Abstract
Does emotion affect how people see? We investigated the effects of emotion and attention, as well as their conjoint effect, on contrast sensitivity, a dimension of early vision. We manipulated the emotional valence and the attentional distribution of cues preceding a target stimulus and asked observers to judge the orientation of the target as contrast varied. This study provides the first behavioral evidence that (a) emotion enhances contrast sensitivity irrespective of attention and (b) emotion potentiates the effect of attention on contrast sensitivity.
Attention allows people to select a subset of information and grant it priority for processing. Attending to information in the environment could play an even more substantial role when that information is emotionally arousing—providing information critical for reacting to changes in the environment. For instance, the sudden appearance of a fearful face in the periphery probably indicates an impending threat or danger, warranting immediate attention. Indeed, there is evidence that emotion facilitates both the speed with which arousing information is processed (Öhman, Flykt, & Esteves, 2001) and the likelihood that it will be processed (Anderson & Phelps, 2001; Vuilleumier & Schwartz, 2001). In a rapid serial visual presentation (RSVP) paradigm, the second of two stimuli presented in rapid succession is often not detected—a phenomenon known as the attentional blink (Raymond, Shapiro, & Arnell, 1992). However, if this second stimulus is emotionally arousing, it is less likely to be missed (Anderson & Phelps, 2001). Although this finding suggests enhanced processing of emotional stimuli, the level at which emotion and attention interact and how they do so is unclear. Could this interaction affect early vision?
In an effort to understand the mechanisms responsible for the emotional modulation of attention, researchers have begun to explore the underlying neural circuitry. This exploration has focused on the amygdala, a medial temporal lobe structure known to be important for processing emotional stimuli. Patients with bilateral amygdala damage show no attenuation of the attentional blink with emotional stimuli. This finding indicates that the amygdala is involved in the enhanced perception of emotional events (Anderson & Phelps, 2001). The amygdala responds to the emotional content of an event rapidly (Ledoux, 2002) and prior to awareness (Whalen et al., 1998). It has been suggested that the amygdala facilitates perception by altering sensory cortical processing via feedback connections to visual cortex (Kapp, Supple, & Whalen, 1994; Ledoux, 2002). In studies consistent with this hypothesis, the amygdala and extrastriate cortex have been shown to correlate in their responses to images of fearful faces (Morris et al., 1998), and this modulation is attenuated in patients with amygdala damage (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). These studies suggest the amygdala may alter attentional processing in emotional contexts, by modulating early visual regions. Although these brain-imaging results suggest an interaction between emotional stimuli and perception, there is no behavioral evidence indicating that emotion directly alters early visual processes. Could the mere presence of an emotional stimulus affect the way one sees?
In contrast, it is well established that attention enhances perception at early visual levels. Attention can be allocated to a relevant location either by overtly moving the eyes or by covertly deploying attention to that location (Posner, 1980). Covert attention allows the observer to monitor the environment and informs subsequent eye movements. Covert attention can be allocated at will in response to task or goal demands (i.e., sustained or endogenous attention), or reflexively (i.e., transient or exogenous attention; Cheal & Lyon, 1991; Nakayama & Mackeben, 1989). Single-cell studies (Martinez-Trujillo & Treue, 2002; McAdams & Maunsell, 1999; Reynolds, Pasternak, & Desimone, 2000) and neuroimaging studies (Brefczynski & DeYoe, 1999; Liu, Pestilli, & Carrasco, 2005) have shown that covert attention modulates contrast sensitivity in early visual areas.
In the present study, we focused on transient covert attention, which is stimulus driven and automatically triggered by the sudden appearance of a peripheral stimulus. Transient attention enhances performance on a variety of perceptual tasks, such as visual search (Carrasco & McElree, 2001; Nakayama & Mackeben, 1989), letter identification (Talgar, Pelli, & Carrasco, 2004), and texture segmentation (Yeshurun & Carrasco, 1998, 2000), and affects processes carried out by primary visual cortex, such as spatial resolution (Carrasco, Williams, & Yeshurun, 2002; Yeshurun & Carrasco, 1998, 2000) and contrast sensitivity (Cameron, Tai, & Carrasco, 2002; Carrasco, Ling, & Read, 2004; Carrasco, Penpeci-Talgar, & Eckstein, 2000; Liu et al., 2005; Lu & Dosher, 2000). Considering the link between emotion and attention (Anderson & Phelps, 2001; Öhman et al., 2001; Vuilleumier & Schwartz, 2001), and knowing that attention affects early visual processes, we investigated the possibility that emotion interacts with attention to further enhance even the earliest levels of visual perception. Because of the reflexive nature of transient covert attention, we propose that it may play a particularly crucial role in selectively processing information when the emotional content of the cue signals potential threat (e.g., a fearful face).
The two goals of the present study were to determine (a) whether emotion influences early visual processes, irrespective of attention, and (b) whether emotion potentiates the effect of attention on contrast sensitivity. Contrast is a natural candidate for understanding the relations among emotion, attention, and early vision. Enhanced contrast sensitivity brought about by an emotionally arousing cue would allow observers to better detect the presence of potentially threatening stimuli and to respond to them more effectively.
EXPERIMENT 1: DOES EMOTION ENHANCE PERCEPTION?
In Experiment 1, we explored the influence of emotion on perception. We manipulated the emotional valence of a face (fearful or neutral valence) appearing at fixation and measured the effect of the face on contrast thresholds for a subsequent discrimination task. We decided to use an orientation discrimination task because performance on this task improves with contrast (e.g., Cameron et al., 2002; Carrasco et al., 2000; Foley & Legge, 1981). We predicted that if emotion enhances perception irrespective of attention, the presence of a fearful face would result in lower contrast thresholds than the presence of a neutral face.
Method
Subjects
Fourteen undergraduates from the New York University Subject Pool participated in Experiment 1. They were all naive as to the purpose of this study and had normal or corrected-to-normal vision.
Apparatus
The stimuli were created using Matlab and the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). Observers viewed the stimuli on a gamma-corrected monitor. A video attenuator was used to drive just the green gun of a 21-in. IBM P260 monitor (1024 × 768 pixels; 120 Hz)—thus providing a larger possible set of distinct luminance levels (∼12.6 bits). Background luminance was set to 12.2 cd/m2.
Stimuli and Design
A black square (0.3° × 0.3°) was presented in the center of a uniform gray background, serving as a fixation point that remained on screen throughout the entire experiment (Fig. 1). The target display consisted of four Gabor patches (sinusoidal gratings enveloped by a Gaussian; 2 cycles/deg, 7.9° × 7.9°), which were centered at 11° eccentricity on the intercardinal meridians. One Gabor was tilted (8°) either clockwise or counterclockwise (target stimulus), and the other three were oriented vertically (distractor stimuli). Both the location and the tilt of the target Gabor were random from trial to trial.
Fig. 1.
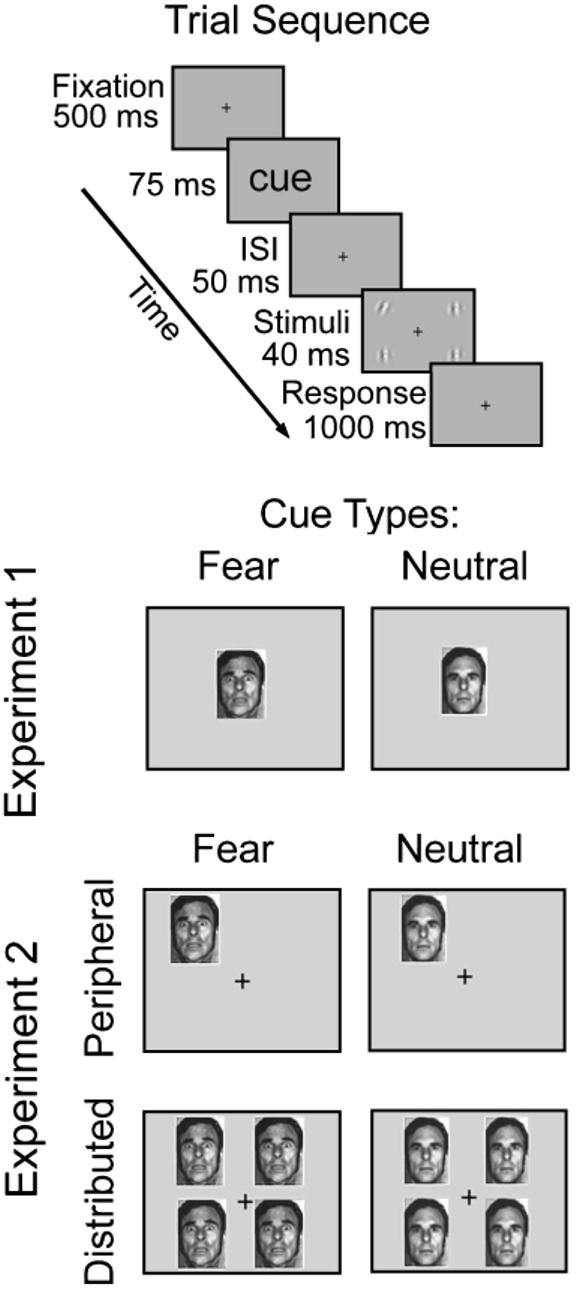
Sequence of events in a trial and illustration of cue types. In Experiment 1, each trial contained a fearful or neutral face at the center of the screen. Shortly after presentation of this cue, four gratings were flashed—a tilted target and three vertical distractors. Observers performed a two-alternative forced-choice orientation discrimination task (left vs. right) for the target. The trial sequence and task in Experiment 2 were similar, except that the cue preceding the target was either peripheral (attracting covert attention) or distributed (baseline). ISI = interstimulus interval.
We used the method of constant stimuli to obtain psychometric functions. In each trial, the contrast of the Gabors was randomly sampled from a set of Michelson contrasts in nine log increments from 2% to 20%. The four Gabor stimuli were preceded by a face at the center of the screen (5° × 6.6° of visual angle). To manipulate emotion, we sampled from the Pictures of Facial Affect series (Ekman & Friesen, 1976) prototypical exemplars of fearful and neutral expressions. The set included 11 unique exemplars of each emotion category (6 female faces and 5 male faces), for a total of 22 unique facial stimuli. Each fearful expression had a neutral counterpart posed by the same person. To ensure that the facial expressions were discriminable, we conducted a preliminary study, which indicated that observers could report the facial expression of both expression types at the size, eccentricity, and timing parameters used in the study with greater than 90% accuracy.
To rule out the possibility that differential effects on performance could be due to low-level characteristics of these face cues (e.g., differences in mean luminance due to different amounts of white in eyes and teeth), we also presented both face types inverted (control condition), such that their physical content remained the same but their emotional content was not readily processed (McKelvie, 1995). In total, Experiment 1 involved four face conditions: fear, neutral, and their respective upside-down counterparts.
Procedure
Observers viewed the display binocularly at a distance of 57 cm from the monitor, with their heads stabilized by a chin rest. They were asked to fixate on the central fixation point throughout the experiment. On each trial, a fixation point appeared for 500 ms and was followed by the brief flash of either a fearful or a neutral face in the center of the screen (75 ms). Following a blank interstimulus interval of 50 ms, the four Gabor stimuli (tilted target and three distractors) were presented for 40 ms. The 165-ms interval between cue onset and stimulus offset was chosen to preclude eye movements, thus ensuring that the observers performed the task under conditions of covert attention (Mayfrank, Kimmig, & Fischer, 1987). Observers performed a two-alternative forced-choice (2AFC) orientation discrimination task for the target Gabor. If the target was tilted to the left, they indicated its orientation by pressing “1.” If it was tilted to the right, they pressed “2.” Feedback was given; correct responses were followed by a tone, and no tone was presented after incorrect responses. Each observer performed 1 training block before the 10 experimental blocks of 120 trials each; half the trials contained fearful faces, and half contained neutral faces. The experiment lasted 1 hr.
Results
The results for this experiment are summarized in the psychometric functions shown in Figure 2. The data were fit to separate Weibull functions, with chance and asymptote constrained to 50% and 100%, respectively. Threshold was taken at 82% accuracy.
Fig. 2.
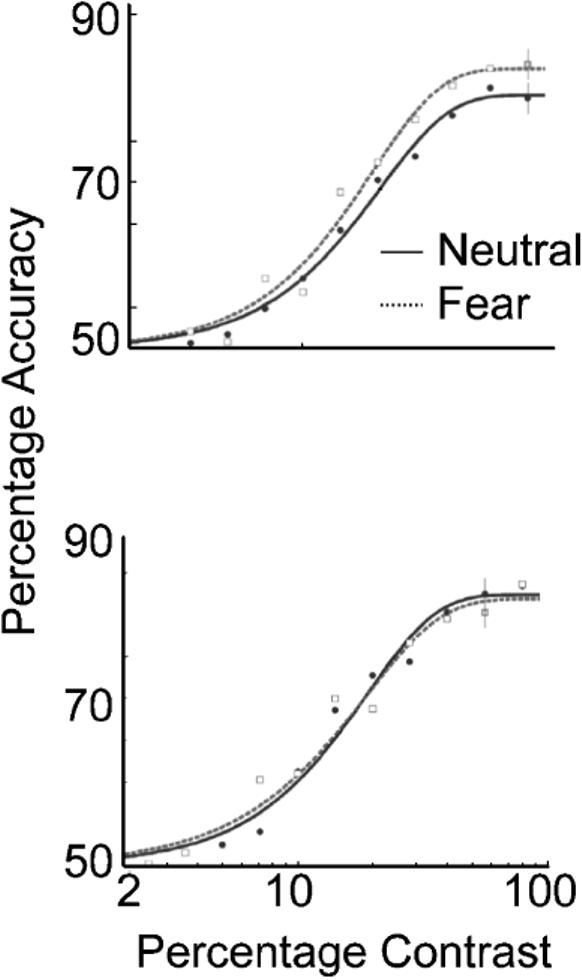
Contrast psychometric functions from Experiment 1: observers' average accuracy as a function of the stimulus contrast level for trials with upright faces as cues (top) and trials with upside-down faces as cues (bottom). The averaged functions reflect the general performance of all observers. Error bars correspond to the average ±1 standard error for each condition.
The top graph in Figure 2 shows the results for trials on which the face cue was presented upright. When observers were presented with a fearful face before the target stimuli appeared, the psychometric function shifted to the left, indicating an increase in contrast sensitivity at threshold (19% contrast for fearful faces vs. 22% contrast for neutral faces). In other words, the level of contrast needed to perform the orientation discrimination task was lower when the stimuli were preceded by a fearful face than when they were preceded by a neutral face. A nested hypothesis test (Weibull with a separate fit for each condition vs. one fit for the two conditions collapsed together) revealed significant differences between the two conditions (p < .01). The observers were more sensitive to contrast when the faces had a fearful expression than when they had a neutral expression; that is, the mere presence of a fearful face heightened contrast sensitivity. This is the first demonstration that emotion can alter an early visual process; emotion actually affects how people see.
In contrast, when the faces were inverted (control condition; Fig. 2, bottom panel), the psychometric functions did not differ between the two face conditions (p > .05; 19 vs. 18.7% contrast). This indicates that the effect obtained was indeed a result of emotional processing of the faces, and not a result of low-level feature differences.
EXPERIMENT 2: DOES EMOTION INTERACT WITH ATTENTION IN AFFECTING EARLY VISION?
Knowing that covert attention enhances early visual processes, and given the link between emotion and attention (Anderson & Phelps, 2001), we investigated what influence emotion may have on the effect of covert attention on perception. Does emotion potentiate the effect of covert attention on perception? Having established that the stimuli used in Experiment 1 lowered contrast threshold, we used the same stimuli as the attentional cues. To explore the possible interaction between attention and emotion, we used a cuing paradigm in which we varied the emotional content of the attentional cues to examine their effect on contrast sensitivity. We manipulated attention prior to the appearance of the Gabor stimuli by presenting a single peripheral cue adjacent to the upcoming target location—drawing attention reflexively to that location. In the baseline condition, a cue appeared at each possible target location (i.e., distributed cue). Although both cue types indicated the temporal onset of the target display, only the peripheral cue drew focused attention exclusively to the target location.
We expected to replicate the typically observed attentional effect; that is, we expected the peripheral-cue condition would lead to lower contrast thresholds than the distributed-cue condition. Additionally, if the effect of emotion observed in Experiment 1 works independently of the effect of covert attention (i.e., the effects are additive), the amount of reduction in threshold due to a fearful face would be the same for the peripheral-cue and distributed-cue conditions. However, if covert attention and emotion interact multiplicatively to enhance perception, the effect of a fearful face on contrast thresholds would differ depending on the type of attentional cue. That is, the effect of emotion on threshold would be greater in the peripheral-cue condition than in the distributed-cue condition.
Method
Subjects
Six observers, all with normal or corrected-to-normal vision, participated in Experiment 2. Five of these observers were naive as to the purpose of the experiment. The sixth observer, S.L., was a coauthor.
Stimuli and Design
The stimuli were almost identical to those in Experiment 1. The only difference was that the faces used in Experiment 1 were used here as attentional precues (Fig. 1). On each trial, the four Gabor stimuli (7.9° × 7.9°; centered at 11° eccentricity on the intercardinal meridians) were preceded by one of two types of attentional precues: peripheral (to elicit focused transient attention) or distributed (baseline; spreading attention across the display). As in Experiment 1, to manipulate emotion, we used faces of either fearful or neutral valence (5° × 6.6°) as cues. In the peripheral-cue condition, a randomly sampled face appeared centered at 5° eccentricity on the intercardinal meridian, adjacent to the location where the target was about to appear. The distributed precue consisted of four faces (identical), simultaneously presented adjacent to all four possible target locations. Whereas the face was always presented at the center of the display in Experiment 1, Experiment 2 utilized a set of distributed cues as a baseline condition. The central cue and distributed cues are equivalent; it has been shown that the performance difference between peripheral-cue and central-cue conditions is comparable to the difference between peripheral-cue and distributed-cue conditions for both contrast sensitivity (Talgar et al., 2004) and spatial resolution (Carrasco et al., 2002; Yeshurun & Carrasco, 1998, 2000).
Experiment 2 had eight precue conditions: fearful peripheral, neutral peripheral, fearful distributed, neutral distributed, and their respective upside-down counterparts.
Procedure
Observers viewed the display binocularly at a distance of 57 cm from the monitor, with their heads stabilized by a chin rest. They were asked to fixate on the central fixation point throughout the experiment. Each trial began with a fixation point for 500 ms, followed by the brief flash of one or four fearful or neutral faces (75 ms). Following an interstimulus interval of 50 ms, the Gabor stimuli were presented for 40 ms. Observers performed a 2AFC orientation discrimination task for the target Gabor. Contrast thresholds at 82% performance were obtained for each condition using the QUEST adaptive staircase procedure (Watson & Pelli, 1983), which is a conventional psychophysical method to obtain thresholds efficiently. Staircases for all eight conditions were interleaved in each run, with 40 trials per condition, for a total of 320 trials per run. Each observer participated in approximately 10 practice runs or more until thresholds stabilized, followed by 25 experimental runs distributed over six 1-hr sessions. For each condition, 25 contrast thresholds were obtained.
Note that the 125-ms interval between the cue and target onsets was chosen to maximize the effect of the peripheral cue in automatically eliciting transient attention (Carrasco et al., 2000, 2004; Carrasco & McElree, 2001; Nakayama & Mackeben, 1989; Yeshurun & Carrasco, 1998). Furthermore, the 165-ms interval between cue onset and stimulus offset was chosen to preclude eye movements, thus ensuring that the observers performed the task under the conditions of covert attention (Mayfrank et al., 1987).
Results
Peripheral cues led to higher contrast sensitivity than distributed cues did (Carrasco et al., 2000, 2004; Carrasco & McElree, 2001; Liu et al., 2005); that is, transient attention itself enhanced contrast sensitivity. Furthermore, observers were more sensitive to contrast when the faces had a fearful expression as opposed to a neutral expression. As in Experiment 1, this result suggests that the mere presence of fearful faces heightens contrast sensitivity. However, there were no systematic differences between the upside-down fearful and neutral faces—again ruling out an alternative, low-level explanation for the results.
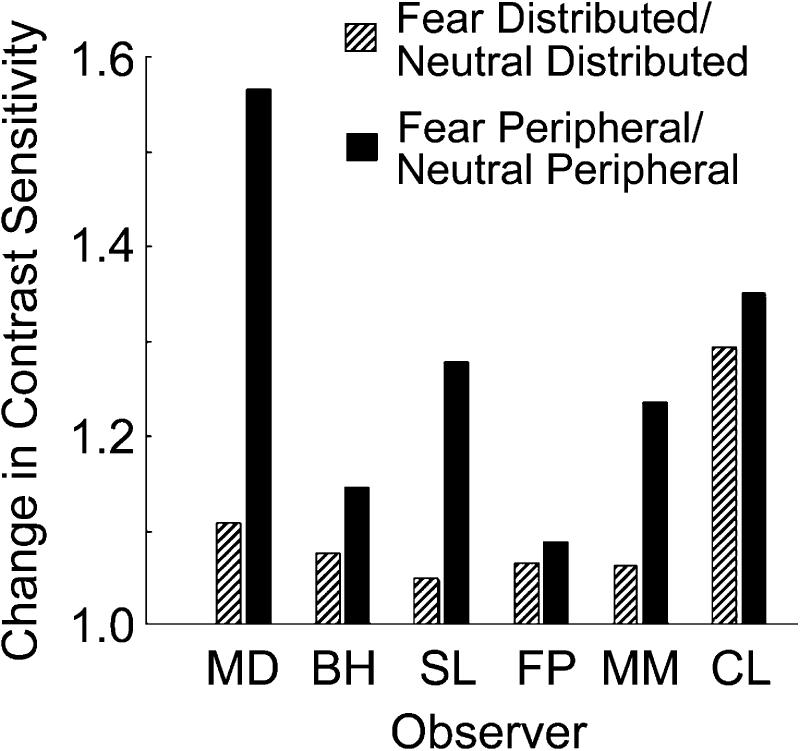
The effects of emotion and attention on contrast sensitivity interacted. Figure 3 illustrates the effect of emotion on sensitivity by plotting the ratio between fearful and neutral faces for the peripheral-cue and distributed-cue conditions separately. A ratio of 1 would indicate no effect of the fearful face on contrast sensitivity, and ratios higher than 1 indicate an increase in contrast sensitivity with fearful faces. All 6 observers showed the same pattern of results: Emotion increased contrast sensitivity. A pure effect of emotion on perception—regardless of attention—would be indicated if the effect of emotion were identical in the peripheral- and distributed-cue conditions. However, there was a greater effect of emotion for the peripheral-cue than the distributed-cue condition across all observers. The presence of a fearful face heightened contrast sensitivity more in the peripheral-cue condition (fearful face, peripheral cue/neutral face, peripheral cue) than in the distributed-cue condition (fearful face, distributed cue/neutral face, distributed cue). The highest sensitivity was induced by the conjunction of fearful face and peripheral cue. Thus, manipulating emotion in conjunction with attention increased contrast sensitivity beyond the mere manipulation of either attention or emotion.
Fig. 3.
Contrast-sensitivity ratios for the 6 observers in Experiment 2 (upright faces). The ratio between contrast sensitivity following a fearful face and contrast sensitivity following a neutral face is shown separately for the peripheral-cue and distributed-cue conditions. A ratio of 1 would indicate no difference between the fearful and neutral conditions; values greater than 1 indicate that fearful faces improved contrast sensitivity more than neutral faces.
A within-subjects three-way analysis of variance (attention: distributed vs. peripheral; emotion: fearful face vs. neutral face; face orientation: upright vs. inverted) on the log-transformed contrast thresholds confirmed these results. When the faces were right side up, there were main effects of emotion, F(1, 591) = 14.70, p < .005, and attention, F(1, 591) = 121.10, p < .0001, as well as an Emotion × Attention interaction, F(1, 591) = 4.82, p < .05. The effect of attention on contrast sensitivity was more pronounced for the right-side-up faces, F(1, 591) = 101.81, p < .0001, than for the upside-down faces, F(1, 591) = 41.38, p < .01. Face orientation interacted with emotion, F(1, 1187) = 6.49, p < .05; the effect of emotion on contrast sensitivity was significant only for right-side-up faces; there was no effect of emotion for upside-down faces, F(1, 591) = 1.09, p > .1.
Figure 4 illustrates the impact of emotion and attention on perception. The gratings depict the average contrast threshold (i.e., the contrast necessary to perform the orientation discrimination task at 82% accuracy) for each of the four experimental conditions using upright faces as precues. The contrast threshold was lower when observers were cued with a fearful face than when the cue was a neutral face (a vs. b, c vs. d). The highest sensitivity was induced by the conjunction of a fearful face and a peripheral cue (a).
Fig. 4.
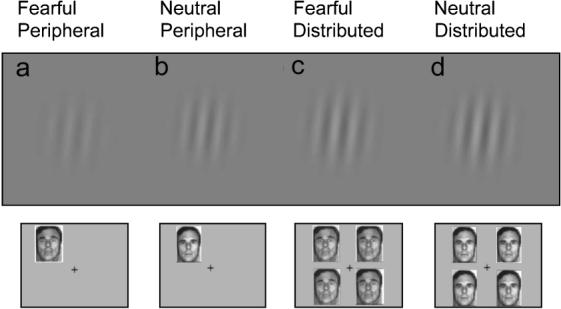
The impact of emotion and attention on perception. The gratings shown represent the contrast threshold (i.e., the contrast necessary to perform the orientation discrimination task at 82% accuracy) in each condition: fearful face, peripheral cue (a); neutral face, peripheral cue (b); fearful face, distributed cue (c); and neutral face, distributed cue (d).
DISCUSSION
The present results are the first to demonstrate that emotion facilitates early visual processing. The mere presence of a fearful face enhances contrast sensitivity (Experiment 1), and this effect is magnified with transient covert attention (Experiment 2). Previous studies have shown that both emotion (Kapp et al., 1994; Kosslyn et al., 1996; Ledoux, 2002; Morris et al., 1998) and covert attention (Brefczynski & DeYoe, 1999; Corbetta & Shulman, 2002; Martinez-Trujillo & Treue, 2002; McAdams & Maunsell, 1999; Reynolds et al., 2000; Saenz, Buracas, & Boynton, 2003) can enhance activity in the visual cortex, suggesting possible neural mechanisms for the independent effects of emotion and attention on perception. In the case of emotion, this modulation may occur via feedback from the amygdala (Anderson & Phelps, 2001; Ledoux, 2002). The amygdala has reciprocal connections with visual processing regions, including the extrastriate cortex (Whalen et al., 1998). It has been suggested that the amygdala, which receives information about the emotional salience of a stimulus quickly and prior to awareness (Amaral, Behniea, & Kelly, 2003), may modulate early visual processes in the presence of emotional stimuli (Ledoux, 2002; Morris et al., 1998). In the case of attention, this modulation may come about via a ventral fronto-parietal network, which includes the temporo-parietal junction (TPJ) cortex and the ventral frontal cortex (VFC; Corbetta & Shulman, 2002). However, the present results indicate that the effects of emotion and attention on early vision are not simply additive; rather, the joint effect of emotion and attention demonstrates that emotion potentiates the perceptual benefits brought about by transient attention.
What mechanism underlies emotion's potentiation of attention? It is possible that there is a multiplicative interactive effect of emotion and attention on processing in early visual cortex, an effect beyond what would be predicted by a simple additive effect of each alone. However, it is also possible that an emotional, fearful face leads to an amygdala response, which in turn provides feedback to both the visual cortex and additional regions that directly enhance attention. The notion that the amygdala may interact with attention directly is supported by the fact that face-processing regions, such as the fusiform gyrus, demonstrate an enhanced response to fearful (compared with neutral) faces, and that attention increases this effect (Anderson, Christoff, Panitz, De Rosa, & Gabrieli, 2003; Vuilleumier, Armony, Driver, & Dolan, 2001).
We chose to use fearful faces to manipulate emotion because these stimuli have been shown previously to lead to correlated responses in the amygdala and extrastriate cortex (Morris et al., 1998). Fearful faces in particular may indicate an ambiguous threat in the environment. Fear in another person suggests the presence of a potential threat, but the source of the threat is unclear (Whalen, 1998). For this reason, a fearful face could be the optimal cue to engage transient covert attention, given its reflexive nature. Although we have clearly demonstrated that emotion facilitates perception and potentiates attention, the magnitude of these effects might vary depending on the type of emotion cue (e.g., anger, happiness) and the type of attention manipulated.
Given that the emotional salience of a stimulus is an indication of its value or importance, one would expect that emotion may influence even the most basic perceptual abilities. Here we have demonstrated for the first time that emotion facilitates early vision: People actually see better in the presence of emotional stimuli. Previous neuroimaging (Morris et al., 1998) and neuroanatomical (Amaral et al., 2003) studies in both humans and nonhumans have implied such a relation between emotion and perception, but none have demonstrated this effect on perceptual behavior. In addition, the current results reveal a perceptual effect that was not considered in these earlier animal and neuroimaging studies—the effect of emotion in potentiating the perceptual benefit of attention. This additional facilitation of perception by emotion when covert attention is engaged indicates that emotion's influence on perception is more pervasive than previously thought.
Acknowledgments
The authors would like to thank the observers who participated in this study. In addition, we would like to thank B. Holmes and D. Valenzuela for assistance in conducting the study, K. Choi for statistical consulting, and A.K. Anderson, M.R. Delgado, A.M. Giordano, J. Gobell, T.S. Liu, F. Loula, and F. Pestilli for helpful discussions. This work was funded by a National Science Foundation grant to M.C. and a National Institute of Mental Health grant to E.A.P.
REFERENCES
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. Journal of Neuroscience. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the ‘spotlight’ of visual attention. Nature Neuroscience. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Cameron EL, Tai JC, Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vision Research. 2002;42:949–967. doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, McElree B. Covert attention accelerates the rate of visual information processing. Proceedings of the National Academy of Sciences, USA. 2001;98:5363–5367. doi: 10.1073/pnas.081074098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein M. Spatial covert attention increases contrast sensitivity across the CSF: Support for signal enhancement. Vision Research. 2000;40:1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Williams PE, Yeshurun Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision. 2002;2:467–479. doi: 10.1167/2.6.4. [DOI] [PubMed] [Google Scholar]
- Cheal M, Lyon D. Central and peripheral precuing of forced-choice discrimination. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1991;43A:859–880. doi: 10.1080/14640749108400960. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Pictures of facial affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Foley JM, Legge GE. Contrast detection and near-threshold discrimination in human vision. Vision Research. 1981;21:1041–1053. doi: 10.1016/0042-6989(81)90009-2. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Supple WF, Jr., Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behavioral Neuroscience. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Shin LM, Thompson WL, McNally RJ, Rauch SL, Pitman RK, Alpert NM. Neural effects of visualizing and perceiving aversive stimuli: A PET investigation. NeuroReport. 1996;7:1569–1576. doi: 10.1097/00001756-199607080-00007. [DOI] [PubMed] [Google Scholar]
- Ledoux JE. The synaptic self. Viking; New York: 2002. [Google Scholar]
- Liu T, Pestilli F, Carrasco M. Transient attention enhances perceptual performance and fMRI response in human visual cortex. Neuron. 2005;45:469–477. doi: 10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z-L, Dosher BA. Spatial attention: Different mechanisms for central and peripheral temporal precues? Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1534–1548. doi: 10.1037//0096-1523.26.5.1534. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo J, Treue S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron. 2002;35:365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- Mayfrank L, Kimmig H, Fischer B. The role of attention in the preparation of visually guided saccadic eye movements in man. In: O'Regan JK, Levy-Schoen A, editors. Eye movements: From physiology to cognition. North-Holland; New York: 1987. pp. 37–45. [Google Scholar]
- McAdams CJ, Maunsell JHR. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. The Journal of Neuroscience. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvie SJ. Emotional expression in upside-down faces: Evidence for configurational and componential processing. British Journal of Social Psychology. 1995;34:325–334. doi: 10.1111/j.2044-8309.1995.tb01067.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Research. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM. Global feature-based attention for motion and color. Vision Research. 2003;43:629–637. doi: 10.1016/s0042-6989(02)00595-3. [DOI] [PubMed] [Google Scholar]
- Talgar CP, Pelli DG, Carrasco M. Covert attention enhances letter identification without affecting channel tuning. Journal of Vision. 2004;4:22–31. doi: 10.1167/4.1.3. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S. Beware and be aware: Capture of spatial attention by fear-related stimuli in neglect. NeuroReport. 2001;12:1119–1122. doi: 10.1097/00001756-200105080-00014. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature. 1998;396:72–75. doi: 10.1038/23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. The locus of attentional effects in texture segmentation. Nature Neuroscience. 2000;3:622–627. doi: 10.1038/75804. [DOI] [PubMed] [Google Scholar]