Wound infections are serious and relatively common postoperative complications. They are generally detected five to nine days after surgery and are usually attributed, even by surgeons, to poor surgical technique or failure to maintain sterility. However, it has been known for decades that all wounds become contaminated, often by bacteria from the skin or within the patient, and that it is host defense mechanisms that prevent most contamination from developing into clinical infections. Host defense is especially important during the initial hours following contamination, i.e., the immediate perioperative period.
As might thus be expected, factors that improve host defense reduce infection risk. Many of these are under the direct control of anesthesiologists and are at least as important as appropriate use of prophylactic antibiotics, which halve infection risk [1]. This article will review non-pharmacologic methods of reducing infection risk, with special emphasis on methods available to anesthesiologists.
Background
Wound infections are among the most common serious complications of anesthesia and surgery [2–4]. For example, based on the CDC’s Study on the Effect of Nosocomial Infection Control (SENIC), the wound infection risk in patients undergoing colon surgery ranges from 9 to 27%, depending on the duration of surgery, degree of contamination of the wound, and number of underlying diseases [5]. On average, the wound infection rate following colon resection lasting > 2 hours is reported to be about 15% in most hospitals [5]. More recent values are somewhat lower, but the risk of infection remains distressingly high.
The morbidity (and related cost) associated with surgical infections is considerable; estimates of prolonged hospitalization vary from 5 to 20 days per infection [2, 5, 6]. Moreover, after-hospital costs are higher because patients recovering from wound infections are usually discharged before the wound closes entirely, and thus require dressing changes 2 to 3 times daily. The required supplies are costly, and home-nursing visits may be necessary. Despite the substantial reduction in wound infection rates resulting from the universal implementation of sterile technique and prophylactic antibiotics, the incidence of perioperative wound infections remains so high, and so costly, that interventions producing even small further decreases in the infection rate must be considered seriously.
Various factors influence development of wound infections, including 1) character and magnitude of contamination; 2) effects of hemostasis, foreign bodies, damaged tissues, etc. on the local milieu; 3) wound perfusion, which delivers immune components such as oxygen, inflammatory cells, growth factors, cytokines, and nutritional components including amino acids, glucose, and insulin; 4) antibiotic administration; and 5) immune function [7, 8]. Non-specific or “natural” immunity is the most important host defense following acute bacterial contamination, particularly with the most common surgical pathogens, including S. Aureus, Klebsiella, E. coli, Candida, and Enterococcus [2, 3]. Non-specific immune responses include opsonization of bacteria, granulocyte demargination, diapedesis, phagocytosis, and both oxygen-dependent and non-oxidative bacterial killing [9]. Among these, oxidative killing by neutrophils dominates.
The first few hours following bacterial contamination constitute a decisive period during which infection is established [10]. The effects of antibiotic administration and of hypoperfusion are especially important during this period. For example, antibiotics limit infection when given within 3 hours of bacterial inoculation but are ineffective when given more than 3 hours after inoculation [7, 11]. Similarly, wound hypoperfusion (achieved by epinephrine infiltration or “dehydration shock”) aggravates test infections when induced up to 2.5 hours after the inoculation, but has no effect when induced later [10]. Techniques aimed at improving resistance to surgical wound infections are thus most likely to succeed if implemented during the decisive period. It is because the decisive period is so important that interventions restricted to the perioperative period influence wound infection risk, even though infections are usually detected clinically 5–10 days after surgery.
Maintaining Normothermia
Perioperative Thermal Homeostasis
General [12] and neuraxial [13] anesthesia profoundly impairs thermoregulatory control. Consequently, nearly all unwarmed surgical patients become hypothermic. Hypothermia results initially from a rapid core-to-peripheral redistribution of body heat [14, 15] and is followed by a linear reduction in core temperature that results from heat loss exceeding heat production. Even mild perioperative hypothermia has been causally linked to numerous severe complications including increased blood loss [16] and transfusion requirement [17], morbid myocardial outcomes [18], prolonged post-anesthetic recovery [19] and hospitalization [6], negative nitrogen balance [20], post-anesthetic shivering [21–23], and thermal discomfort [24]. Hypothermia also increases the risk of surgical wound infection.
Hypothermia Reduces Host Defense
Hypothermia may facilitate perioperative wound infections in two ways. First, sufficient intraoperative hypothermia triggers thermoregulatory vasoconstriction [25, 26]. Furthermore, vasoconstriction during recovery is universal in hypothermic patients because brain anesthetic concentration decreases rapidly, allowing re-emergence of thermoregulatory responses [27]. Thermoregulatory vasoconstriction decreases subcutaneous oxygen tension in humans [28], and the risk of wound infection correlates with subcutaneous oxygen tension [29, 30].
Second, considerable evidence indicates that mild core hypothermia directly impairs immune function including T-cell-mediated antibody production [31, 32] and “non-specific” oxidative bacterial killing by neutrophils [8]. Bacterial killing by neutrophils is apparently reduced as temperature decreases from 41 to 26°C [33, 34], although in vitro results depend critically on the model used [35]. Decreased killing results at least in part because production of oxygen and nitroso free radicals is oxygen-dependent in the range of oxygen partial pressures found in wounds [36, 37].
Patients having initial postoperative temperature near 34.5°C — a typical core temperature in unwarmed patients undergoing major surgery [25, 26, 38] — require several hours to restore core normothermia. Bacterial fixation, that is the conversion of contamination into an infection, will thus typically occur while unwarmed patients remain hypothermic. Perioperative hypothermia may thus contribute to surgical wound infections even though the infections usually are not detected until days after surgery. In contrast, it is unlikely that exaggerated bacterial growth aggravates infections in hypothermic patients because the small differences in in vitro growth rates within the tested temperature range would decrease bacterial growth during hypothermia [39].
Normothermia Reduces Infection Risk
Taken together, these in vitro results suggest that hypothermia may directly impair neutrophil function, or impair it indirectly by triggering subcutaneous vasoconstriction and subsequent tissue hypoxia. Consistent with this theory, mild hypothermia reduces resistance to test infections in animals [40, 41]. More importantly, 1.9°C core hypothermia (core temperature of 34.7°C) triples the incidence of surgical wound infection following colon resection [6]. These infections were clinically important as indicated by the fact that infected patients, on average, were hospitalized one week longer than the uninfected patients.
A subsequent, uncontrolled, retrospective trial failed to identify a correlation between temperature and infection [42]. This study, though, suffered such serious methodological flaws that it is difficult to interpret [43]. In contrast, a subsequent randomized trial confirmed that both local and systemic warming reduces infection risk — although this may be the only thermoregulatory trial ever published in which core temperature is not reported [44].
Interestingly, hypothermia also increases the duration of hospitalization by 20% even when infected patients are excluded from the analysis — apparently because healing per se was significantly impaired (Table 1) [6]. This result is consistent with studies by Carli et al. showing that mild hypothermia aggravates postoperative protein wasting [20] and that mild hypothermia reduces collagen deposition (i.e., scar formation) [6].
Table 1.
Maintaining Perioperative Normothermia Reduces Wound Infection Risk and Shortens Hospitalization.
| Normothermic | Hypothermic | P | |
|---|---|---|---|
| Number | 104 | 96 | |
| Temperature (°C) | 36.6 ± 0.5 | 34.7 ± 0.6 | <0.001 |
| Infection (%) | 6 | 19 | <0.01 |
| Hospitalization (days) | 12.1 ± 4.4 | 14.7 ± 6.5 | 0.001 |
A randomized, blinded trial from Kurz et al. [6]. Maintaining normothermia reduced infection risk by a factor of three and reduced the duration of hospitalization by 20%. The reduction in hospitalization persisted even when analysis was restricted to uninfected patients.
Excluding brain injury, the major causes of morbidity and mortality in trauma patients are coagulopathy and infection. Since both coagulation [16, 17] and resistance to infection [6, 44] are profoundly influenced by hypothermia, it is unsurprising that outcome would be improved in normothermic trauma patients [45]. The difficulty with this study, however, is that it is a retrospective analysis. This is a grave limitation because the most seriously injured patients are likely to become most hypothermic. It is thus difficult to be sure that adverse outcomes result from hypothermia per se rather than underlying injury. Nonetheless, the result is consistent with known effects of hypothermia.
Supplemental Oxygen
Tissue Oxygenation
Oxidative killing of pathogenic bacteria by neutrophils is the most important immune defense against surgical pathogens [46]. Oxidative killing depends on the production of bactericidal superoxide radicals from molecular oxygen. The rate of this reaction, catalyzed by the NADPH-linked oxygenase, is PO2-dependent. Our studies indicate that neutrophil superoxide production has a Km for oxygen of the NADPH-linked oxygenase of at least 60 mmHg [47]. Consistent with this observation, oxidative killing is oxygen-dependent from 0 to ≥150 mmHg [48].
Inadequate tissue oxygen also impairs tissue repair. Scar formation requires hydroxylation of abundant proline and lysine residues [49]. The prolyl and lysyl hydroxylases that catalyze this reaction depend on the substrate oxygen [49]. The Km for O2 of prolyl hydroxylase has been variously estimated at 20, 25, and 100 mmHg [50–52]. Even using the most conservative estimate, proline hydroxylation of collagen will be PO2-dependent through the range of 0 to at least 200 mmHg, with 90% of the effect occurring by 90 mmHg. Consistent with this estimate, hydroxyproline deposition is proportional to arterial PO2 in rabbits [53] and surgical patients [54].
Oxygen partial pressure in wounds also has a regulatory component [55, 56]. For example, it has been known since the 1980’s that oxygen regulates angiogenesis [57, 58]. Angiogenesis is mediated by micromolar concentrations of H2O2 and other reactive oxygen species that activate vascular endothelial growth factor [59, 60].
The partial pressure of oxygen in subcutaneous tissues varies widely, even in patients whose arterial hemoglobin is fully saturated. Many factors are known to influence tissue oxygen tension, including systemic and local temperature [28], smoking [61], anemia [62], perioperative fluid management [54], and uncontrolled surgical pain [63]. But as might be expected, one of the most effective (and least expensive) ways of increasing tissue oxygenation is to simply augment inspired oxygen concentration (Fig. 1) [29].
Fig. 1.
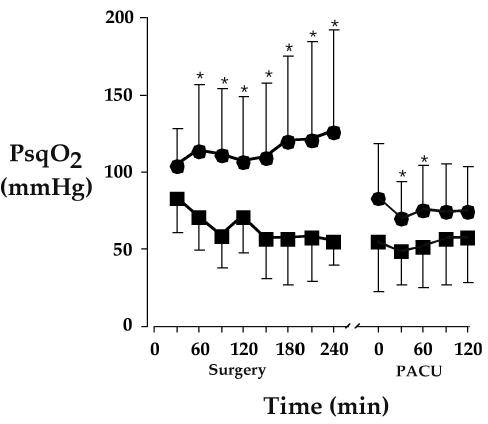
Subcutaneous oxygen tension, the primary determinant of wound infection risk, during surgery and in the postoperative care unit (*P < 0.01) [29]. Tissue oxygenation was measured in a surrogate wound on the upper arm. Intraoperative tissue oxygen partial pressure was doubled by supplemental oxygen (FIO2 = 80% vs. 30%); the effect was less during the postoperative period. Results are expressed as means ± SDs.
Supplemental Oxygen Reduces Infection Risk
The concept that oxygen is an antibiotic was developed by Knighton and colleagues in a series of in vitro and animal studies in the 1980’s [64, 65]. That tissue oxygenation might have a clinically important effect on wound infection risk was first identified by Hopf et al. [30]. In an observational study, they found that infection risk was inversely proportional to postoperative tissue oxygenation. As a natural consequence of its observational design, this study was confounded by the possibility that tissue oxygenation was worse in sicker patients who had the largest operations — and thus the greatest infection risk — but that there might not be a causal link between the two observations.
The first randomized trial of supplemental oxygen and wound infection risk, Greif et al. [29] involved 500 patients undergoing elective colon resection who were randomly assigned to an inspired oxygen concentration of 30% (n = 246) or 80% (n = 254) intraoperatively and for two hours after surgery. Wounds were evaluated daily by blinded investigators; both pus and a positive culture were required for diagnosis of infection. Wound scores [66] were 5 ± 9 in the patients given 30% oxygen and 3 ± 7 in those given 80%, P = 0.019. (All results are expressed as means ± SDs.). There were 13 surgical wound infections in the patients given 80% oxygen and 28 in those given 30% (P = 0.01). Supplemental oxygen thus halved infection risk.
In contrast, a subsequent report by Pryor et al. [67] with only 160 patients reported that supplemental oxygen increases the risk of infection. It is thus worth considering why the results of Pryor et al. differ so markedly from those of Greif et al. [29]. Pryor et al. [67] did not specify the baseline infection rate they used, making it impossible to confirm their estimate that 300 patients would be required to detect a 40% reduction in the infection rate. But to have an 80% power to detect the 40% risk reduction that they specified from 25% (our baseline) or from 11% (baseline from Greif et al. [29]) would require 540 or 651 patients, respectively; to detect a 40% increase would require 698 or 930 patients, respectively. The study thus appears to have been underpowered and then stopped after only 160 patients were randomized. The authors specify that 160 patients was an a priori stopping point, although 53.3% of the anticipated sample size is a curious a priori stopping point [68].
A second factor is that Pryor’s [67] treatment groups were not homogeneous. For example, in their study patients assigned to 80% oxygen weighed more and were more than twice as likely to have a body-mass index (BMI) exceeding 30 kg/m2. Patients assigned to 80% oxygen also had longer operations, lost significantly more blood, and required significantly more fluid replacement. Furthermore, Pryor et al. failed to control many variables believed to influence infection risk including anesthetic, fluid, antibiotic, and pain management. A third limitation of Pryor’s study is that wound infections were determined by retrospective chart review; a review that was apparently conducted by unblinded investigators. This insensitive methodology contrasts markedly with the daily wound evaluations by blinded investigators used by Greif et al. It is possible that these methodological problems contributed to a result that is inconsistent with considerable in vitro, in vivo, and clinical data [69].
The most recent randomized trial of supplemental oxygen, Belda et al. [70] involved 300 patients undergoing colon resection who were randomly assigned to 30% or 80% FiO2 intraoperatively and 6 hours postoperatively. Blinded investigators diagnosed all wound infections using Centers for Disease Control criteria. Baseline patient characteristics, anesthetic management, and potential confounding factors were recorded. Wound infection rates were compared with chi-square analysis. Logistic regression was used to assess the contribution of potential confounding factors. Surgical wound infection occurred in 24.4% of patients receiving 30% oxygen, but only 14.9% of those receiving 80% oxygen (P=0.04). After adjustment the relative risk of infection in patients given supplemental oxygen was 0.46 (P=0.04). Supplemental inspired oxygen thus reduced wound infection risk by roughly a factor of two.
The most recent trial related to supplemental oxygen and wound infection by Myles et al. evaluated substituting supplemental oxygen (80%) for 70% nitrous oxide in 30% oxygen [71]. Infection risk in the patients given supplemental oxygen was significantly reduced, by about 25%. This study differs from previous ones, though, in varying both nitrous oxide and oxygen concentration. It is thus impossible to determine from the results of Myles, et al whether the observed reduction in infection risk resulted from avoidance of nitrous oxide or the beneficial effects of supplemental oxygen.
There are at least three reasons why nitrous oxide might increase infection risk and the authors’ hypothesis was that nitrous oxide would reduce host resistance. However, another recent outcome trial, Fleishmann et al. [72], specifically compared infection risk in more than 400 patients who were randomly assigned to 65% nitrous oxide or 65% nitrogen; there was no significant difference between the groups. It thus seems likely that reduced infection risk in the patients of Myles et al. [71] results from supplemental oxygen rather than nitrous oxide toxicity per se. This trial thus provides additional support for the antibiotic effect of supplemental oxygen.
There have thus now been three randomized trials specifically evaluating the effect of supplemental oxygen on surgical wound infection. Two trials, with a total of 800 patients, each found that 80% FiO2 reduced infection risk by a factor of two. In contrast, one small study —with only 160 patients and substantial methodological problems — found just the opposite. Furthermore, an additional study with 2,000 patients that found that substituting supplemental oxygen for nitrous oxide significantly reduces infection risk [71]. Since nitrous oxide, per se, does not increase infection risk [72], it is reasonable to consider this study as additional confirmation that supplemental oxygen reduces infection risk. Supplemental oxygen should thus be provided when practical (Table 2).
Table 2.
Randomized Trials Evaluating the Effect of Supplemental Oxygen on Wound Infection Risk.
| Number | FIO2= 30% (%infected) | FIO2= 80% (% infected) | P | |
|---|---|---|---|---|
| Greif et al. [29] | 500 | 11 | 5 | 0.01 |
| Pryor et al. [67] | 160 | 11 | 25 | 0.02 |
| Belda et al. [70] | 300 | 24 | 15 | 0.04 |
| Myles et al. [71] | 2000 | 10 | 7.7 | 0.03 |
With the exception of a small study by Pryor, et al, available randomized trials show that supplemental oxygen significantly reduces infection risk.
In each trial, supplemental oxygen was provided intraoperatively; however, postoperative treatments differed. In Greif et al. [29] and Pryor et al. [67] supplemental oxygen was continued for two postoperative hours. In contrast, oxygen was continued for six postoperative hours in Belda et al. [70] and was restricted to the intraoperative period in Myles et al. [71]. There is currently no study that directly compares intraoperative oxygen only with the combination of intraoperative and postoperative oxygen. The extent to which supplemental postoperative oxygen contributes to reduced infection risk thus remains unclear.
Supplemental Oxygen is Safe
The major complication associated with brief periods of oxygen administration is pulmonary atelectasis. Concern about atelectasis is appropriate because it occurs in up to 85% of patients undergoing lower abdominal surgery and is thought by some to be an important cause of morbidity [73–75]. Two mechanisms contribute to perioperative atelectasis: compression and absorption. Compression results from cephalad displacement of diaphragm, decreased compliance, and reduced functional residual capacity [76]. To some extent, these factors contribute with any anesthetic technique. Absorption, in contrast, is defined by uptake of oxygen from isolated alveoli and results from administration of high oxygen partial pressures. Administration of 100% oxygen, even for a few minutes, causes significant postoperative atelectasis via this mechanism [74, 77].
It is important, though, to distinguish between 100% intraoperative oxygen, which does produce atelectasis, and 80% oxygen, which does not [78]. Akça, et al. have shown that 80% perioperative oxygen does not cause atelectasis. Atelectasis was evaluated with computerized tomography the morning after open colon resection. Relatively small amounts of pulmonary atelectasis were observed on the CT scans, and the percentages did not differ significantly in the patients given 30% oxygen (2.5 ± 3.2%) or 80% oxygen (3.0 ± 1.8%, Fig. 2). Pulmonary function was virtually identical in the two groups.
Fig. 2.
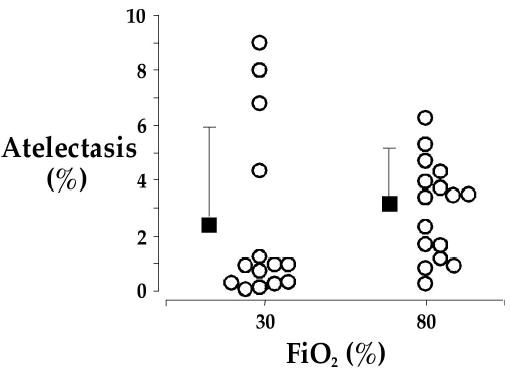
From Akça et al. [114] Relatively small amounts of pulmonary atelectasis were observed on the CT scans, and the percentages did not differ significantly in the patients given 30% oxygen (2.5 ± 3.2%) or 80% oxygen (3.0 ± 1.8%). Results are shown for individual patients, along with the group means and SDs. These data provided a 99% chance of detecting a 2% difference in atelectasis volume at an alpha level of 0.05. Poorly-aerated regions were also comparable between the groups (9.5 ± 4.4% in the patients given 30% oxygen vs. 10.3 ± 4.2% in the patients given 80% oxygen).
Hyperoxia causes peripheral vasoconstriction, reduced cardiac output, and slight bradycardia [79], a response that is not sympathetically mediated [80]. In contrast, hypoxia — of a magnitude that is probably common in postoperative patients — is associated with cardiac rhythm disturbances [81] that are prevented by supplemental oxygen [82].
Surgery, anesthesia, cardiopulmonary bypass, and mechanical ventilation each independently impair pulmonary immune defenses [83–85]. Hyperoxia, in contrast, provokes pulmonary expression of inflammatory cytokines which in turn helps maintain phagocytosis and oxidative killing by alveolar macrophages (Fig. 3) [86]. It is likely that this response helps patients resist pneumonia, but could well become harmful over long periods of time or in the context of other factors promoting pulmonary inflammation.
Fig. 3.
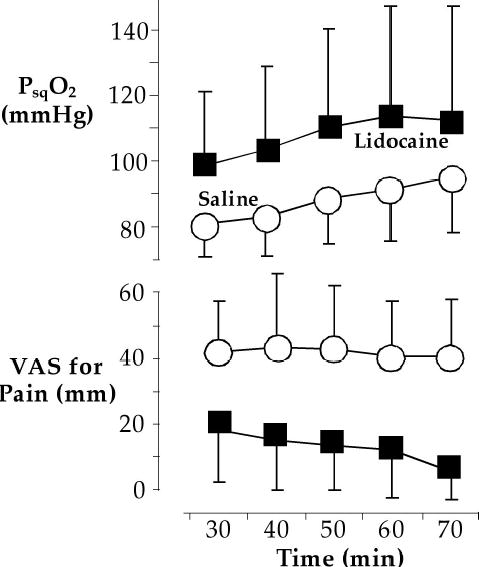
From Kotani et al. [86] the fraction of alveolar macrophages ingesting opsonized and non-opsonized particles during anesthesia with 100% (n = 30, circles) and 30% (n = 30, squares) inspired oxygen. Asterisks (*) indicate statistically significant differences (P < 0.05) from elapsed time zero in each group; pounds signs (#) identify significant differences (P < 0.01) between the two groups. Data are expressed as means ± SDs.
Operating room fires can result in substantial injury to the patient and health care providers. In United States, there are approximately 2,260 reported hospital fires per year, resulting in 1 death and 130 injuries. But among these, fewer than 100 occur in operating rooms and of those, only a small fraction result in injury.
As might be expected, oxygen facilitates ignition of flammable material such as operating room draping and speeds propagation of fire once ignited. However, oxygen is normally contained within an anesthesia circuit or well-away from ignition sources such as electrocautery devices. Concerns about operating fire should thus not normally prevent clinicians from providing supplemental oxygen, and especially not in patients at risk for wound infection since infections are much more common than fires. Even open oxygen (such as provided by nasal prongs) dissipates in less than 10 cm and is unlikely to contribute to fire risk unless the ignition source is immediately proximate to the oxygen source [87].
Surgical Site Preparation
It is widely believed that removing hair at the operative site reduces contamination and, therefore, infection risk. It thus remains routine to shave surgical sites. In fact, it is well established that avoiding hair removal or using depilatories rather than shaving reduces infection rates after clean operations [88]. Furthermore, infection rates are reduced when hair is clipped rather than shaved, even when hair is removed on the day of surgery [89]. The reason, presumably, is that shaving injures skin, thus allowing surface bacteria to penetrate. If hair removal at the incision site is considered necessary, it should thus be performed with clippers during the immediate preoperative period. A corollary is that patients can be warned not to shave their operative sites before surgery, as some shave in an effort to be helpful.
Smoking
Two European studies, published in 1993 and 1996, each showed that smokers have a markedly increased risk of surgical wound infection. These results were considered unsurprising since smoking a single cigarette markedly reduces tissue oxygenation for one hour [61]; tissues are thus nearly constantly hypoxic in “pack-a-day” smokers.
Interestingly, though, three subsequent large trials published in 2000 and later, again from Europe, show no relationship between smoking and infection risk (Table 3) [29, 70, 72]. The reason, presumably, is that smoking is no longer permitted in hospitals. While smoking obviously produces numerous adverse effects, it no longer appears to be a specific risk factor for development of surgical wound infection.
Table 3.
Smoking and Wound Infection Risk.
| Year | Non-Smokers (%infected / n) | Smokers (%infected / n) | P | |
|---|---|---|---|---|
| Kurz et al. [6] | 1996 | 7% / 148 | 22% / 76 | <0.001 |
| Greif et al. [29] | 2000 | 8% / 283 | 8% / 122 | NS |
| Fleishmann et al. [72] | 2005 | 16% / 335 | 17% / 81 | NS |
| Belda et al. [70] | 2005 | 22% / 187 | 30% / 46 | NS |
A study published in 1996 observed that infection risk was tripled in smokers. In an additional study from 1993, bacterial infection risk was doubled from 15 ± 3% to 33 ± 8%, but these values include pneumonia as well as surgical wound infections [113]. In contrast, three subsequent studies observed no relationship between smoking and infection risk. The probable explanation is that after 2000, smoking was no longer allowed in hospitals. Smoking thus no longer appears to be an important risk factor for development of surgical wound infection.
Glucose Control
Diabetic patients are at increased risk for all kinds of infectious complications and have two-to-three times the risk of surgical wound infection as nondiabetic patients after cardiac operations. In diabetic patients having gastrointestinal or cardiac operations, hyperglycemia (blood glucose exceeding either 200 or 220 mg/dL) is associated with wound infection risk [90, 91]. However, it is important to distinguish the long-term peripheral micro-vascular disease of diabetes (which cannot be acutely reversed) with the immediate effects of perioperative hyperglycemia.
There are nonetheless reasons to believe that hyperglycemia per se increases infection risk. For example, the risk of SSI for both diabetic and nondiabetic patients is doubled in cardiac surgical patients when blood glucose exceeds 200 mg/dL in the first 48 hours; interestingly, half of the observed hyperglycemic episodes occurred in nondiabetic patients [92].
In an observational trial, Furnary et al. demonstrated a significant reduction in deep sternal wound infections when perioperative insulin management was switched from subcutaneous administration using a sliding scale to a continuous insulin infusion [93]. Rigorous postoperative glucose control using an aggressive insulin infusion protocol has also been shown to reduce multiple organ failure, sepsis, and mortality in critical care patients [94]. This study, though, is the only published prospective evidence that tight perioperative glucose control improves outcome — and focused on cardiac surgical patients admitted to a critical care unit. Whether this finding can be extrapolated to other surgical patients remains to be determined.
It is worth noting, though, that glucose control differs from the other interventions discussed in this article. The others are all simple-to-implement, inexpensive, and pose little or no risk. Tight glucose control, in contrast, requires critical care with all the expense that implies, and includes a distinct risk of hypoglycemia. Further study will be required to determine which patients benefit from tight glucose control and whether outcomes improve sufficiently to justify the difficulty and expense.
Potential, But Unproven Interventions
Vascular Volume
Mild-to-moderate reductions in vascular volume trigger peripheral vasoconstriction to maintain nearly normal blood pressure. However, well-maintained arterial pressure and central organ perfusion comes at the expense of peripheral perfusion, which can be reduced substantially by even small volume deficits. Blood pressure (and urine output) are thus poor indicators of peripheral perfusion [95].
As might thus be expected, peripheral perfusion and oxygenation were better in surgical patients given 16–18 mL·kg−1·h−1 than in those given 8 mL·kg−1·h−1. The tissue oxygen tension was greater in the high volume group in both the intraoperative (81 ± 26 mmHg vs. 67 ± 18 mmHg, P = 0.03) postoperative periods (77 ± 26 mmHg vs. 59 ± 15 mmHg, P = 0.03). These results suggested that simply providing supplemental fluid might reduce infection risk. There is also evidence that titrating perioperative hydration to tissue oxygenation results in more fluid administration and better wound healing [96].
Unfortunately, the results of a subsequent clinical outcome study were less encouraging [97]. Patients undergoing open colon resection were randomly assigned to small (8 mL·kg−1·h−1) or large volume (16–18 mL·kg−1·h−1) fluid management. Infection rates were nearly identical and the study was stopped on a futility basis after about 250 patients were enrolled. It is important to recognize, though, that this study was underpowered and a clinically important effect of fluid management on infection risk remains possible. Other studies identify either improved [98, 99] or worsened [100] composite complication rates in patients given larger fluid volumes. It is thus unclear from available literature how fluids should be managed to minimize infection risk. Furthermore, the results are likely to vary as a function of type of surgery, type of fluid, or by dosing scheme (i.e., goal-directed vs. mL/kg).
Pain Relief
Postoperative pain provokes an autonomic response that markedly increases adrenergic nerve activity and plasma catecholamine concentrations [101]. A consequence is arteriolar vasoconstriction. Reduced peripheral perfusion, in turn, would thus be expected to decrease tissue oxygen partial pressure.
In fact, this theory was confirmed by Akça et al. [63] who showed that tissue oxygen partial pressures were 25 mmHg greater in patients having knee arthroplasty when their pain was aggressively treated (Fig. 4). Whether this translates into lower infection risk has yet to be demonstrated, although a 25-mm increase is probably clinically important [30]. But, of course, patients deserve adequate analgesia even if pain relief proves not to reduce infection risk.
Fig. 4.
A of pain scores and tissue oxygenation in patients given intra-articular lidocaine (squares) or saline (circles). Pain scores, on a 100-mm visual-analog scale, were much larger in patients given saline, and their tissue oxygen partial pressures averaged 25 mmHg less. All values differed significantly between the two groups; data are presented as means ± SDs. Reprinted from The Lancet, 354, Akça O, Melischek M, Scheck T, et al. Postoperative pain and subcutaneous oxygen tension. Page 41, Copyright (1999), with permission from Elsevier [63].
Hypercapnia
The primary determinants of tissue oxygen availability are arterial oxygen content, cardiac output, and local perfusion [102–104]. An important, but often overlooked, influence on cardiac output is arterial carbon dioxide partial pressure [105]. For example, hyperventilation and hypocapnia decrease cardiac output, which in turn decreases blood flow and oxygen tension in brain and splanchnic organs [106–108]. Hypocapnia also shifts the oxyhemoglobin curve leftward and restricts oxygen unloading at the tissue level.
Hypercapnia, in contrast, increases cardiac output, apparently via sympathetic nervous system activation, and also improves oxygen extraction. Consequently, hypercapnia increases oxygen availability to tissue [109]. Since hypercapnia during cardiopulmonary bypass does increase tissue oxygenation (Akça, et al; unpublished data), the increase observed in volunteers and routine surgical patients presumably results largely from an increase in cardiac output, rather than primary vasodilation per se.
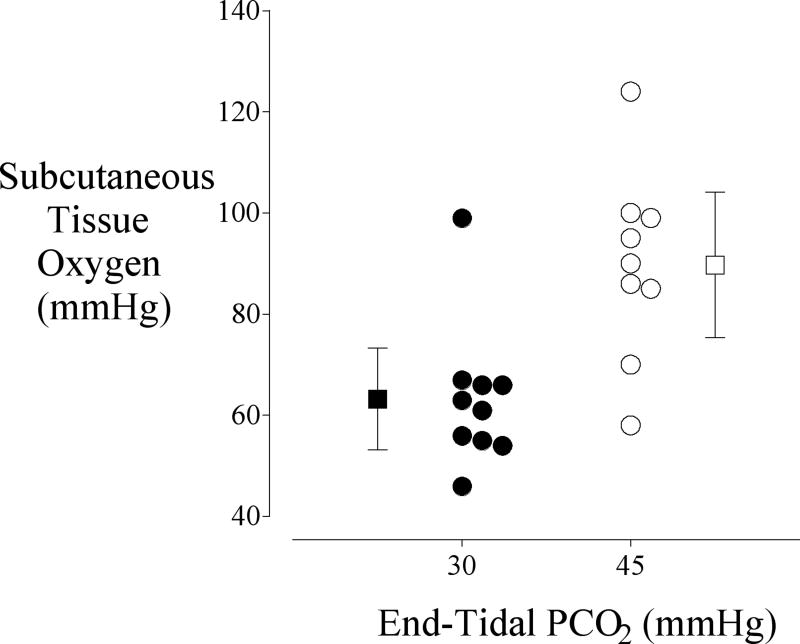
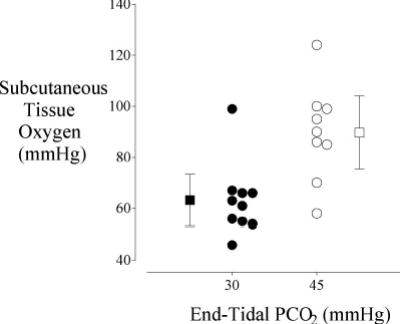
Hypercapnia also causes a complex interaction between altered cardiac output, hypoxic pulmonary vasoconstriction, and intrapulmonary shunt with the result being a net increase in PaO2 at a given inspired oxygen concentration [110]. But even at a given PaO2, there is a linear relationship between arterial carbon dioxide tension and cardiac output and subcutaneous oxygenation. In fact, each mmHg increase in arterial carbon dioxide resulted in a 0.8 mmHg increase in subcutaneous oxygenation in volunteers [111]. The increase was even more impressive in surgical patients, with subcutaneous oxygenation increasing from 63 ± 14 at a PaCO2 of 30 mmHg to 89 ± 19 at a PaCO2 of 45 mmHg (Fig. 5) [112]. These data suggest that maintaining slight hypercapnia, a simple and inexpensive maneuver, may reduce infection risk. However, this theory has yet to be confirmed.
Fig. 5.
From Akça et al. [112] a study subcutaneous tissue oxygen as a function of end-tidal PCO2 in patients undergoing major surgery. Measurements were made on the lateral aspect of the upper arm with a polargraphic electrode system. The mean oxygen tension in the group given 45 mmHg C02 was significantly greater (P = 0.014) than the group given 30 mmHg. Results are presented as means ± SDs.
Summary
Surgical site infections are among the most common serious perioperative complications. Infections are established during a decisive period lasting a few hours after contamination. Adequacy of host immune defenses is the primary factor determining whether inevitably wound contamination progresses into a clinical infection. As it turns out, many determinants of infection risk are under the direct control of anesthesiologists — factors that are at least as important as prophylactic antibiotics.
Major outcome studies demonstrate that the risk of surgical wound infection is reduced three-fold simply by keeping patients normothermic. Infection risk is reduced by an additional factor of two by providing supplemental oxygen (80% vs. 30%) during surgery and for the initial hours after surgery. The contribution, if any, of other factors including tight glucose control, fluid management, and mild hypercapnia have yet to be suitably tested. But it is clear that anesthesiologists can substantially reduce the risk of wound infection simply by providing supplemental oxygen and keeping patients normothermic.
Footnotes
Received from the Department of Outcomes Research, Cleveland Clinic Foundation; and the Outcomes Research Institute and Department of Anesthesiology, University of Louisville, Louisville, KY.
Supported by NIH Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). Dr. Sessler has no personal financial interest related to any of the work presented here.
References
- 1.Platt R, Zaleznik DF, Hopkins CC, et al. Perioperative antibiotic prophylaxis for herniorrhaphy and breast surgery. N Engl J Med. 1990 Jan 18;322(3):153–160. doi: 10.1056/NEJM199001183220303. [DOI] [PubMed] [Google Scholar]
- 2.Bremmelgaard A, Raahave D, Beir-Holgersen R, Pedersen JV, Andersen S, Sorensen AI. Computer-aided surveillance of surgical infections and identification of risk factors. J Hosp Infect. 1989;13:1–18. doi: 10.1016/0195-6701(89)90090-x. [DOI] [PubMed] [Google Scholar]
- 3.Coles B, van Heerden JA, Keys TF, Haldorson AI. Incidence of wound infection for common general surgical procedures. Surg Gynecol Obstet. 1982;154:557–560. [PubMed] [Google Scholar]
- 4.Polk HC, Simpson CJ, Simmons BP, Alexander JW. Guidelines for prevention of surgical wound infection. Arch Surg. 1983;118:S1213–1217. doi: 10.1001/archsurg.1983.01390100075019. [DOI] [PubMed] [Google Scholar]
- 5.Haley RW, Culver DH, Morgan WM, White JW, Emori TG, Hooton TM. Identifying patients at high risk of surgical wound infection: A simple multivariate index of patient susceptibility and wound contamination. Am J Epidemiol. 1985;121:206–215. doi: 10.1093/oxfordjournals.aje.a113991. [DOI] [PubMed] [Google Scholar]
- 6.Kurz A, Sessler DI, Lenhardt RA Study of wound infections and temperature group. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 7.Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:161–168. [PubMed] [Google Scholar]
- 8.Van Oss CJ, Absolam DR, Moore LL, Park BH, Humbert JR. Effect of temperature on the chemotaxis, phagocytic engulfment, digestion and O2 consumption of human polymorphonuclear leukocytes. J Reticuloendothel Soc. 1980;27:561–565. [PubMed] [Google Scholar]
- 9.Benhaim P, Hunt TK. Natural resistance to infection: Leukocyte functions. J Burn Care Rehabil. 1992;13:287–292. doi: 10.1097/00004630-199203000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Miles AA, Miles EM, Burke J. The value and duration of defence reactions of the skin to the primary lodgement of bacteria. Br J Exp Pathol Feb. 1957;38(1):79–96. [PMC free article] [PubMed] [Google Scholar]
- 11.Classen DC, Evans RS, Pestotnik R, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical wound infection. N Engl J Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 12.Xiong J, Kurz A, Sessler DI, et al. Isoflurane produces marked and non-linear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1996;85:240–245. doi: 10.1097/00000542-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki M, Kurz A, Sessler DI, et al. Thermoregulatory thresholds during spinal and epidural anesthesia. Anesthesiology. 1994;81:282–288. doi: 10.1097/00000542-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Matsukawa T, Sessler DI, Christensen R, Ozaki M, Schroeder M. Heat flow and distribution during epidural anesthesia. Anesthesiology. 1995;83:961–967. doi: 10.1097/00000542-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Matsukawa T, Sessler DI, Sessler AM, et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82:662–673. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Winkler M, Akça O, Birkenberg B, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91(4):978–984. doi: 10.1097/00000539-200010000-00039. [DOI] [PubMed] [Google Scholar]
- 17.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild intraoperative hypothermia increases blood loss and allogeneic transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 18.Frank SM, Fleisher LA, Breslow MJ, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: A randomized clinical trial. JAMA. 1997;277:1127–1134. [PubMed] [Google Scholar]
- 19.Lenhardt R, Marker E, Goll V, et al. Mild intraoperative hypothermia prolongs postoperative recovery. Anesthesiology. 1997;87:1318–1323. doi: 10.1097/00000542-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Carli F, Emery PW, Freemantle CAJ. Effect of peroperative normothermia on postoperative protein metabolism in elderly patients undergoing hip arthroplasty. Br J Anaesth. 1989;63:276–282. doi: 10.1093/bja/63.3.276. [DOI] [PubMed] [Google Scholar]
- 21.Alfonsi P, Adam F, Passard A, Guignard B, Sessler DI, Chauvin M. Nefopam, a non-sedative benzoxazocine analgesic, selectively reduces the shivering threshold. Anesthesiology. 2004;100:37–43. doi: 10.1097/00000542-200401000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alfonsi P, Hongnat JM, Lebrault C, Chauvin M. The effects of pethidine, fentanyl and lignocaine on postanesthetic shivering. Anaesthesia. 1995;50:214–217. doi: 10.1111/j.1365-2044.1995.tb04559.x. [DOI] [PubMed] [Google Scholar]
- 23.Alfonsi P, Nourredine KE, Adam F, Chauvin M, Sessler DI. Effect of postoperative skin-surface warming on oxygen consumption and the shivering threshold. Anaesthesia Dec. 2003;58(12):1228–1234. doi: 10.1046/j.1365-2044.2003.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurz A, Sessler DI, Narzt E, Bakar A, Lenhardt R, Huemer G. Postoperative hemodynamic and thermoregulatory consequences of intraoperative core hypothermia. J Clin Anesth. 1995;7:359–366. doi: 10.1016/0952-8180(95)00028-g. [DOI] [PubMed] [Google Scholar]
- 25.Sessler DI, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69:357–364. doi: 10.1097/00000542-198809000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Sessler DI, Olofsson CI, Rubinstein EH, Beebe JJ. The thermoregulatory threshold in humans during halothane anesthesia. Anesthesiology. 1988;68:836–842. doi: 10.1097/00000542-198806000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Sessler DI, Rubinstein EH, Moayeri A. Physiological responses to mild perianesthetic hypothermia in humans. Anesthesiology. 1991;75:594–610. doi: 10.1097/00000542-199110000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Sheffield CW, Sessler DI, Hopf HW, et al. Centrally and locally mediated thermoregulatory responses alter subcutaneous oxygen tension. Wound Rep Reg. 1997;4:339–345. doi: 10.1046/j.1524-475X.1996.40310.x. [DOI] [PubMed] [Google Scholar]
- 29.Greif R, Akça O, Horn E-P, Kurz A, Sessler DI Outcomes Research™ Group. Supplemental perioperative oxygen to reduce the incidence of surgical wound infection. N Engl J Med. 2000;342:161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 30.Hopf HW, Hunt TK, West JM, et al. Wound tissue oxygen tension predicts the risk of wound infection in surgical patients. Arch Surg. 1997 Sep;132(9):997–1004. doi: 10.1001/archsurg.1997.01430330063010. discussion 1005. [DOI] [PubMed] [Google Scholar]
- 31.Farkas LG, Bannantyne RM, James JS, Umamaheswaran B. Effect of two different climates on severely burned rats infected with pseudomonas aeruginosa. Eur Surg Res. 1974;6:295–300. doi: 10.1159/000127732. [DOI] [PubMed] [Google Scholar]
- 32.Saririan K, Nickerson DA. Enhancement of murine in vitro antibody formation by hyperthermia. Cell Immunol. 1982;74:306–312. doi: 10.1016/0008-8749(82)90031-4. [DOI] [PubMed] [Google Scholar]
- 33.Leijh CJ, Van den Barselaar MT, Van Zwet TL, Dubbeldeman-Rempt I, Van Furth R. Kinetics of phagocytosis of staphylococcus aureus and escherichia coli by human granulocytes. Immunology. 1979;37:453–465. [PMC free article] [PubMed] [Google Scholar]
- 34.Wenisch C, Narzt E, Sessler DI, et al. Mild intraoperative hypothermia reduces production of reactive oxygen intermediates by polymorphonuclear leukocytes. Anesth Analg. 1996;82(4):810–816. doi: 10.1097/00000539-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 35.Frohlich D, Wittmann S, Rothe G, Sessler DI, Vogel P, Taeger K. Mild hyperthermia down-regulates receptor-dependent neutrophil function. Anesth Analg Jul. 2004;99(1):284–292. doi: 10.1213/01.ane.0000117142.28174.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hohn DC, MacKay RD, Halliday B, Hunt TK. The effect of oxygen tension on the microbicidal function of leukocytes in wound and in vitro. Surg Forum. 1976;27:18–20. [PubMed] [Google Scholar]
- 37.Mader JT. Phagocytic killing and hyperbaric oxygen: Antibacterial mechanisms. HBO Reviews. 1982;2:37–49. [Google Scholar]
- 38.Kurz A, Sessler DI, Narzt E, Lenhart R. Morphometric influences on intraoperative core temperature changes. Anesth Analg. 1995;80:562–567. doi: 10.1097/00000539-199503000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Mackowisk PA. Direct effects of hyperthermia on pathogenic microorganisms: Teleologic implications with regard to fever. Rev Infect Dis. 1981;3:508–520. doi: 10.1093/clinids/3.3.508. [DOI] [PubMed] [Google Scholar]
- 40.Sheffield CW, Sessler DI, Hunt TK. Mild hypothermia during isoflurane anesthesia decreases resistance to E. Coli dermal infection in guinea pigs. Acta Anaesthesiol Scand. 1994;38:201–205. doi: 10.1111/j.1399-6576.1994.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 41.Sheffield CW, Sessler DI, Hunt TK, Scheuenstuhl H. Mild hypothermia during halothane anesthesia decreases resistance to S. Aureus dermal infection in guinea pigs. Wound Rep Reg. 1994;2:48–56. doi: 10.1046/j.1524-475X.1994.20108.x. [DOI] [PubMed] [Google Scholar]
- 42.Barone JE, Tucker JB, Cecere J, et al. Hypothermia does not result in more complications after colon surgery. Am Surg. 1999;65:356–359. [PubMed] [Google Scholar]
- 43.Sessler DI, Kurz A, Lenhardt R. Hypothermia reduces resistance to surgical wound infections [letter] Am Surg. 1999;65(12):1193–1196. [PubMed] [Google Scholar]
- 44.Melling AC, Ali B, Scott EM, Leaper DJ. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001 Sep 15;358(9285):876–880. doi: 10.1016/S0140-6736(01)06071-8. [DOI] [PubMed] [Google Scholar]
- 45.Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: An ominous predictor of survival. J Trauma. 1987;27:1019–1024. [PubMed] [Google Scholar]
- 46.Babior BM. Oxygen-dependent microbial killing by phagocytes. N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 47.Allen DB, Maguire JJ, Mahdavian M, et al. Wound hypoxia and acidosis limit neutrophil bacterial killing mechansims. Arch Surg. 1997;132:991–996. doi: 10.1001/archsurg.1997.01430330057009. [DOI] [PubMed] [Google Scholar]
- 48.Togawa T, Nemoto T, Yamazaki T, Kobayashi T. A modified internal temperature measurement device. Med Biol Eng. 1976;14:361–364. doi: 10.1007/BF02478138. [DOI] [PubMed] [Google Scholar]
- 49.Prockop DJ, Kivirikko KI, Tuderman L, Guzman NA. The biosynthesis of collagen and its disorders: Part one. N Engl J Med. 1979;301:13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- 50.De Jong L, Kemp A. Stoichiometry and kinetics of the prolyl 4-hydroxylase partial reaction. Biochim Biophys Acta. 1984;787:105–111. doi: 10.1016/0167-4838(84)90113-4. [DOI] [PubMed] [Google Scholar]
- 51.Hutton JJ, Tappel AL, Udenfriend S. Cofactor and substrate requirements of collagen proline hydroxylase. Arch Biochem Biophys. 1967;118:231–240. [Google Scholar]
- 52.Myllyla R, Tuderman L, Kivirikko K. Mechanism of the prolyl hydroxylase reaction 2: Kinetic analysis of the reaction sequence. Eur J Biochem. 1977;80:349–357. doi: 10.1111/j.1432-1033.1977.tb11889.x. [DOI] [PubMed] [Google Scholar]
- 53.Goodson WH, Hunt TK. Development of a new miniature method for the study of wound healing in human subjects. J Surg Res. 1982;33:394–401. doi: 10.1016/0022-4804(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 54.Jönsson K, Jensen JA, Goodson WH, et al. Tissue oxygenation, anemia, and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214:605–613. doi: 10.1097/00000658-199111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg Sep. 2003;186(3):259–263. doi: 10.1016/s0002-9610(03)00211-3. [DOI] [PubMed] [Google Scholar]
- 56.Davidson JD, Mustoe TA. Oxygen in wound healing: more than a nutrient. Wound Repair Regen. 2001 May–Jun;9(3):175–177. doi: 10.1046/j.1524-475x.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- 57.Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. Oxygen regulates the expression of angiogenesis factor by macrophages. Science. 1983;221:1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- 58.Knighton DR, Silver IA, Hunt TK. Regulation of wound-healing angiogenesis: Effect of oxygen gradients and inspired oxygen concentration. Surgery. 1981;90:262–270. [PubMed] [Google Scholar]
- 59.Sen CK, Khanna S, Babior BM, Hunt TK, Ellison EC, Roy S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J Biol Chem. 2002 Sep 6;277(36):33284–33290. doi: 10.1074/jbc.M203391200. [DOI] [PubMed] [Google Scholar]
- 60.Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol May. 2001;280(5):H2357–2363. doi: 10.1152/ajpheart.2001.280.5.H2357. [DOI] [PubMed] [Google Scholar]
- 61.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg. 1991;126:1131–1134. doi: 10.1001/archsurg.1991.01410330093013. [DOI] [PubMed] [Google Scholar]
- 62.Gosain A, Rabin J, Reymond JP, Jenson JA, Hunt TK, Upton RA. Tissue oxygen tension and other indicators of blood loss or organ perfusion during graded hemorrhage. Surgery. 1991:523–532. [PubMed]
- 63.Akça O, Melischek M, Scheck T, et al. Postoperative pain and subcutaneous oxygen tension. Lancet. 1999;354:41–42. doi: 10.1016/S0140-6736(99)00874-0. [DOI] [PubMed] [Google Scholar]
- 64.Knighton DR, Fiegel VD, Halverson T, Schneider S, Brown T, Wells CL. Oxygen as an antibiotic. The effect of inspired oxygen on bacterial clearance. Arch Surg Jan. 1990;125(1):97–100. doi: 10.1001/archsurg.1990.01410130103015. [DOI] [PubMed] [Google Scholar]
- 65.Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic: The effect of inspired oxygen on infection. Arch Surg. 1984;119:199–204. doi: 10.1001/archsurg.1984.01390140057010. [DOI] [PubMed] [Google Scholar]
- 66.Byrne DJ, Malek MM, Davey PG, Cuschieri A. Postoperative wound scoring. Biomed Pharmacother. 1989;43:669–673. doi: 10.1016/0753-3322(89)90085-1. [DOI] [PubMed] [Google Scholar]
- 67.Pryor KO, Fahey TJ, 3rd, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population: a randomized controlled trial. JAMA. 2004 Jan 7;291(1):79–87. doi: 10.1001/jama.291.1.79. [DOI] [PubMed] [Google Scholar]
- 68.Greif R, Sessler DI. Supplemental oxygen and risk of surgical site infection (letter) JAMA. 2004 Apr 28;291(16):1957. doi: 10.1001/jama.291.16.1957-a. author reply 1958–1959. [DOI] [PubMed] [Google Scholar]
- 69.Akca O, Sessler DI. Supplemental oxygen and risk of surgical site infection (letter) JAMA. 2004 Apr 28;291(16):1956–1957. doi: 10.1001/jama.291.16.1956-b. author reply 1958–1959. [DOI] [PubMed] [Google Scholar]
- 70.Belda FJ, Aguilera L, Garcia de la Asuncion J, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005 Oct 26;294(16):2035–2042. doi: 10.1001/jama.294.16.2035. [DOI] [PubMed] [Google Scholar]
- 71.Myles PS, Leslie K, silbert B, Paech M, Peyton P. Evaluation of nitrous oxide in the gas mixture for anesthesia: A randomized controlled trial (the ENIGMA trial) Anesthesiology. 2005;103:A681. [Google Scholar]
- 72.Fleischmann E, Lenhardt R, Kurz A, et al. Nitrous oxide and risk of surgical wound infection: a randomised trial. Lancet. 2005 Sep 24;366(9491):1101–1107. doi: 10.1016/S0140-6736(05)67422-3. [DOI] [PubMed] [Google Scholar]
- 73.Lindberg P, Gunnarsson L, Tokics L, et al. Atelectasis and lung function in the postoperative period. Acta Anaesthesiol Scand. 1992;36(6):546–553. doi: 10.1111/j.1399-6576.1992.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 74.Joyce CJ, Baker AB. Effects of inspired gas composition during anaesthesia for abdominal hysterectomy on postoperative lung volumes. Br J Anaesth. 1995;75(4):417–421. doi: 10.1093/bja/75.4.417. [DOI] [PubMed] [Google Scholar]
- 75.Schwieger I, Gamulin Z, Suter PM. Lung function during anesthesia and respiratory insufficiency in the postoperative period: pysiological and clinical implications. Acta Anaesthesiol Scand. 1989;33(7):527–534. doi: 10.1111/j.1399-6576.1989.tb02960.x. [DOI] [PubMed] [Google Scholar]
- 76.Krayer S, Rehder K, Vettermann J, Didier EP, Ritman EL. Position and motion of the human diaphragm during anesthesia-paralysis. Anesthesiology Jun. 1989;70(6):891–898. doi: 10.1097/00000542-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Rothen HU, Sporre B, Engberg G, Wegenius G, Hedenstierna G. Reexpansion of atelectasis during general anaesthesia may have a prolonged effect. Acta Anaesthesiol Scand. 1995;39(1):118–125. doi: 10.1111/j.1399-6576.1995.tb05602.x. [DOI] [PubMed] [Google Scholar]
- 78.Edmark L, Kostova-Aherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology Jan. 2003;98(1):28–33. doi: 10.1097/00000542-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 79.Lodato RF, Jubran A. Response time, autonomic mediation, and reversibility of hyperoxic bradycardia in conscious dogs. J Appl Physiol. 1993;74(2):634–642. doi: 10.1152/jappl.1993.74.2.634. [DOI] [PubMed] [Google Scholar]
- 80.Haque WA, Boehmer J, Clemson BS, Leuenberger UA, Silber DH, Sinoway LI. Hemodynamic effects of supplemental oxygen administration in congestive heart failure. J Am Coll Cardiol Feb. 1996;27(2):353–357. doi: 10.1016/0735-1097(95)00474-2. [DOI] [PubMed] [Google Scholar]
- 81.Rosenberg J, Rasmussen V, von Jessen F, Ullstad T, Kehlet H. Late postoperative episodic and constant hypoxaemia and associated ECG abnormalities. Br J Anaesth Nov. 1990;65(5):684–691. doi: 10.1093/bja/65.5.684. [DOI] [PubMed] [Google Scholar]
- 82.Stausholm K, Kehlet H, Rosenberg J. Oxygen therapy reduces postoperative tachycardia. Anaesthesia Aug. 1995;50(8):737–739. doi: 10.1111/j.1365-2044.1995.tb06109.x. [DOI] [PubMed] [Google Scholar]
- 83.Kotani N, Hashimoto H, Sawamura D, Sessler DI, Muraoka M, Matsuki A. Expression of genes for pro-inflammatory cytokines in alveolar macrophages during propofol and isoflurane anesthesia. Anesth Analg. 1999;89:1250–1256. [PubMed] [Google Scholar]
- 84.Kotani N, Hashimoto H, Sessler DI, et al. Intraoperative modulation of alveolar macrophage function during isoflurane and propofol anesthesia. Anesthesiology. 1998;89:1125–1132. doi: 10.1097/00000542-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 85.Kotani N, Hashimoto H, Sessler DI, et al. Cardiopulmonary bypass produces greater pulmonary than systemic proinflammatory cytokines. Anesth Analg. 2000;90(5):1039–1045. doi: 10.1097/00000539-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Kotani N, Hashimoto H, Sessler DI, et al. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology. 2000;93(1):15–25. doi: 10.1097/00000542-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 87.Reyes RJ, Smith AA, Mascaro JR, Windle BH. Supplemental oxygen: ensuring its safe delivery during facial surgery. Plast Reconstr Surg Apr. 1995;95(5):924–928. [PubMed] [Google Scholar]
- 88.Cruse PJE, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206–210. doi: 10.1001/archsurg.1973.01350200078018. [DOI] [PubMed] [Google Scholar]
- 89.Ko W, Lazenby WD, Zelano JA, Isom OW, Krieger KH. Effects of shaving methods and intraoperative irrigation on suppurative mediastinitis after bypass operations. Ann Thorac Surg Feb. 1992;53(2):301–305. doi: 10.1016/0003-4975(92)91337-9. [DOI] [PubMed] [Google Scholar]
- 90.Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356–361. doi: 10.1016/s0003-4975(96)01044-2. [DOI] [PubMed] [Google Scholar]
- 91.Pomposelli JJ, Baxter JK, 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998 Mar–Apr;22(2):77–81. doi: 10.1177/014860719802200277. [DOI] [PubMed] [Google Scholar]
- 92.Dellinger EP. Preventing surgical-site infections: the importance of timing and glucose control. Infect Control Hosp Epidemiol. 2001;22(10):604–606. doi: 10.1086/501829. [DOI] [PubMed] [Google Scholar]
- 93.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures [see comments] Ann Thorac Surg. 1999;67(2):352–360. doi: 10.1016/s0003-4975(99)00014-4. discussion 360–352. [DOI] [PubMed] [Google Scholar]
- 94.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the surgical intensive care unit. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 95.Jonsson K, Jensen JA, Goodson WH, 3rd, West JM, Hunt TK. Assessment of perfusion in postoperative patients using tissue oxygen measurements. Br J Surg Apr. 1987;74(4):263–267. doi: 10.1002/bjs.1800740413. [DOI] [PubMed] [Google Scholar]
- 96.Hartmann M, Jonsson K, Zederfeldt B. Effect of tissue perfusion and oxygenation on accumulation of collagen in healing wounds. Randomized study in patients after major abdominal operations. Eur J Surg. 1992;158(10):521–526. [PubMed] [Google Scholar]
- 97.Kabon B, Akca O, Taguchi A, et al. Supplemental intravenous crystalloid administration does not reduce the risk of surgical wound infection. Anesth Analg Nov. 2005;101(5):1546–1553. doi: 10.1213/01.ANE.0000180217.57952.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology Oct. 2002;97(4):820–826. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Holte K, Klarskov B, Christensen DS, et al. Liberal versus restrictive fluid administration to improve recovery after laparoscopic cholecystectomy: a randomized, double-blind study. Ann Surg Nov. 2004;240(5):892–899. doi: 10.1097/01.sla.0000143269.96649.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg Nov. 2003;238(5):641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halter JB, Pflug AE, Porte D. Mechanism of plasma catecholamine increases during surgical stress in man. J clin Endocrin and Metab. 1977;45:936–944. doi: 10.1210/jcem-45-5-936. [DOI] [PubMed] [Google Scholar]
- 102.Rooth G, Hedstrand U, Tyden H, Ogen C. The validity of transcutaneous oxygen tension method in adults. Crit Care Med. 1976;4:162–165. doi: 10.1097/00003246-197605000-00009. [DOI] [PubMed] [Google Scholar]
- 103.Tremper K, Shoemaker W. Transcutaneous oxygen monitoring of critically ill adults with, and without low flow shock. Crit Care Med. 1981;9:706–709. doi: 10.1097/00003246-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 104.Tremper K, Waxman K, Shoemaker W. Effects of hypoxia and shock on transcutaneous PO2 values in dogs. Crit Care Med. 1979;7:526–531. doi: 10.1097/00003246-197912000-00003. [DOI] [PubMed] [Google Scholar]
- 105.Laffey JG, Kavanagh BP. Carbon dioxide and the critically ill--too little of a good thing? Lancet. 1999;354(9186):1283–1286. doi: 10.1016/S0140-6736(99)02388-0. [DOI] [PubMed] [Google Scholar]
- 106.Barker S, Hyatt J, Clarke C, Tremper K. Hyperventilation reduces transcutaneous oxygen tension and skin blood flow. Anesthesiology. 1991;75:619–624. doi: 10.1097/00000542-199110000-00011. [DOI] [PubMed] [Google Scholar]
- 107.Domino K, Lu Y, Einstein B, Hlastala M. Hypocapnia worsens arterial blood oxygenation and increases VA/Q heterogeneity in canine pulmonary edema. Anesthesiology. 1993;78(1):91–99. doi: 10.1097/00000542-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 108.Gustaffson U, Sjoberg F, Lewis D, Thorborg P. The effect of hypocapnia on skeletal muscle microcirculatory blood flow, oxygenation, and pH. Int J Microcirc Clin Exp. 1993;12(2):131–141. [PubMed] [Google Scholar]
- 109.Mas A, Saura P, Joseph D, et al. Effects of acute moderate changes in PaCO2 on global hemodynamics and gastric perfusion. Crit Care Med. 2000;28(2):360–365. doi: 10.1097/00003246-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 110.Hickling K, Joyce C. Permissive hypercapnia in ARDS and its effects on tissue oxygenation. Acta Anaesthesiol Scand. 1995;107:201–208. doi: 10.1111/j.1399-6576.1995.tb04359.x. [DOI] [PubMed] [Google Scholar]
- 111.Akça O, Doufas AG, Morioka N, Iscoe S, Fisher J, Sessler DI. Hypercapnia improves tissue oxygenation. Anesthesiology Oct. 2002;97(4):801–806. doi: 10.1097/00000542-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 112.Akça O, Liem E, Suleman MI, Doufas AG, Galandiuk S, Sessler DI. Effect of intra-operative end-tidal carbon dioxide partial pressure on tissue oxygenation. Anaesthesia Jun. 2003;58(6):536–542. doi: 10.1046/j.1365-2044.2003.03193.x. [DOI] [PubMed] [Google Scholar]
- 113.Stopinski J, Weissbach M. [Do abuse of nicotine and alcohol have an effect on the incidence of postoperative bacterial infections?] J Chirurgie. 1993;130:422–425. [PubMed] [Google Scholar]
- 114.Akça O, Podolsky A, Eisenhuber E, et al. Comparable postoperative pulmonary atelectasis in patients given 30% or 80% oxygen during and for two hours after colon resection. Anesthesiology. 1999;91:991–998. doi: 10.1097/00000542-199910000-00019. [DOI] [PubMed] [Google Scholar]