. Perhaps in the parkinsonian state, a given tremor-related GPi unit can dynamically change its limb preference. Filion and collaborators (27) demonstrated in parkinsonian monkeys an increase in the number of GPi cells that are kinesthetically responsive to multiple joints, suggesting a loss of joint selectivity. Perhaps this enhanced responsiveness is expressed during tremor by alternate phase locking to different joints, as we report here (Fig. 3). However, this conclusion requires further experimental support, as our data, consisting of multiunit recording, leave open the possibility that we were sampling the activity of different subgroups of cells at that site at different times.
Finally, it is conceivable that GPi generates synchronous tremor-related activity on its own as a result of changes arising from the striatal dopamine depletion. This idea is consistent with previous reports of enhanced rhythmic synchronization in the pallidum of MPTP-treated monkeys, as compared with normal (1, 28). However, our findings suggest that increased correlated activity occurs in functionally related neurons and that such synchronization is a dynamic phenomenon.
Acknowledgments
We thank Hélène Richard for her valuable assistance throughout much of this work. We also thank Dr. Vicky Wheelock for her helpful comments on an earlier version of the manuscript.
ABBREVIATIONS
- PD
Parkinson’s disease
- EMG
electromyogram
- GPe and GPi
globus pallidus external and internal segments
- FFT
fast Fourier transform
References
- 1.Nini A, Feingold A, Slovin H, Bergman H. J Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- 2.Denny-Brown D. The Basal Ganglia and Their Relation to Disorders of Movement. Oxford: Oxford Univ. Press; 1962. [Google Scholar]
- 3.Elble R, Koller W. Tremor. Baltimore: Johns Hopkins Univ. Press; 1990. [Google Scholar]
- 4.Lenz F A, Dostrovsky J O, Tasker R R, Yamashiro K, Kwan H C, Murphy J T. J Neurophysiol. 1988;59:299–316. doi: 10.1152/jn.1988.59.2.299. [DOI] [PubMed] [Google Scholar]
- 5.Lenz F A, Kwan H C, Martin R L, Tasker R R, Dostrovsky J O, Lenz Y E. Brain. 1994;117:531–543. doi: 10.1093/brain/117.3.531. [DOI] [PubMed] [Google Scholar]
- 6.Lamarre Y, Joffroy A, Dumont M, De Montigny C, Grou F, Lund J. Can J Neurol Sci. 1975;2:227–233. doi: 10.1017/s0317167100020321. [DOI] [PubMed] [Google Scholar]
- 7.Lamarre Y. In: Movement Disorders: Tremor. Findley L J, Capileo R, editors. New York: Oxford Univ. Press; 1984. pp. 183–194. [Google Scholar]
- 8.Llinás R. In: Movement Disorders: Tremor. Findley L J, Capileo R, editors. New York: Oxford Univ. Press; 1984. pp. 165–182. [Google Scholar]
- 9.Lenz F A, Vitek J L, DeLong M R. Stereotact Funct Neurosurg. 1993;60:94–103. doi: 10.1159/000100595. [DOI] [PubMed] [Google Scholar]
- 10.Pare D, Curro’Dossi R, Steriade M. Neuroscience. 1990;35:217–226. doi: 10.1016/0306-4522(90)90077-h. [DOI] [PubMed] [Google Scholar]
- 11.Hutchison W D, Lozano A M, Tasker R R, Lang A E, Dostrovsky J O. Exp Brain Res. 1997;113:557–563. doi: 10.1007/pl00005606. [DOI] [PubMed] [Google Scholar]
- 12.Guiot G, Hardy J, Albe-Fressard D. Neurochirurgia (Stuttg) 1962;5:1–18. doi: 10.1055/s-0028-1095441. [DOI] [PubMed] [Google Scholar]
- 13.Lenz F A, Tasker R R, Kwan H C, Schnider S, Kwong R, Murayama Y, Dostrovsky J O, Murphy J T. J Neurosci. 1988;8:754–764. doi: 10.1523/JNEUROSCI.08-03-00754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohye C, Shibazaki T, Hirai T, Wada H, Hirato M, Kawashima Y. J Neurophysiol. 1989;61:488–500. doi: 10.1152/jn.1989.61.3.488. [DOI] [PubMed] [Google Scholar]
- 15.Pollock L, Davis L. Arch Neurol Psychiat. 1930;23:303–319. [Google Scholar]
- 16.Alberts W W, Libet B, Wright E W, Jr, Feinstein B. Confin Neurol. 1965;26:318–327. doi: 10.1159/000104047. [DOI] [PubMed] [Google Scholar]
- 17.Schaltenbrand G, Bailey P. Introduction to Stereotaxis with an Atlas of the Human Brain. Stuttgart: Thieme; 1959. [Google Scholar]
- 18.Gray C M, Maldonado P E, Wilson M, McNaughton B. J Neurosci Methods. 1995;63:43–54. doi: 10.1016/0165-0270(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 19.Brillinger D R. Time Series: Data Analysis and Theory. San Francisco: Holden-Day; 1981. [Google Scholar]
- 20.Rosenberg J R, Amjad A M, Breeze P, Brillinger D R, Halliday D M. Prog Biophys Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- 21.Kimura M, Matsumoto N. Adv Neurol. 1997;74:111–118. [PubMed] [Google Scholar]
- 22.Umbach W, Ehrhardt K J. Arch Psychiat Nervenkr. 1965;207:106–113. doi: 10.1007/BF00343773. [DOI] [PubMed] [Google Scholar]
- 23.Jasper H, Bertrand G. In: The Thalamus. Purpura D P, Yahr M D, editors. New York: Columbia Univ. Press; 1966. pp. 365–390. [Google Scholar]
- 24.Hurtado J, Lachaux J-P, Beckley D, Gray C, Sigvardt K. Soc Neurosci Abstr. 1998;24:1719. [Google Scholar]
- 25.Bergman H, Wichmann T, Karmon B, DeLong M R. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 26.Alberts W W, Wright E W, Jr, Feinstein B. Nature (London) 1969;221:670–672. doi: 10.1038/221670a0. [DOI] [PubMed] [Google Scholar]
- 27.Filion M, Tremblay L, Bedard P. Brain Res. 1988;444:165–176. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- 28.Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Trends Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]