Abstract
Risk of wound infection is increased in morbidly obese surgical patients, in part because a major determinant of wound infection risk, tissue oxygenation, is marginal. Unlike in lean patients, supplemental inspired oxygen (FIO2) only slightly improves tissue oxygenation in obese patients. Mild hypercapnia improves tissue oxygenation in lean, but has not been evaluated in obese patients. We thus tested the hypothesis that mild hypercapnia markedly improves tissue oxygenation in morbidly obese patients given FIO2 80% during major abdominal surgery. Thirty obese patients (body mass index 61.5±17 kg/m2) scheduled for open gastric bypass were randomly assigned to normocapnia (n=15, end-tidal PCO2 35 mmHg) or hypercapnia (n=15, end-tidal PCO2 50 mmHg); FIO2 was 80%. Anesthetic management and other confounding factors were controlled. Tissue oxygen tension was measured subcutaneously at the upper arm using a polarographic probe in a silastic tonometer. Demographic characteristics, cardiovascular measurements, and PaO2 (222±48 versus 230±68 mmHg in normocapnic versus hypercapnic; mean±SD, P=0.705) were comparable in the groups. Tissue oxygen tension, however, was greater in hypercapnic than in normocapnic patients (78±31 versus 56±13 mmHg, P=0.029). Mild hypercapnia increased tissue oxygenation by an amount believed to be clinically important and could potentially reduce the risk of surgical wound infection in morbidly obese patients.
Keywords: obesity, hypercapnia, tissue oxygenation, and wound infection
Introduction
Obesity is a rapidly increasing health care problem worldwide. In obese surgical patients, wound infections are common and associated with considerable morbidity (1). A contributing factor is almost certainly reduced subcutaneous wound tissue oxygenation (PsqO2). The primary defense against surgical pathogens is oxidative killing by neutrophils (2); hence, infection risk is inversely related to tissue oxygen partial pressure (3–5). For instance, the incidence of infection can be increased by either perioperative cigarette smoking or hypothermia (6), both of which reduce PsqO2 (7,8).
PsqO2 in healthy lean and obese awake volunteers is approximately 60 mmHg (9). PsqO2 remains near that concentration during anesthesia and surgery in lean patients, but it is dramatically decreased — in some patients almost by half — in obese patients during anesthesia and surgery (10). Furthermore, in obese patients providing 80% inspired oxygen during surgery only slightly increases perioperative PsqO2 (10), whereas it doubles PsqO2 in lean patients (11).
Two factors contribute to diminished PsqO2 in obese patients. One is that arterial oxygen (PaO2) is reduced in obese patients at any given inspired oxygen concentration (10). The second factor is that obesity increases the size of individual fat cells without changing blood flow (12). Although cardiac output, the amount of circulating blood, and resting oxygen consumption are all elevated in obese patients (13), tissue perfusion remains abnormally low in relation to body weight (14,15) and, therefore, poorly oxygenated. Thus, even at a given PaO2, PsqO2 is significantly less in obese patients than in lean patients (10). All the above factors result in PsqO2 that is critically low in the obese individual undergoing anesthesia and surgery, i.e., approximately 40 mmHg (10), leading to an increased risk of wound infections (4).
Mild hypercapnia (i.e., an end-tidal PCO2 of 50 mmHg) increases cardiac index (16) and may induce peripheral vasodilation (17). Hypercapnia thus significantly increases PsqO2 in lean volunteers (16) and surgical patients (18), and does so at no cost and with minimal side effects. Because supplemental oxygen is relatively ineffective in obese patients (10), hypercapnia remains an attractive option for improving PsqO2 in this high-risk population. It remains unknown, though, whether hypercapnia can overcome the relative hypoperfusion of the adipose tissue. We, therefore, tested the hypothesis that hypercapnia (end-tidal PCO2 of 50 mmHg versus 35 mmHg) increases PsqO2 in obese patients given 80% inspired oxygen.
Methods
With approval of the local human studies committee and written informed patient consent, we enrolled 30 patients undergoing open gastric bypass surgery. A patient had to have a body mass index (BMI) of more than 50 kg/m2 to qualify for the study. A single surgeon performed all operations. Exclusion criteria were documented coronary or peripheral artery disease, insulin-dependent diabetes mellitus, history of recent cigarette smoking, and any symptoms of infection or sepsis.
Protocol
General anesthesia was induced with sodium thiopental (3–5 mg/kg) and fentanyl (1–3 μg/kg). Vecuronium (0.1 mg/kg) or succinylcholine (0.8–1 mg/kg) was given to facilitate endotracheal intubation. After induction of anesthesia, patients were randomly assigned to one of two groups: normocapnia (n=15) or hypercapnia (n=15). The patients randomized to normocapnia were maintained at an end-tidal carbon dioxide partial pressure (ETCO2) of 35 mmHg, which we considered routine care; those randomized to hypercapnia were maintained at an ETCO2 of 50 mmHg.
The lungs of the patients in both groups were ventilated with a tidal volume of 6–8 mL/kg with a respiratory rate of approximately 10 breaths per minute. Positive end-expiratory pressure was maintained at 5 mmHg, and peak ventilatory pressure was kept less than 45 mmHg. We induced hypercapnia by removing the soda lime from the rebreathing system and increasing or decreasing the fresh gas flow as necessary to maintain the ETCO2 partial pressure near 50 mmHg. EtCO2 partial pressure was continuously monitored using infrared side stream capnography.
Anesthesia was maintained with sevoflurane (1–2%) in 80% oxygen, balance nitrogen. Sevoflurane administration was adjusted to maintain mean arterial blood pressure within 20% of the preinduction value. A supplemental bolus dose of fentanyl (100 μg) was given when heart rate or arterial blood pressure exceeded 120% of their baseline values. No vasoactive drugs were given.
In all patients, normal body weight in kg was estimated according to an average normal BMI of 22.5 kg/m2. A fluid bolus of 10 mL/kg normal body weight was given before induction of anesthesia. Subsequently, patients were given a maintenance dose of at least 10–12 mL·kg normal body weight−1·hour−1. Additionally boluses of colloids were administered as necessary to maintain mean arterial blood pressure within 20% of baseline and to maintain urine output more than 1 mL·kg normal body weight−1·hour−1. Blood loss was replaced with crystalloid at a 4:1 ratio or colloid at a 2:1 ratio. Allogenic blood was administered as necessary to maintain hematocrit more than 26%. A forced-air warmer was placed over the lower body of each patient to keep core temperature near 36°C.
Measurements
Demographic data, ASA Physical Status, preoperative laboratory values, and type and duration of surgery were recorded. All routine anesthetic, respiratory, and hemodynamic variables during the surgery were also recorded. Detailed records of fluid management, including urine output, were kept. Inspired oxygen, end-tidal sevoflurane, and ETCO2 concentrations were measured continuously during anesthesia. Oxygen saturation was measured with a pulse oximeter throughout anesthesia. Intraoperative core temperature was measured in the distal esophagus (Mon-a-therm, Tyco-Mallinckrodt Anesthesiology Products, St. Louis, MO).
After induction of anesthesia, a 20-g cannula was inserted into a radial artery. Pressure from this catheter was monitored continuously during surgery and in the postanesthesia care unit. Cardiac output was measured noninvasively using the partial rebreathing Fick method (NICO, Novametrix Medical System Inc, Wallingford, CT). Arterial blood for gas analysis was obtained intraoperatively in each patient at least once during the study during stabilized conditions approximately one hour after the beginning of surgery.
After induction of anesthesia, a silastic tonometer was inserted into the lateral left upper arm for measurement of subcutaneous PsqO2 and temperature. The silastic tonometer consisted of 15 cm of tubing filled with hypoxic saline; 10 cm of the tubing was tunneled subcutaneously. A polarographic oxygen sensor and thermistor (Licox, Integra Life Science, CA, USA) were inserted into the subcutaneous portion of the tonometer as previously described (19). PsqO2 (PsqO2) was recorded at 10-min intervals during the intraoperative period, and the probe was removed before discontinuation of anesthesia.
In vitro accuracy of the oxygen sensors is ± 3 mmHg for the range from 0 to 100 mmHg and ± 5% for 100 to 360 mmHg in a water bath at 37°C. Temperature sensitivity is 0.25%/ºC. However, thermistors were incorporated into the sensors, and temperature compensation was included in the PsqO2 calculations. Oxygen sensor calibration remains stable (within 8% of baseline value for room air) in vivo for at least 8 hours. The electrodes were individually factory-calibrated, but calibration was confirmed by exposing the electrode to room air (ambient pO2 of 154 mmHg); in all cases, measurements in air were within 10% of 154 mmHg. To exclude a significant drift of the oxygen sensor, probes were again exposed to room air after each surgery; none differed by more than 10% from its baseline value.
Forearm-minus-fingertip temperature gradient was measured as an indicator of peripheral arteriovenous shunt vasoconstriction. As in previous studies (20), we defined a gradient more than zero an indication of significant vasoconstriction.
Data analysis
The major purpose of our study was to test the hypothesis that mild hypercapnia increases intraoperative subcutaneous oxygen partial pressure in morbidly obese patients given 80% inspired oxygen. Based on the results of a previous study in lean volunteers (16), we estimated that hypercapnia would increase subcutaneous oxygen partial pressure by 15 mmHg, with a standard deviation of 15 mmHg. A sample-size estimate based on these data suggested that 13 patients per group would provide 90% power to detect a significant difference in PsqO2 at an alpha level of 0.05. Therefore, we studied 30 morbidly obese patients (15 per group).
Demographic and morphometric factors and major and minor outcomes in the two groups were compared with unpaired, two-tailed t or Fisher exact tests. Intraoperative values were averaged over the entire operation for each patient, and then averaged among patients in each group. Nonparametrically distributed data were analyzed by Wilcoxon Rank Sum tests. Results are presented as means ± SDs or medians [25th percentile, 75th percentile]; P < 0.05 was considered statistically significant.
Results
The study was performed at the Washington University, St. Louis, Missouri. All 30 enrolled patients completed the study according to protocol. Demographic and morphometric factors were similar in the two groups (Table 1). Intraoperative anesthetic management was similar in each group (Table 2), with one exception: only 2 of 15 hypercapnic patients required colloids to maintain hemodynamic stability whereas 9 of 15 normocapnic patients were given colloids (P = 0.008) No blood transfusions were needed to keep the hematocrit above 30%.
Table 1.
Demographic and Morphometric Characteristics.
| Normocapnia | Hypercapnia | P | |
|---|---|---|---|
| Number of Patients | 15 | 15 | — |
| Age (y) | 49 ± 12 | 45 ± 7 | 0.215 |
| Weight (kg) | 157 ± 33 | 189 ± 57 | 0.068 |
| Height (cm) | 168 ± 10 | 169 ± 10 | 0.854 |
| Body Mass Index (kg/m2) | 60 ± 11 | 67 ± 19 | 0.081 |
| Sex (M/F) | 5 / 10 | 3 / 12 | 0.409 |
| Preoperative Hemoglobin (mg/dL) | 13.7 ± 1.1 | 13.2 ± 1.4 | 0.273 |
| ASA Status (II/III/IV) | 4/11/0 | 4/11/0 | 1.000 |
| Duration of Surgery (min) | 200 ± 38 | 225 ± 69 | 0.226 |
| Duration of Anesthesia (min) | 202 ± 65 | 233 ± 54 | 0.133 |
Data are presented as means ± SDs or as counts for the categorical outcomes. Means were compared with unpaired, two-tailed t tests, and counts were compared with chi-square or Fisher’s exact tests.
Table 2.
Intraoperative Management
| Normocapnia | Hypercapnia | P | |
|---|---|---|---|
| Core Temperature (°C) | 36.3 ± 0.5 | 36.2 ± 0.4 | 0.731 |
| End-tidal Sevoflurane (%) | 2.1 ± 1.0 | 1.9 ± 0.7 | 0.571 |
| Fentanyl (μg) | 460.0 ± 172.4 | 530.0 ± 295.1 | 0.434 |
| Blood Loss (mL) | 350 [250, 500] | 300 [200, 700] | 0.496 |
| Crystalloid (mL/h) | 4066 ± 1226 | 4020 ± 1258 | 0.919 |
| Colloid (mL/h) | 500 [0, 1000] | 0 [0, 0] | 0.025 |
| Urine Output (mL/h) | 425 [260, 700] | 300 [250, 450] | 0.180 |
| Hemoglobin (mg/dL) | 12.4 ± 1.3 | 12.0 ± 1.6 | 0.549 |
Data are presented as means ± SDs or medians [25th percentile, 75th percentile]. Means were compared with unpaired, two-tailed t tests, medians with Wilcoxon rank-sum tests.
Rebreathing carbon dioxide induced mild hypercapnia without causing any adverse effects. All hemodynamic variables were comparable in the two groups (Table 3). ETCO2 partial pressures were 35 ± 2 mmHg in the normocapnic group and 52 ± 6 mmHg in the hypercapnic group (P < 0.001). PaCO2 also was significantly less in the normocapnic patients (42 ± 8 mmHg) than in the hypercapnic patients (58 ± 7 mmHg; P < 0.001); pH was significantly less in the hypercapnic patients (Table 3).
Table 3.
Intraoperative Hemodynamics, Respiratory Variables, and Tissue Oxygenation.
| Normocapnia | Hypercapnia | P | |
|---|---|---|---|
| End-tidal PCO2 (mmHg) | 35 ± 2 | 52 ± 6 | <0.001 |
| Arterial PCO2 (mmHg) | 42 ± 8 | 58 ± 7 | <0.001 |
| Arterial pH | 7.38 ± 0.08 | 7.29 ± 0.05 | <0.001 |
| MAP (mmHg) | 88 ± 15 | 87 ± 9 | 0.817 |
| Heart Rate (beats/min) | 83 ± 18 | 81 ± 10 | 0.676 |
| Cardiac Index (L/m2) | 3.6 ± 0.8 | 3.7 ± 0.8 | 0.740 |
| PaO2 (mmHg) | 222 ± 48 | 230 ± 68 | 0.705 |
| TsqO2 (°C) | 34.3 ± 2.2 | 34.1 ± 1.8 | 0.797 |
| PsqO2 (mmHg) | 56 ± 13 | 78 ± 31 | 0.029 |
Data are presented as means ± SDs. Means were compared with unpaired, two-tailed t tests. End-tidal PCO2 = end tidal carbon dioxide partial pressure. Arterial PCO2 = arterial carbon dioxide pressure. MAP = mean arterial pressure. PaO2 = arterial oxygen partial pressure TsqO2 = tissue temperature in the PsqO2 measurement area. PsqO2 = tissue oxygen partial pressure.
PaO2 was virtually identical in the two treatment groups. However, subcutaneous tissue oxygen tension (PsqO2) was significantly greater in patients assigned to mild hypercapnia (78 ± 31 mmHg) than in those assigned to normocapnia (56 ± 13 mmHg, P < 0.029, Table 3 and Figure 1).
Figure 1.
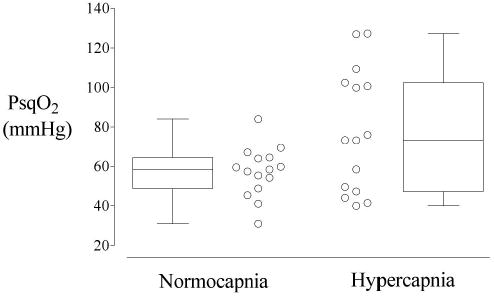
Subcutaneous tissue oxygen tension (PsqO2) averaged over the entire operation for each patient (open circles) and then summarized with a box and whisker plot for each treatment group. The Normocapnia group had an end-tidal PCO2 of 35 mmHg (n=15). The Hypercapnia group had an end-tidal PCO2 of 50 mmHg (n=15); the FIO2 in both groups was 80%. The difference between the groups was statistically significant, P = 0.029.
Patients in both groups were slightly vasodilated, but forearm-minus-fingertip temperature gradients were similar in the hypercapnic patients (−1.0 ± 2.6°C) and the normocapnic patients (−1.0 ± 2.8°C, P = 0.99).
Discussion
Wound infection risk is inversely related to PsqO2 (4). Supplemental (80%) oxygen, which doubles PsqO2 in lean patients, halves the risk of surgical wound infection in this patient population (11). However, supplemental oxygen only slightly increases PsqO2 in morbidly obese patients (10). It is thus apparent that providing supplemental oxygen alone will only partially address the overwhelming infection risk that occurs in obese patients.
Our results suggest an additional strategy: we found that in morbidly obese patients having major abdominal surgery, mild intraoperative hypercapnia significantly increased PsqO2 by 22 mmHg from 56 ± 13 to 78 ± 31 mmHg (≈40%; P = 0.03). This is a clinically important increase, since tissue oxygen tension near 80 mmHg almost eliminates the risk of wound infection (4). Our results are notable in that our patients were all given 80% oxygen; our data thus indicate that mild hypercapnia provides a substantial incremental improvement in PsqO2 beyond that provided by supplemental oxygen alone.
Nearly all mechanistic, animal, and clinical evidence suggests that PsqO2 is an important determinant of infection risk. The exception is a recent study by Pryor et al. (21); however, this study was under powered and had methodological problems. Thus, the results are difficult to interpret (11,22,23) and should not be considered proof that PsqO2 is unimportant.
Mild hypercapnia increases cardiac index in lean patients (16) and may induce peripheral vasodilation (17). Which factor contributes most to improved PsqO2 remains unknown. In contrast, in our study, hypercapnic and normocapnic obese patients had similar cardiac output during the study period. A possible, but unlikely, explanation is that the NICO partial rebreathing Fick system, which has not been validated in morbidly obese patients, may simply have missed a true increase. Another possibility is that cardiac output does not increase in the morbidly obese during hypercapnia, possibly because cardiac reserve is limited in these patients or they are normally hyperdynamic (24). It is also possible that cardiac output in the normocapnic patients was relatively high as a result of supplemental colloid administration. However, in the hypercapnic patients, hypercapnia itself probably increased cardiac output and, consequently, they did not need additional fluids to maintain arterial blood pressure. Thus, arterial blood pressure, heart rate, and cardiac index were comparable in each treatment group.
As expected, arterial pH was slightly less in the hypercapnic patients, but the reduction was of questionable clinical importance. There was thus little or no evidence of hypercapnia-induced toxicity in our patients.
We provided mild intraoperative hypercarbia by removing the soda lime CO2 absorbent from the circle system of the anesthesia machine. We used this approach so that minute ventilation and respiratory rate would be comparable in each treatment group. However, it is obviously easier to simply reduce the respiratory rate until the desired end-tidal PCO2 accumulates. This is presumably the approach that would be used clinically. Hypercapnia by hypoventilation also increases cardiac output by increasing venous return as a result of the decreased mean intrathoracic pressure. Because no drugs or special equipment is required, there is hardly any cost associated with inducing mild hypercapnia with either approach.
There are many ways to improve PsqO2 during surgery, including maintenance of normothermia, adequate pain treatment, regional anesthesia, and adequate volume and fluid substitution. Fluid administration in morbidly obese patients might be difficult to evaluate. However, in critical patient populations prone to wound infections such as morbidly obese patients, a combination of all the above treatments might be necessary to adequately increase PsqO2 and, thus, decrease wound infection rate.
We studied a population of morbidly obese patients who had a BMI near 63 kg/m2. Such patients have a frequent incidence of sleep apnea and, thus, presumably normally maintain abnormally high arterial PaCO2 concentrations. Therefore, an ETCO2 of 50 mmHg may have been normal for our study participants while 35 mmHg was too low. However, these characteristics are typical of the morbidly obese population and do not reduce the validity of our results or the conclusions we draw from them.
The BMI in our hypercapnic patients was 67 kg/m2 while the BMI in the normocapnic patients was 60 kg/m2. This difference was not significant and is not likely to be clinically important. Furthermore, since a greater BMI reduces PsqO2, a totally equal BMI in the hypercapnic and normocapnic group would have increased the difference in subcutaneous PsqO2 and further strengthened our results.
In summary, intraoperative mild hypercapnia (ETCO2 = 52 mmHg) significantly increased PsqO2 by 22 mmHg (≈40%) in morbidly obese patients having major abdominal surgery. Hypercapnia is easy to provide, involves no additional costs, and causes minimal side effects. We thus conclude the intraoperative mild hypercapnia could be a useful addition to supplemental oxygen in morbidly obese patients at risk for surgical wound infection. However, further studies are needed to evaluate the impact of our results on patient outcome.
Footnotes
Received from Department of Anesthesiology, Washington University, St. Louis, MO; Department of Anesthesiology and General Intensive Care, University of Vienna, Austria; the Outcomes Research Institute, University of Louisville, Louisville, KY; the Department of Outcomes Research, Cleveland Clinic Foundation, Cleveland, OH; and the Department of Anesthesiology, University of Bern, Switzerland.
Presented, in part, at the annual meeting of the American Society of Anesthesiologists in San Francisco, California, October 2003.
Implications: Wound and tissue oxygenation are critically low in morbidly obese patients, even with supplemental oxygen administration. Mild hypercapnia combined with supplement oxygen during the perioperative period significantly increased tissue oxygenation in obese patients.
Supported by NIH Grant GM 61655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). CRD Grant #931504, Clinical Research Division, Department of Anesthesiology, Washington University School of Medicine (St. Louis, MO). Tyco-Mallinckrodt Anesthesiology Products, Inc. (St. Louis, MO) donated the thermocouples we used. None of the authors has a personal financial interest related to this research. Gilbert Haugh, M.S., helped with the statistical analysis and Nancy Alsip, Ph.D., edited the manuscript (both of the University of Louisville).
References
- 1.Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. 2003;361:2032–5. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 2.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–68. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 3.Gottrup F. Physiology and measurement of tissue perfusion. Ann Chir Gynaecol. 1994;83:183–9. [PubMed] [Google Scholar]
- 4.Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997;132:997–1004. doi: 10.1001/archsurg.1997.01430330063010. discussion 5. [DOI] [PubMed] [Google Scholar]
- 5.Van Esbroeck G, Gys T, Hubens A. Evaluation of tissue oximetry in perioperative monitoring of colorectal surgery. Br J Surg. 1992;79:584–7. doi: 10.1002/bjs.1800790640. [DOI] [PubMed] [Google Scholar]
- 6.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334:1209–15. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 7.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126:1131–4. doi: 10.1001/archsurg.1991.01410330093013. [DOI] [PubMed] [Google Scholar]
- 8.Sheffield CW, Sessler DI, Hopf HW, et al. Centrally and locally mediated thermoregulatory responses alter subcutaneous oxygen tension. Wound Rep Reg. 1997;4:339–45. doi: 10.1046/j.1524-475X.1996.40310.x. [DOI] [PubMed] [Google Scholar]
- 9.Hiltebrand LB, Kaiser HA, Niedhart DJ, Kurz A. Obesity Does Not Decrease Subcutaneous Tissue Oxygenation Annual Meeting of the American Society of Anesthesiologists. New Orleans, LA, 2005:A365.
- 10.Kabon B, Nagele A, Reddy D, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–80. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greif R, Akca O, Horn EP, et al. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. Outcomes Research Group. N Engl J Med. 2000;342:161–7. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 12.Jansson PA, Larsson A, Smith U, Lonnroth P. Glycerol production in subcutaneous adipose tissue in lean and obese humans. J Clin Invest. 1992;89:1610–7. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Divitiis O, Fazio S, Petitto M, et al. Obesity and cardiac function. Circulation. 1981;64:477–82. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 14.Cheymol G. Drug pharmacokinetics in the obese. Fundam Clin Pharmacol. 1988;2:239–56. doi: 10.1111/j.1472-8206.1988.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 15.Di Girolamo M, Skinner NS, Jr, Hanley HG, Sachs RG. Relationship of adipose tissue blood flow to fat cell size and number. Am J Physiol. 1971;220:932–7. doi: 10.1152/ajplegacy.1971.220.4.932. [DOI] [PubMed] [Google Scholar]
- 16.Akca O, Doufas AG, Morioka N, et al. Hypercapnia improves tissue oxygenation. Anesthesiology. 2002;97:801–6. doi: 10.1097/00000542-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Mas A, Saura P, Joseph D, et al. Effect of acute moderate changes in PaCO2 on global hemodynamics and gastric perfusion. Crit Care Med. 2000;28:360–5. doi: 10.1097/00003246-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Akca O, Liem E, Suleman MI, et al. Effect of intraoperative end-tidal carbon dioxide partial pressure on tissue oxygenation. Anaesthesia. 2003;58:536–42. doi: 10.1046/j.1365-2044.2003.03193.x. [DOI] [PubMed] [Google Scholar]
- 19.Hopf HW, Viele M, Watson JJ, et al. Subcutaneous perfusion and oxygen during acute severe isovolemic hemodilution in healthy volunteers. Arch Surg. 2000;135:1443–9. doi: 10.1001/archsurg.135.12.1443. [DOI] [PubMed] [Google Scholar]
- 20.Kurz A, Xiong J, Sessler DI, et al. Desflurane reduces the gain of thermoregulatory arteriovenous shunt vasoconstriction in humans. Anesthesiology. 1995;83:1212–9. doi: 10.1097/00000542-199512000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Pryor KO, Fahey TJ, 3rd, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population: a randomized controlled trial. JAMA. 2004;291:79–87. doi: 10.1001/jama.291.1.79. [DOI] [PubMed] [Google Scholar]
- 22.Greif R, Sessler DI. Supplemental oxygen and risk of surgical site infection (letter) JAMA. 2004;291:1957. doi: 10.1001/jama.291.16.1957-a. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 23.Akca O, Sessler DI. Supplemental oxygen and risk of surgical site infection (letter) JAMA. 2004;291:1956–7. doi: 10.1001/jama.291.16.1956-b. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 24.Kanoupakis E, Michaloudis D, Fraidakis O, et al. Left ventricular function and cardiopulmonary performance following surgical treatment of morbid obesity. Obes Surg. 2001;11:552–8. doi: 10.1381/09608920160556715. [DOI] [PubMed] [Google Scholar]


