Abstract
Many autoimmune diseases are thought to be precipitated by viral infections. In this issue of the JCI, Lang et al. demonstrate that, in a mouse model of autoimmune hepatitis, viral infections not only trigger expansion of self-reactive T cells but also activate antigen-presenting cells through TLR stimulation (see the related article beginning on page 2456). Activated cells then secrete IFN-α and TNF-α, which trigger tissue release of chemokines that attract self-reactive CD8+ T cells, ultimately leading to liver damage.
Autoimmune diseases result from the propagation of T and B cells that recognize self antigens and mediate tissue destruction. Normally, self-reactive lymphocytes are deleted in central lymphoid organs, the thymus and bone marrow, during development (1). In the periphery, multiple safeguards exist to further prevent activation of self-reactive lymphocytes that have eluded central elimination (2). Thus, autoimmunity is fundamentally due to failure of central and/or peripheral mechanisms of immunological tolerance. Viral infections have long been suspected to instigate or overtly precipitate autoimmunity (3–5). Viral antigens can trigger autoimmune responses by molecular mimicry of self structures. Virus-mediated tissue destruction may also generate novel tissue-specific antigens to which T cells are not tolerant. Moreover, antiviral immune responses can trigger the release of cytokines that induce bystander activation of autoreactive T cells. In this issue of the JCI, Lang et al. demonstrate that viruses can initiate autoimmune damage through yet another mechanism (6). In a mouse model of hepatitis, lymphocytic choriomeningitis virus (LCMV) induced IFN-α secretion through TLR3. In turn, IFN-α triggered secretion of chemokines that attract autoreactive T cells into the liver, thereby causing hepatitis.
To model liver-specific expression of a self-antigen, Lang et al. (6) used transgenic mice that express the LCMV-glycoprotein1–60 (LCMV-GP) under the control of the mouse albumin promoter (Alb-1 mice). The LCMV-glycoprotein peptide33–41 (gp33) is presented to CD8+ T cells by H-2Db MHC class I molecules. Because the albumin promoter drives LCMV-GP expression in hepatocytes, but not in the thymus, gp33-specific CD8+ T cells are not subjected to central deletion and are present in the periphery. Infection of Alb-1 mice with live LCMV caused expansion and activation of T cells specific for the viral/self LCMV-GP antigen, resulting in hepatitis. Immunization of Alb-1 mice with gp33 peptide, however, induced expansion of autoreactive T cells but did not result in liver damage. Similarly, transfusion of gp33-specific transgenic CD8+ T cells into Alb-1 mice with or without administration of gp33 did not elicit hepatitis. Thus, expansion of autoreactive T cells is necessary, but not sufficient, to induce hepatitis in this model.
Autoimmunity is induced by a cascade of cytokines and chemokines triggered by TLR3
Lang et al. (6) went on to identify the signal that promotes liver damage by autoreactive T cells during a viral infection. It is known that viruses elicit cytokine responses through several TLRs, including TLR3, TLR7, and TLR9 (7). TLRs trigger secretion of type I IFNs, i.e., IFN-α and IFN-β, as well as proinflammatory cytokines such as TNF-α. By testing these candidates, Lang et al. found that hepatitis was triggered when gp33 was administered along with a TLR3 agonist, polyinosinic–polycytidylic acid [poly(I:C)]. A TLR9 agonist, CpG, was only weakly effective. Despite its ability to trigger autoimmunity, poly(I:C) did not appear to directly activate self-reactive CD8+ T cells, as indicated by assessment of activation markers and effector functions. In fact, poly(I:C) predominantly acted on the liver, where it induced remarkably high secretion of CXC chemokine ligand 9 (CXCL9). The liver CXCL9 response to poly(I:C) required bone marrow–derived cells, as no CXCL9 induction was observed in irradiated TLR3 competent mice reconstituted with TLR3–/– bone marrow cells. Moreover, CXCL9 induction was secondary to poly(I:C)-induced secretion of IFN-α and TNF-α, as no CXCL9 induction was observed in IFN receptor α–deficient (IFNAR–/–) and TNF receptor 1–deficient (TNFR1–/–) mice.
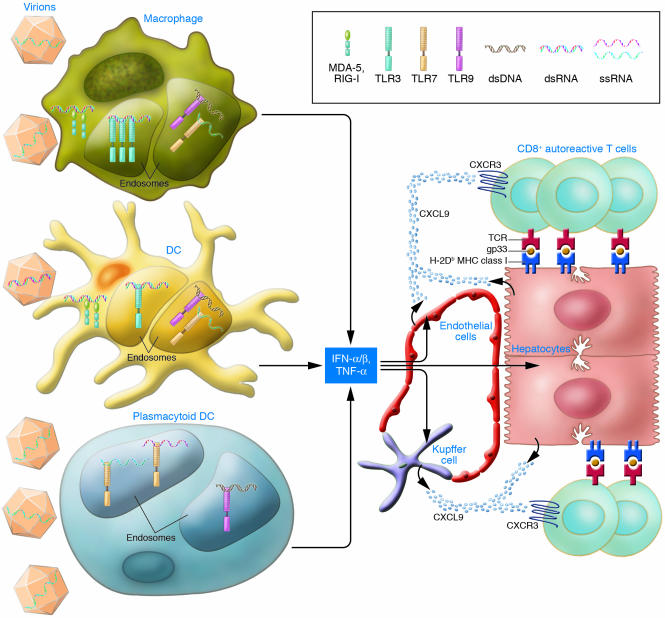
CXCL9 is an effective chemoattractant for T cells expressing the receptor CXCR3, including antigen-primed CD8+ T cells (8). Indeed, when primed gp33-specific transgenic CD8+ T cells were transferred into Alb-1 mice, the majority migrated into the liver following administration of poly(I:C), resulting in notable liver damage (6). T cell migration was reduced in Alb-1/ IFNAR–/– mice as well as following treatment with pertussis toxin, which blocks chemokine receptors. In summary, these results demonstrate that viral infection can precipitate autoimmunity by provoking TLR3-mediated secretion of IFN-α and TNF-α from macrophages, DCs, or other bone marrow–derived cells. In turn, IFN-α and TNF-α induce secretion of CXCL9 by hepatocytes and other cells, which attract self-reactive T cells into the liver, where they cause the damage associated with hepatitis (Figure 1).
Figure 1. Virus-induced mechanisms of autoimmune hepatitis.
In this issue of the JCI, Lang et al. (6) demonstrate that LCMV infection precipitates autoimmune hepatitis in a mouse model through activation of TLR3 in antigen-presenting cells, such as macrophages and DCs. Upon activation, cells secrete IFN-α/β and TNF-α, which trigger release of CXCL9 by hepatocytes, Kupffer cells, endothelial cells, and possibly other cells. CXCL9 attracts CXCR3-positive self-reactive CD8+ T cells that cause liver damage. As individual viruses can be detected in multiple antigen-presenting cells by different sensors, the figure also illustrates the potential involvement of other antigen-presenting cells, such as plasmacytoid DCs, and other viral sensors, such as TLR7, TLR9, and the RNA helicases melanoma differentiation–associated protein 5 (MDA-5) and retinoic acid–inducible gene I (RIG-I) in triggering autoimmunity. dsRNA, double-stranded RNA; ssRNA, single-stranded RNA.
IFN-α promotes autoimmunity: multiple mechanisms contribute to the general paradigm
The study by Lang et al. (6) indicates that virally-induced autoimmunity requires 2 steps: expansion of self-reactive T cells and TLR triggering in antigen-presenting cells. A role for TLR triggering in autoimmune diseases has recently been confirmed in other models. Poly(I:C) stimulation of TLR3 promotes diabetes in some mouse models (9, 10), although results appear to vary in the diabetes-prone BB rat and the NOD mouse model (11, 12). Similarly, TLR9 stimulation by DNA–anti-DNA immune complexes contributes to the activation of autoreactive B cells (13). That IFN-α promotes autoimmunity is consistent with the detection of an IFN-α signature in the transcriptome of blood leukocytes from SLE patients, along with their elevated IFN-α serum levels (14, 15). Moreover, therapeutic administration of IFN-α can induce SLE-like symptoms (16), and blockade of IFN-α/β signaling prevents autoimmunity in several animal models (9, 10, 17, 18).
Lang et al. (6) demonstrate that IFN-α acts by inducing CXCL9, which serves as a chemoattractant for autoimmune T cells into the liver. This is consistent with a previous study showing that CXCR3, the receptor for CXCL9, is required for a T cell–mediated attack of pancreatic β cells in a mouse model of diabetes (19). However, IFN-α also has other effects that can contribute to autoimmunity: it induces expression of MHC class I on tissues, thereby increasing susceptibility to CD8+ T cell attack (9) and also enhances CD8+ T cell cytolytic capacity. The IFN-α–induced upregulation of CD69 on CD8+ T cells observed by Lang et al. may promote their retention in lymph nodes (20), prolonging T cell interaction with antigen-presenting cells and enhancing T cell priming. IFN-α also facilitates T cell priming by promoting presentation of exogenous antigens through MHC class I (21).
Potential roles for cytosolic sensors of RNA and plasmacytoid DCs in autoimmunity
While the study by Lang et al. (6) establishes a clear role for TLR-mediated activation of macrophages, DCs, and IFN-α secretion, additional molecules and cells are likely to serve as viral triggers of autoimmunity. In addition to TLRs, cells rely on cytosolic double-stranded RNA–dependent protein kinase (PKR) and RNA helicases to detect RNA viruses and poly(I:C). PKR can elicit apoptosis of pancreatic β cells (22). RNA helicases, which include retinoic acid–inducible gene I (RIG-I) and melanoma differentiation–associated protein 5 (MDA-5), elicit secretion of IFN-α and proinflammatory cytokines, but each specifically detects only certain viruses (23, 24). Thus, in humans and relevant mouse models, distinct viruses may precipitate autoimmune diseases by triggering PKR or RNA helicases rather than TLRs (Figure 1). Consistent with this, a recent study has shown significant association of human type 1 diabetes with a polymorphism of MDA-5, suggesting that genetic predisposition to diabetes may result from an altered capacity to detect viruses and/or elicit IFN-α responses (25). While the study by Lang et al. (6) clearly suggests a major role for macrophages and DCs in IFN-α responses to viruses, other antigen-presenting cells may also contribute to IFN-α responses and autoimmunity (Figure 1). Indeed, plasmacytoid DCs, which specialize in the secretion of IFN-α in response to viruses, abundantly infiltrate skin lesions in SLE (26, 27).
Collectively, these studies underscore the importance of viruses in inciting self reactivity. Because individual viruses are detected in antigen-presenting cells by unique sensors, a full understanding of the role of viruses in human autoimmune diseases awaits the identification of these novel pathways and the viruses responsible for triggering them.
Footnotes
Nonstandard abbreviations used: CXCL9, CXC chemokine ligand 9; GP, glycoprotein1–60; gp33, LCMV-glycoprotein peptide33–41; IFNAR, IFN receptor α; LCMV, lymphocytic choriomeningitis virus; PKR, double-stranded RNA–dependent protein kinase; poly(I:C), polyinosinic–polycytidylic acid.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:2319–2322 (2006). doi:10.1172/JCI29879.
See the related article beginning on page 2456.
References
- 1.Mathis D., Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 2.Abbas A.K., Lohr J., Knoechel B., Nagabhushanam V. T cell tolerance and autoimmunity. Autoimmun. Rev. 2004;3:471–475. doi: 10.1016/j.autrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Fujinami R.S., von Herrath M.G., Christen U., Whitton J.L. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin. Microbiol. Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz M.S., Sarvetnick N. Viruses, host responses, and autoimmunity. Immunol. Rev. 1999;169:241–253. doi: 10.1111/j.1600-065X.1999.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Von Herrath M.G., Holz A., Homann D., Oldstone M.B. Role of viruses in type I diabetes. Semin. Immunol. 1998;10:87–100. doi: 10.1006/smim.1997.0108. [DOI] [PubMed] [Google Scholar]
- 6.Lang K.S., et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki A., Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 8.Loetscher M., et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang K.S., et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- 10.Devendra D., et al. Interferon-alpha as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes. 2005;54:2549–2556. doi: 10.2337/diabetes.54.9.2549. [DOI] [PubMed] [Google Scholar]
- 11.Sobel D.O., et al. Low dose poly I:C prevents diabetes in the diabetes prone BB rat. J. Autoimmun. 1998;11:343–352. doi: 10.1006/jaut.1998.0203. [DOI] [PubMed] [Google Scholar]
- 12.Sobel D.O., Ahvazi B. Alpha-interferon inhibits the development of diabetes in NOD mice. Diabetes. 1998;47:1867–1872. doi: 10.2337/diabetes.47.12.1867. [DOI] [PubMed] [Google Scholar]
- 13.Rifkin I.R., Leadbetter E.A., Busconi L., Viglianti G., Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol. Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 14.Bennett L., et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baechler E.C., et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause I., Valesini G., Scrivo R., Shoenfeld Y. Autoimmune aspects of cytokine and anticytokine therapies. Am. J. Med. 2003;115:390–397. doi: 10.1016/s0002-9343(03)00390-5. [DOI] [PubMed] [Google Scholar]
- 17.Kono D.H., Baccala R., Theofilopoulos A.N. Inhibition of lupus by genetic alteration of the interferon-alpha/beta receptor. Autoimmunity. 2003;36:503–510. doi: 10.1080/08916930310001624665. [DOI] [PubMed] [Google Scholar]
- 18.Theofilopoulos A.N., Baccala R., Beutler B., Kono D.H. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 19.Frigerio S., et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat. Med. 2002;8:1414–1420. doi: 10.1038/nm1202-792. [DOI] [PubMed] [Google Scholar]
- 20.Shiow L.R., et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 21.Le Bon A., et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 22.Scarim A.L., et al. Mechanisms of beta-cell death in response to double-stranded (ds) RNA and interferon-gamma: dsRNA-dependent protein kinase apoptosis and nitric oxide-dependent necrosis. . Am. J. Pathol. 2001;159:273–283. doi: 10.1016/s0002-9440(10)61693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato H., et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 24.Gitlin L., et al. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth D.J., et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat. Genet. 2006;38:617–619. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- 26.Ronnblom L., Alm G.V. Systemic lupus erythematosus and the type I interferon system. Arthritis Res. Ther. 2003;5:68–75. doi: 10.1186/ar625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jahnsen F.L., Farkas L., Lund-Johansen F., Brandtzaeg P. Involvement of plasmacytoid dendritic cells in human diseases. Hum. Immunol. 2002;63:1201–1205. doi: 10.1016/s0198-8859(02)00759-0. [DOI] [PubMed] [Google Scholar]