Abstract
Inborn errors of cholesterol synthesis cause human malformation syndromes, including Smith-Lemli-Opitz syndrome, lathosterolosis, desmosterolosis, X-linked dominant chondrodysplasia punctata type 2, and congenital hemidysplasia with ichthyosiform erythroderma and limb defects. Because adequate cholesterol is not transported across the placenta, low cholesterol and elevated sterol precursor levels are present during embryogenesis. It has been debated whether the malformations result from low cholesterol or the buildup of sterol precursors. In this issue of the JCI, Engelking et al. provide evidence that sterol precursor accumulation plays a pivotal role in the genesis of facial malformations (see the related article beginning on page 2356).
Inborn errors of cholesterol synthesis
Smith-Lemli-Opitz syndrome (SLOS; see ref. 1) is due to mutation of the gene coding for 7-dehydrocholesterol reductase (DHCR7). DHCR7 reduces the Δ7 double bond in 7-dehydrocholesterol (7-DHC) to yield cholesterol in the final step of cholesterol synthesis. Impaired DHCR7 activity causes an accumulation of 7-DHC and cholesterol deficiency. Clinical manifestations of SLOS (Figure 1) are variable, and the phenotypic spectrum is broad. Severely affected infants have multiple congenital anomalies and devastating neurological impairment. In contrast, mild SLOS combines learning and autistic-like behavioral problems with minor physical anomalies. Typical craniofacial findings include microcephaly; ptosis; a short, upturned nose; and micrognathia. Cleft palate occurs in approximately half of the patients. Limb anomalies include short thumbs, postaxial polydactyly, and 2-3 toe syndactyly. SLOS is a relatively common genetic disorder. The clinical incidence has been estimated to be on the order of 1 in 20,000 to 1 in 60,000. However, the carrier frequency for SLOS mutations suggests an incidence on par with more well-known genetic disorders, such as phenylketonuria (1 in 14,000) and osteogenesis imperfecta (1 in 20,000).
Figure 1. SLOS phenotype.
Typical facial features of a SLOS patient as an infant (A) and young child (B). Cutaneous syndactyly of the second and third toes (C) is the most frequently reported finding in SLOS. Hand anomalies (D and E) may include single palmar creases, short thumbs, and postaxial polydactyly. Some degree of cleft palate (F) is observed in approximately half of the patients.
SLOS was identified as a defect of cholesterol synthesis in 1993 (2). Subsequently, 4 additional human malformation syndromes have been shown to be due to impaired cholesterol synthesis (reviewed in ref. 3). These include the “SLOS-like” syndromes desmosterolosis and lathosterolosis, which are due to deficiencies of 3β-hydroxysterol-Δ24-reductase and lathosterol Δ5-desaturase, respectively. Two skeletal dysplasia syndromes with dermatologic manifestations, X-linked dominant chondrodysplasia punctata type 2 and congenital hemidysplasia with ichthyosiform erythroderma and limb defects, result from deficiency of 3β-hydroxysterol-Δ8, Δ7-sterol isomerase and NADPH sterol dehydrogenase, respectively. Hydrops-ectopic calcification-moth-eaten dysplasia and some cases of Antley-Bixler syndrome may include a minor impairment of cholesterol synthesis (3). However, the contribution, if any, of impaired cholesterol synthesis to these latter 2 syndromes is not clear.
Cholesterol deficiency and precursor toxicity
Understanding the pathophysiological processes that underlie the developmental defects in SLOS is complicated because cholesterol is important in multiple biological processes. Cholesterol is a structural lipid in cellular membranes and an obligatory biogenic precursor for steroid, oxysterol, and bile acid synthesis, and cholesterol modification of hedgehog morphogenic proteins is required for normal hedgehog signaling. Further complicating our understanding of the pathophysiology is that each of these biological processes could be perturbed by either cholesterol deficiency or excessive sterol precursor accumulation.
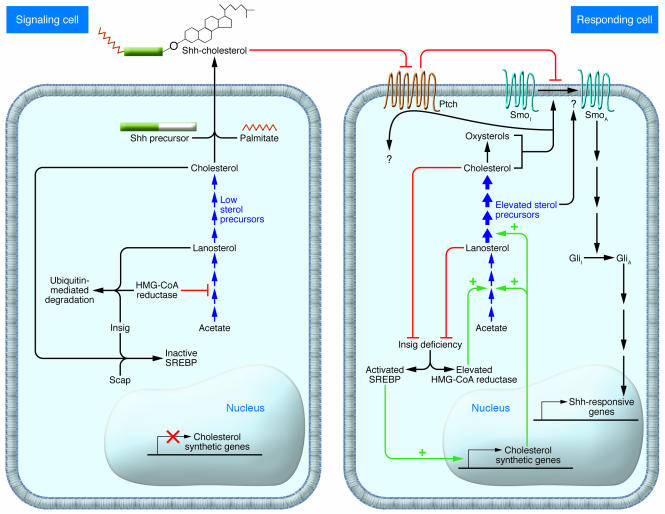
It has been debated whether the malformations in SLOS and related disorders are due to low cholesterol, low total sterol, or elevated sterol precursor levels. Precursor sterols could be bioactive themselves or give rise to bioactive sterol products, including cytotoxic oxysterols (4, 5). The study by Engelking et al. in this issue of the JCI (6) helps separate these factors. This group disrupted both Insig-1 and Insig-2 and studied the developmental consequences in double-knockout (DKO) mice. Insig proteins play a pivotal role in the regulation of cholesterol synthesis by modulating the activity of 2 sterol-sensing domain–containing proteins, Scap and HMG-CoA reductase (reviewed in ref. 7) (Figure 2). When cellular cholesterol levels are low, Scap escorts SREBPs from the endoplasmic reticulum to the Golgi apparatus, where they are activated by proteolytic processing. Activated SREBPs translocate to the nucleus and promote the expression of cholesterologenic genes. When cellular cholesterol levels rise, Insig proteins bind Scap and inhibit the translocation of SREBPs to the Golgi apparatus. Insig proteins also modulate HMG-CoA reductase activity. When the levels of lanosterol, a precursor sterol, increase, Insig binds to and mediates the proteolytic destruction of HMG-CoA reductase.
Figure 2. Insig regulation of cholesterol synthesis and Shh signaling.
When cholesterol levels are sufficient, Insig proteins regulate cholesterol synthesis by preventing the activation of SREBPs and promoting the degradation of HMG-CoA reductase (left). Cholesterol synthesis becomes dysregulated in Insig-DKO embryos (right). Even when cholesterol levels are elevated, activated SREBPs continue to promote the transcription of cholesterol synthetic genes, and HMG-CoA reductase activity remains high. This results in an accumulation of sterol precursors. The Shh signaling pathway is sensitive to perturbation of cholesterol homeostasis. In the signaling cell (left), Shh protein is modified by the addition of cholesterol. In the responding cell (right), Ptch maintains Smo in an inactive state (SmoI), perhaps by preventing sterol binding. Shh binding to Ptch relieves this inhibition. Activation of Smo (SmoA) promotes transcription of Shh-responsive genes through the Gli family of transcription factors. Adequate cholesterol or oxysterol levels appear to be necessary for Smo activation. Accumulating sterol precursors in the Insig-DKO embryos could, perhaps via a direct interaction with Smo, alter Shh signaling and underlie the genesis of facial clefting reported by Engelking et al. in Insig-DKO mice (6). GliI, inactive Gli; GliA, activated Gli.
When both Insig-1 and Insig-2 are disrupted in the liver, cholesterol synthesis becomes dysregulated. Even though cholesterol levels rise significantly above normal, activated SREBPs continue to promote the transcription of cholesterol synthetic genes, and HMG-CoA reductase activity remains high (8). Engelking et al. (6) now report the developmental consequences of Insig deficiency. Most Insig-DKO embryos have midline facial clefting ranging from cleft palate to a complete facial cleft. Cleft palate is a malformation found in both humans and mice with inborn errors of cholesterol synthesis. Mouse models of SLOS (9), lathosterolosis (10), and desmosterolosis (11) have been produced by targeted homologous recombination. All 3 of these mouse models exhibit abnormally low tissue cholesterol levels; however, the accumulating precursors differ. Cleft palate is found in SLOS and lathosterolosis mice at a frequency of 9% and 88% respectively, but is not found in desmosterolosis mice. These differences suggest that cleft palate frequency may be dependent on the nature of the accumulating sterol precursor. However, because all of these mouse models have decreased cholesterol levels, one cannot exclude the possibility that low cholesterol combined with the differential abilities of 7-DHC, lathosterol, and desmosterol to substitute for cholesterol is the critical factor. Insig-DKO embryos accumulate sterol precursors but are not cholesterol deficient. Hence, the Engelking et al. study implicates sterol precursor accumulation, rather than diminished cholesterol or total sterol levels, as a key factor in the genesis of facial malformations.
Sonic hedgehog and cleft palate
The discovery that cholesterol is necessary for the maturation of hedgehog proteins (12) provided insight into a developmental pathway that is likely perturbed due to inborn errors of cholesterol synthesis. The hedgehog proteins play a major role in multiple developmental fields. Sonic hedgehog (Shh) is involved in palate development, and disruption of Shh causes cleft palate (13). Shh undergoes autocatalytic processing, whereby a molecule of cholesterol becomes covalently attached to its signaling domain (12). Cholesterol modification is necessary for normal Shh signaling (14) (Figure 2). Shh binds to the receptor Patched (Ptch). Ptch, like Scap and HMG-CoA reductase, contains a sterol-sensing domain. In the absence of Shh, Ptch functions to maintain the signal-transducing protein Smoothened (Smo) in an inactive state. Initial hypotheses predicted that 7-DHC would disrupt the processing of Shh. However, Cooper et al. (15) showed that Shh signaling in SLOS fibroblasts is impaired at the level of Smo, due to decreased membrane sterol levels. This group hypothesized that total sterol levels may affect the balance of active and inactive forms of Smo. Recently, Corcoran and Scott (16) showed that Smo signal transduction is stimulated by either cholesterol or oxysterols, possibly by binding directly to Smo and stabilizing the active form.
It is plausible that altered Shh signaling contributes to the facial clefting found in Insig-DKO embryos. Two potential mechanisms have been proposed. First, Insig proteins could directly interact with Ptch. This would be consistent with the known interaction of Insig with sterol-sensing domain–containing proteins. Second, an accumulating sterol precursor could modulate Smo function. Small molecule modulators, both agonists and antagonists, of the hedgehog signaling pathway have been identified (17). Thus, either a gain or loss of Smo function could be proposed. In the current study, Engelking et al. (6) were able to decrease the frequency and severity of facial clefting in Insig-DKO mice by treating them with lovastatin. Lovastatin is an HMG-CoA reductase inhibitor and lowers sterol precursor levels in Insig-DKO embryos. This experiment strongly supports the idea that elevated sterol precursors underlie the facial clefting. Future work will investigate whether this is mediated through an alteration of Shh signaling.
Acknowledgments
I would like to thank Steven Fliesler for his critical review of this manuscript. I also would like to express my gratitude to my patients and their parents, whose participation allows my group’s research to proceed. This work was supported by the intramural program of the National Institute of Child Health and Human Development, NIH.
Footnotes
Nonstandard abbreviations used: 7-DHC, 7-dehydrocholesterol; DHCR7, 7-dehydrocholesterol reductase; DKO, double-knockout; Ptch, Patched; Shh, Sonic hedgehog; SLOS, Smith-Lemli-Opitz syndrome; Smo, Smoothened.
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 116:2322–2325 (2006). doi:10.1172/JCI29872.
See the related article beginning on page 2356.
References
- 1.Kelley R.I., Hennekam R.C. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 2000;37:321–335. doi: 10.1136/jmg.37.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irons M., Elias E.R., Salen G., Tint G.S., Batta A.K. Defective cholesterol biosynthesis in Smith-Lemli-Opitz syndrome. Lancet. 1993;341:1414. doi: 10.1016/0140-6736(93)90983-n. [DOI] [PubMed] [Google Scholar]
- 3.Porter F.D. Malformation syndromes due to inborn errors of cholesterol synthesis. J. Clin. Invest. 2002;110:715–724. doi: 10.1172/JCI200216386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shackleton C., Roitman E., Guo L.W., Wilson W.K., Porter F.D. Identification of 7(8) and 8(9) unsaturated adrenal steroid metabolites produced by patients with 7-dehydrosterol-delta7-reductase deficiency (Smith-Lemli-Opitz syndrome). J. Steroid Biochem. Mol. Biol. 2002;82:225–232. doi: 10.1016/s0960-0760(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 5.Wassif C.A., Yu J., Cui J., Porter F.D., Javitt N.B. 27-Hydroxylation of 7- and 8-dehydrocholesterol in Smith-Lemli-Opitz syndrome: a novel metabolic pathway. Steroids. 2003;68:497–502. doi: 10.1016/s0039-128x(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 6.Engelking L.J., et al. Severe facial clefting in Insig-deficient mouse embryos caused by sterol accumulation and reversed by lovastatin. J. Clin. Invest. 2006;116:2356–2365. doi: 10.1172/JCI28988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein J.L., DeBose-Boyd R.A., Brown M.S. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Engelking L.J., et al. Schoenheimer effect explained — feedback regulation of cholesterol synthesis in mice mediated by Insig proteins. J. Clin. Invest. 2005;115:2489–2498. doi: 10.1172/JCI25614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wassif C.A., et al. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum. Mol. Genet. 2001;10:555–564. doi: 10.1093/hmg/10.6.555. [DOI] [PubMed] [Google Scholar]
- 10.Krakowiak P.A., et al. Lathosterolosis: an inborn error of human and murine cholesterol synthesis due to lathosterol 5-desaturase deficiency. Hum. Mol. Genet. 2003;12:1631–1641. doi: 10.1093/hmg/ddg172. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler A., et al. Generation of viable cholesterol-free mice. Science. 2003;302:2087. doi: 10.1126/science.1090776. [DOI] [PubMed] [Google Scholar]
- 12.Porter J.A., Young K.E., Beachy P.A. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 13.Rice R., et al. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J. Clin. Invest. 2004;113:1692–1700. doi: 10.1172/JCI200420384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis P.M., et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. [DOI] [PubMed] [Google Scholar]
- 15.Cooper M.K., et al. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat. Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 16.Corcoran R.B., Scott M.P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank-Kamenetsky M., et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J. Biol. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]