Abstract
The ubiquitously expressed, error-prone DNA polymerase β (polβ) plays a role in base excision repair, and the involvement of this molecule in the nonhomologous end joining (NHEJ) process of DNA repair has recently been demonstrated in yeast. Polβ-deficient mice are not viable, and studies on conditional mutants revealed a competitive disadvantage of polβ−/− vs. wild-type cells. We show here that polβ-deficient mice survive up to day 18.5 postcoitum, but die perinatally; a circumstance that allowed the investigation of a potential role of polβ in lymphocyte development by transfer of fetal liver cells (FLC) derived from polβ−/− embryos into lethally irradiated hosts. FLC transfers using mutant cells lead to an almost normal reconstitution of the lymphocyte compartment, indicating that polβ-deficiency does not prevent V(D)J recombination, which is known to employ factors of the NHEJ pathway. Mice reconstituted with polβ−/− FLC mount a normal T cell-dependent immune response against the hapten (4-hydroxy-3-nitrophenyl) acetyl (NP). Moreover, germinal center B cells from NP-immunized reconstituted mice show normal levels and patterns of somatic point mutations in their rearranged antibody genes, demonstrating that polβ is not critically involved in somatic hypermutation.
The ubiquitously expressed DNA polymerase β (polβ) is the smallest DNA polymerase described, consisting of a single 39-kDa polypeptide that has both nucleotidyltransferase and 5′-deoxyribose phosphodiesterase activities. Polβ is essential for base excision repair (BER) (1), and recently it has been localized along synaptonemal complexes, suggesting that polβ may participate in meiotic recombinatorial events (2). More recently, a role for the yeast homologue of polβ (pol IV) in the nonhomologous end joining (NHEJ) pathway of DNA double-strand break repair has been demonstrated (3). In BER, polβ is capable of filling small gaps and nicks in DNA (4). Polβ acts in an error-prone fashion; it shows an average mutation rate of 10−3-10−4/bp/generation (5), and transitions are more frequently introduced than transversions (6). These features mark polβ as a candidate for the elusive “somatic hypermutase,” the hypothetical enzyme responsible for the diversification of antibody genes in T cell-dependent immune responses.
Upon antigen encounter in T cell-dependent immune responses, B cells are driven into specific structures in secondary lymphoid organs, the germinal centers (GC) (7). Here, they refine their antibodies through somatically hypermutating their rearranged V genes to generate high-affinity antibodies against the immunizing antigen (8). Antigen-selected memory B cells and plasma cells leave the GC and provide the organism with life-long immunity. The somatic hypermutation process introduces mainly single nucleotide exchanges, but also deletions and duplications (9, 10), at a high rate (10−3–10−4/bp/generation) into rearranged V region genes (11, 12). The nucleotide changes do not occur at random: transitions dominate over transversions, the four nucleotides do not mutate at equal rates, and in a particular sequence context, mutations occur more frequently than in other positions (hot spots of mutation) (see ref. 13). While advances have been made in identifying cis-acting elements that are required for the hypermutation process (14, 15), the molecular mechanism of somatic hypermutation is still poorly understood, as reflected by the number of models that have been put forward to explain the hypermutation phenomenon (see refs. 16 and 17). One line of thinking draws a connection between the introduction of point mutations and error-prone DNA repair (18). Consequently, the potential role of DNA-repair factors in hypermutation became the focus of investigations in recent years (19–27). While several groups report changes in the level and/or pattern of somatic point mutations in mice deficient for the mismatch repair factors PMS2 and MSH2 and invoke the involvement of these molecules in somatic hypermutation (19, 20, 21, 24, 26), other studies come to the opposite conclusion (22, 23, 25). Moreover, various other molecules acting in different pathways of DNA repair do not appear to play a role in hypermutation (23). All of the DNA repair factors analyzed so far are likely to exhibit their function only secondary to the introduction of somatic mutations into the V region through error-prone DNA polymerization.
DNA polymerases acting in short-patch DNA repair represent salient hypermutase candidates (28). In the light of the recent findings that DNA strand breaks occur during hypermutation (9, 29), it may be envisaged, along the lines of Brenner and Milstein (18), that a nick introduced into the V region serves as an entry point for an error-prone DNA polymerase like polβ.
While polβ-deficient mice are not viable, mutant embryos were present in expected Mendelian ratios at day 10.5 postcoitum (p.c.) (30). The analysis of conditional polβ mutant animals revealed a competitive disadvantage of polβ−/− compared with wild-type cells during T cell development and embryonic development (30, 31). In the present study, we first determined the stage of development at which death of polβ-deficient embryos occurs, and found that the mutant embryos are viable up to day 18.5 p.c., but die at birth. Thus it was possible to reconstitute irradiated wild-type mice with fetal liver cells (FLC) obtained from polβ−/− embryos to test the ability of the mutants to achieve reconstitution of the hematopoietic system. Since polβ-deficient FLC were only slightly impaired in this respect, it was possible to study the potential involvement of polβ in somatic hypermutation.
Materials and Methods
Mice.
C57BL/6 mice heterozygous for a deletion mutation in the 5′ region of the polβ gene (30) (IgHb allotype and H-2b haplotype) were bred to obtain homozygous embryos. Twelve-week-old 129/Sv females (IgHa allotype, H-2b haplotype) were used as recipients for FLC transfers. For timed pregnancies, the appearance of a vaginal plug after overnight mating was labeled as day 0.5 of gestation. 129/SV hosts were purchased from Charles River Breeding Laboratories (Lyon, France).
FLC Transfers.
polβ−/−, polβ+/−, and wild-type FLC were isolated from embryos of IgHb allotype at day 18.5 p.c. Embryos were obtained by intercrossing polβ+/− mice of IgHb allotype. Mice and embryos were genotyped by Southern blot analysis as described previously (30). Single cell suspensions of fetal livers were obtained, and 106 FLC in Hanks' balanced salt solution in a volume of 200 μl were injected i.v. into 129/Sv female mice (IgHa allotype), which were lethally irradiated (1,000 rad) the previous day. Recipients were treated with antibiotics (30 mg/liter kanamycinsulfate, 30 mg/liter neomycinsulfate; and 50 mg/liter gentamycinsulfate) in the drinking water for 2 wk after irradiation. Reconstitution was examined 1 and 2 mo after FLC transfer by staining peripheral blood with anti-IgMa and anti-IgMb antibodies, Southern blot analysis of thymus and spleen, and FACS analysis of thymocytes and splenocytes.
Cell Staining and Flow Cytometry.
The following antibody conjugates were used: phycoerythrin (PE)-, allophycocyanin-, and biotin-conjugated RA3–6B2 (anti-220) (32); FITC-conjugated R33-24-12 (anti-IgM) (33); FITC and biotin-conjugated 1.3-5 (anti-IgD) (34); PE-RS3.1 (anti-IgMa) (35); FITC-MB86 (anti-IgMb) (36); CyChrome (CyC)-conjugated GK1.5/4 (anti-CD4) (37); FITC-53–6.7 (anti-CD8) (38); FITC-conjugated peanut agglutinin (PNA) (Vector Laboratories).
Single cell suspensions from spleen were treated with ACT buffer (0.8% NH4Cl) to eliminate erythrocytes and resuspended in staining buffer (PBS/BSA/NaN3). A total of 106 cells were incubated on ice with FITC-, PE-, CyC- or biotin-conjugated antibodies for 15 min. Biotin-conjugated antibodies were developed with streptavidin-CyC (PharMingen). Flow cytometric analysis was performed on a FACScan (Becton Dickinson).
Immunization and Serum IgG1a and IgG1b Determination.
Six weeks after FLC transfer, recipient mice were immunized i.p. with 100 μg of alum-precipitated NP-CG [(4-hydroxy-3-nitrophenyl) acetyl coupled to chicken γ globulin]. Eleven days after immunization, mice were bled from the tail vein. The serum concentrations of NP-specific IgG1a and IgG1b were assayed by ELISA. Plastic plates were coated with NIP15-BSA (10 μg/ml), and serial serum dilutions were applied onto the plate. Bound antibodies were revealed by biotinylated Ig(4a)20.9 (39) and Ig(4b) (PharMingen) antibodies for IgG1a and IgG1b, respectively. The limit of detection for NP-specific IgG1a and IgG1b ELISA is 0.08 μg/ml.
Purification of GC B Cells and Naive B Cells from Mouse Spleens.
Fourteen days after NP-immunization, splenic GC B cells were enriched by magnetic cell separation by using the MACS system (Miltenyi Biotech, Bergisch Gladbach, Germany). Following erythrocyte lysis, splenic cells were first incubated with anti-IgD-biotin, and after washing with streptavidin-, anti-CD5-, and CD11b-microbeads (Miltenyi Biotech), and applied to a Vs separation column (Miltenyi Biotech). The fraction of cells that remained bound to the separation column was eluted outside the magnetic field and used for the isolation of naive B cells. The flow-through, enriched for GC B cells, was stained with anti-B220-PE and PNA-FITC. B220+PNA+ cells were isolated on a FACS 440 (Becton Dickinson). Naive B cells were isolated according to their B220+IgD+ expression.
Amplification of V186.2 Rearrangements from Genomic DNA of Splenic GC and Naive B Cells.
The V186.2 variable gene segment is used by B cells of C57BL/6 mice in the antibody response against the hapten NP. V186.2 belongs to a family of closely related gene segments, most, but presumably not all, of which have been identified. To specifically amplify V186.2 rearrangements from (sometimes few) GC or naive B cells, a two-step PCR strategy was established that excludes the recognition of most V186.2-related gene segments. The parallel amplification of V186.2 rearrangements from naive B cells carrying unmutated V regions (40) ascertains that no as yet unknown V186.2-related genes are amplified.
Sorted cell suspensions were incubated in 50 μl 10 mM Tris⋅HCl, 0.1 mM EDTA per 105 cells containing 0.5 mg/ml proteinase K for 2.5 h at 50°C. The enzyme was inactivated by denaturation at 95°C (10 min). For the first round of amplification, a primer that recognizes a sequence in the promoter region of V186.2 (outer primer: 5′-TCTTTACAGTTACTGAGCACACAGGAC-3′), and a downstream primer that hybridizes in the intron following JH2 fragment (5′-GGGTCTAGAGGTGTCCCTAGTCCTTCATGACC-3′) was used. At the last 3′ nucleotide, the V186.2 outer primer carries a mismatch to the corresponding sequences of most other known V186.2-related genes. The first round of amplification was performed with 20 μl of the proteinase K-treated cell suspension in a 50-μl volume containing Klentherm buffer (Genecraft, Münster, Germany), 3.5 mM MgCl2, 100 μM dATP, dGTP, dTTP, and dCTP, respectively, 0.4 μM of each primer, and 0.4 μl a mixture of Klentherm DNA polymerase (Genecraft) with native Pyrococeus furosus (Pfu) DNA polymerase (Stratagene) at a ratio of 60 to 1 units. The enzyme mixture was added after the first denaturation step (5 min at 95°C). The amplification program consisted of 20 cycles of 30 s at 95°C, 30 s at 70°C, and 2 min at 72°C, followed by a final incubation step at 72°C for 5 min. For the second round of amplification, the same JH2-specific primer was used together with an internal V186.2-specific primer that carries a mismatch at the last but one 3′ position to all other known V186.2-related genes (5′-CAGTAGCAGGCTTGAGGTCTGGAC-3′). The second round of amplification was performed by using 3 μl of the first-round reaction mixture in a 50 μl volume containing Pfu buffer (Stratagene), 2.0 mM MgCl2, 100 μM dATP, dGTP, dTTP, and dCTP, respectively, 0.4 μM of each primer, and 2.5 units native Pfu DNA polymerase. The amplification conditions were as described above, except that 30 cycles were applied. PCR products were purified by gel electrophoresis, incubated with Klentherm DNA polymerase and dNTPs for 15 min at 72°C to add overhang As, and ligated into the pGEM-T easy vector (Stratagene). Following transformation, plasmids were isolated by using the Qiagen Plasmid-Mini-Kit (Qiagen, Hilden, Germany), and sequenced by dye-terminated automatic sequencing (Applied Biosystems). Sequence analysis was performed with the IMGT-database (http://www.genetik.uni-koeln.de/dnaplot/) and the dnasis software (Pharmacia, Freiburg, Germany). Unreadable sequences and hybrid sequences (in all cases hybrids between V186.2 and 4m4) were excluded from the analysis.
Results and Discussion
polβ-Deficient Mice Die Perinatally.
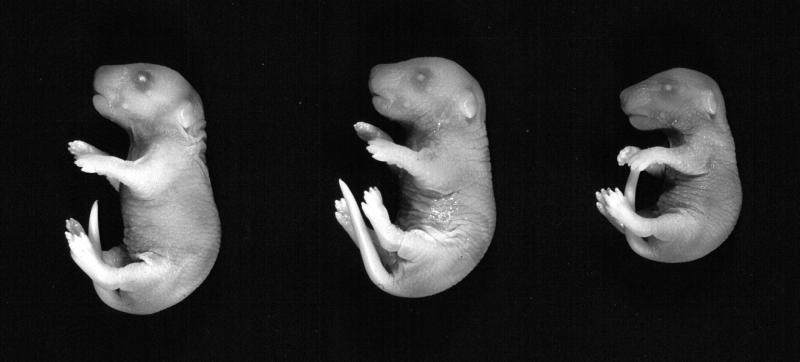
Polβ-deficient mice are not viable (30). To determine the stage of embryonic development when polβ−/− mice die, polβ+/− mice were intercrossed, and the genotype of the offspring was determined by Southern blot analysis at various stages of embryonic development. Mutant embryos were present at the expected Mendelian ratios up to day 18.5 p.c. (Table 1). Although mutant embryos were reduced in size compared with heterozygous or wild-type littermates (Fig. 1), most of them were alive and moving even when prepared at day 18.5 p.c. This shows that death of polβ-deficient mice occurs perinatally. Analysis of the morphology of the mutant embryos failed to reveal distinct gross defects; at day 18.5 p.c., however, the characteristic alveolar explosion did not occur in the mutant embryos (J. Löhler, unpublished results). Extension of pregnancy by administration of progesterone or delivery of pups by Caesarean section failed to produce viable polβ-deficient animals, possibly because the mutants fail to breathe.
Table 1.
Frequencies of polβ+/+, polβ+/−, and polβ−/− embryos at different stages of gestation, derived from intercross between polβ+/− animals
| d.p.c. | polβ+/+ | polβ+/− | polβ−/− | No. of embryos |
|---|---|---|---|---|
| 13.5 | 4 | 7 | 0 | 11 |
| 14.5 | 6 | 8 | 5 | 19 |
| 15.5 | 4 | 12 | 3 | 19 |
| 16.5 | 4 | 8 | 2 | 14 |
| 17.5 | 4 | 10 | 3 | 17 |
| 18.5 | 27 | 53 | 27 | 107 |
| Total | 49 | 98 | 40 | 187 |
d.p.c., days postcoitum.
Figure 1.
polβ−/− embryos (Right) at day 18.5 p.c. are growth retarded compared with their polβ+/− (Center) and polβ+/+ (Left) littermates. Homozygous mutant embryos appeared to be cyanotic.
polβ-Deficient FLC Are Able to Reconstitute Lethally Irradiated Mice and Generate B and T Cells.
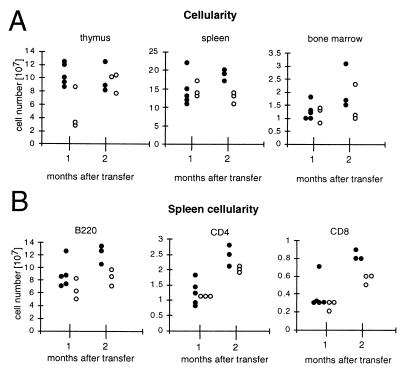
The fetal liver is the major site of hematopoiesis in the mouse embryo at day 18.5 p.c. (41). To generate mice carrying polβ-deficient B cells, 106 FLC from day 18.5 p.c. polβ−/− and, for control, polβ+/− and polβ+/+ embryos, were transferred into lethally irradiated 129/Sv recipient mice. Since recipient B cells bear the IgHa and donor cells bear the IgHb allotype, successful reconstitution was verified by peripheral blood staining with antibodies recognizing IgMa and IgMb, respectively. The vast majority of the animals carried only B cells derived from the donors (data not shown). In addition, reconstitution was confirmed by Southern blot analysis of thymus and spleen (data not shown). Reconstituted mice were sacrificed 1 and 2 mo after FLC transfer and analyzed for thymic, splenic, and bone marrow cellularity. The proportions of splenic B and CD4+ and CD8+ T cells were also determined. The results are depicted in Fig. 2. Polβ-deficient FLC were able to reconstitute the hematopoietic system of lethally irradiated recipients, although less efficiently than the heterozygous control cells.
Figure 2.
Lethally irradiated recipient 129/Sv mice were reconstituted with 106 FLC prepared from day 18.5 p.c. polβ+/− (●) and polβ−/− (○) embryos. One and two months after reconstitution, the total number of thymocytes, splenocytes, and femur bone marrow cells (A) was determined as well as the number of splenic B220+ B cells, CD4+ T cells, and CD8+ T cells (B).
B Cells Derived from polβ-Deficient FLC Are Able to Participate in T Cell-Dependent Immune Responses.
To investigate whether polβ−/− B cells are able to clonally expand and differentiate into plasma cells secreting antigen-specific Ig in the course of a T cell-dependent immune response, reconstituted mice were immunized with NP-CG, and the levels of NP-specific IgG1a and IgG1b were determined 11 days after immunization. Mice reconstituted with polβ-deficient FLC showed serum concentrations of (donor-derived) IgG1b that were in the same range as those of the mice reconstituted with polβ-expressing FLC (Table 2). Thus, polβ-deficient B cells participate in T-dependent immune responses comparably to control B cells.
Table 2.
Immune response of FLC-derived B cells (IgHb) to NP-CG
| FLC genotype | NP-IgG1a μg/ml | NP-IgG1b μg/ml |
|---|---|---|
| polβ−/− | <nd | 926 |
| <nd | 728 | |
| polβ−/− | <nd | 530 |
| 0.6 | 690 | |
| <nd | 820 | |
| polβ+/− | <nd | 790 |
| <nd | 738 | |
| <nd | 2416 |
Reconstituted mice were immunized with NP-CG and the serum concentrations of NP-specific IgG1a and IgG1b were measured 11 days after immunization. nd, below limit of detection (0.08 μg/ml).
polβ-Deficient GC B Cells Show Normal Levels and Patterns of Somatic Point Mutations.
To investigate whether polβ is involved in somatic hypermutation of Ig genes, FLC from polβ−/− and wild-type embryos were transferred into irradiated hosts. Six weeks after reconstitution of the hematopoietic system, the mice were immunized with NP-CG (see Material and Methods), and 14 days later, GC B cells were analyzed for the level and pattern of somatic mutations in rearranged V186.2 genes. The T-dependent immune response against the hapten NP in C57BL/6 mice has been extensively characterized (42–45). B cells responding to NP characteristically express a rearranged V186.2 gene, a member of the J558 gene family, together with λ1 light chains. A point mutation in codon 33 of the complementarity determining region 1 of V186.2, leading to a tryptophan to leucine exchange, increases the affinity of the anti-NP antibody 10-fold (46). Thus, the occurrence of a V186.2 rearrangement bearing this mutation indicates positive selection of the corresponding B cell for NP binding. V186.2 is present in the germ line of C57BL/6 mice (IgHb allotype) but not 129/Sv mice (IgHa allotype), the hosts in the transfer experiments (47). Therefore, the specific amplification of V186.2-carrying rearrangements ensures that B cells derived from the FLC of donor embryos are analyzed, rather than B cells that potentially persisted in the recipient mouse after irradiation.
GC B cells (B220+PNA+) isolated from the spleens of NP-CG-immunized mice reconstituted with FLC from polβ−/− (polβ−/−1 and polβ−/−2) embryos and from a wild-type (reWT) embryo were investigated for somatic mutations in their V regions. Samples of GC B cells came from pairs of mice reconstituted with FLC derived from a single embryo. In one case, cells derived from two polβ−/− embryos were pooled (polβ−/−2). Naive B cells (B220+ IgD+) derived from polβ−/− FLC (polβ−/−2N) were analyzed in parallel. In addition, splenic GC B cells were purified from a similarly immunized wild-type C57BL/6 mouse (WT). Rearranged V186.2 genes from 40,000 cell equivalents were amplified in a semi-nested PCR approach using V186.2-specific primers and a JH2 intron primer (see Material and Methods). JH2 is frequently used in V186.2-rearrangements in the anti-NP response (44). PCR products were cloned and sequenced. The vast majority of the sequences could be assigned to V186.2. Some sequences were mostly homologous to the V186.2-related gene segment 4m4 (40). Only 4 of the altogether 141 sequences showed highest homology to other gene segments in the IMGT database (not shown). In the analysis of GC B cells for somatic mutation, only V186.2-carrying rearrangements were considered. Some sequences showed deletions of a variable number of nucleotides (data not shown) that may represent either deletions introduced during somatic hypermutation in vivo (9) or artifacts that arose during cloning. Rearrangements bearing a 4m4 gene segment were included only in the analysis of naive B cells (9/24 sequences showed a rearranged 4m4).
A total of 9 of 22 sequences derived from the WT mouse showed a unique rearrangement, 13 sequences could be assigned to either of 4 clonally related rearrangements ranging from 2 to 5 members. Of the 30 sequences derived from reWT, 9 could be assigned to either of 4 clonally related rearrangements (2–3 members each). A total of 6 of 25 sequences derived from Pβ−/−1, and 5 of 30 sequences from Pβ−/−2 could be assigned to 3 and 2 clonally related rearrangements, respectively (2–3 members each). In all cases, the sequences bearing the same rearrangement showed (sometimes extensive) intraclonal diversity, indicating that those sequences were derived from independent members of V186.2-bearing B cell clones expanding within the GC as described earlier (48). A total of 4 of 24 sequences derived from naive B cells (Pβ−/−2N) could be assigned to 2 clonally related rearrangements.
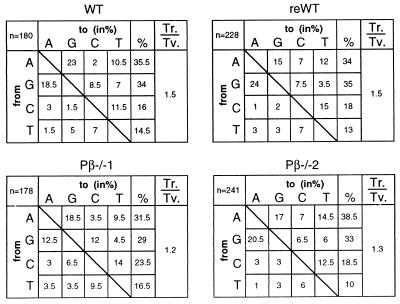
Thirteen sequences derived from the naive B cells were unmutated, and of the remaining sequences, 9 showed 1, and 2 showed 3 nucleotide exchanges, altogether yielding a mutation frequency of 0.15%. Thus, as expected (40), the vast majority of naive B cells carried unmutated rearrangements. In contrast, all sequences derived from GC B cells showed somatic mutations in the V186.2 and/or JH2 gene segments. Among the four sets of cells analyzed, both the range of mutations per rearrangement and the average mutation frequency (around 2%) were similar (Table 3). In determining the pattern of nucleotide substitutions, shared mutations between clonally related rearrangements were counted only once, and the G to T exchange in codon 33 that occurs in the majority of rearrangements was not considered because it is known to result from selection for NP-binding (see above). The pattern of nucleotide substitutions does not reveal differences between wild-type and polβ-deficient GC B cells (Fig. 3). In accord with this observation, the ratios of transitions over transversions are in a similar range in the cell populations (Fig. 3), as is the mutation frequency of the 2nd position in the RGYW hotspots (7.8% and 8.5% in WT and reWT, respectively, and 5.8% and 8.5% in Pβ−/−1 and Pβ−/−2, respectively). Also, the distribution of mutations along the V gene was similar in wild-type and knockout B cells (data not shown).
Table 3.
Analysis of splenic GC B cells from 129 mice reconstituted with FLC from wild-type and polβ−/− embryos (C57BL/6) for somatic mutation of V186.2 gene
| Clones | Mutated | Range | Mut./bp | % mutation | |
|---|---|---|---|---|---|
| WT | 22 | 22 | 4–15 | 205 /9660 | 2.1 |
| reWT | 30 | 30 | 1–18 | 276 /13080 | 2.1 |
| polβ−/−1 | 25 | 25 | 1–13 | 188 /10820 | 1.7 |
| polβ−/−2 | 30 | 30 | 3–15 | 255 /13050 | 1.95 |
| polβ−/−2N* | 24 | 11 | 1–3 | 15 /10180 | 0.15 |
The germline Ig sequences of the V186.2 and 4m4 genes can be retrieved from EBI FTP file server at ftp://.ebi.ac.uk/pub/database/embl/align/ds6748.dat.
*Nine 4m4 rearranged sequences have been included.
Figure 3.
Pattern of nucleotide exchanges in the V186.2 rearrangements of GC B cells derived from a wild-type C57BL/6 mouse and from mice reconstituted with wild-type and polβ-deficient day 18.5 p.c. FLC, respectively. n, the number of mutations; shared mutations in clonally related sequences were counted only once. The G to T exchange in codon 33, which represents a result of selection for NP-binding, was not considered to avoid skewing of the analysis (see text). Ts., transitions; Tv., transversions; Ts./Tv., the transitions over transversions ratio.
A difference between wild-type and polβ−/− GC B cells was observed concerning the fraction of rearrangements showing the characteristic nucleotide exchange in codon 33. Whereas around 70% of the rearrangements derived from wild-type GC B cells carried a mutation in this codon, it occurred in <50% of those from polβ−/− GC B cells. (WT, 78.5% (n = 14); reWT, 67% (n = 27); Pβ−/−1, 26% (n = 23); Pβ−/−2, 46% (n = 24); when the respective mutation was shared among clonally related rearrangements, mutation and rearrangement were counted only once.) It is known that the frequency of rearrangements bearing the affinity-enhancing mutation in codon 33 increases in the course of the anti-NP response (44, 45). While the present observation indicates that the GC response might be delayed in mice reconstituted with polβ-deficient B cells (in accordance with the less efficient reconstitution of the hematopoietic system, see above), the occurrence of a sizeable fraction of rearrangements showing a mutated codon 33, together with the normal anti-NP serum levels upon immunization (see above), shows that polβ-deficient B cells are able to mount an efficient anti-NP response, in which high-affinity somatic antibody mutants are generated and selected.
Conclusions
Polβ deficiency results in growth retardation and perinatal death, the latter most likely resulting from a failure in breathing as suggested by the lack of alveolar explosion in the lungs of day 18.5 p.c. embryos. In a previous study involving the generation of mosaic embryos (31), we noticed that the fraction of polβ-deficient cells in the mosaic mutants decreased during development, indicating a general disadvantage of polβ-deficient vs. wild-type cells. When mosaic adults were obtained, many were reduced in size and weight when compared with heterozygous mosaic littermates. Intriguingly, the fraction of polβ-deleted cells in the brain correlated inversely with the size of the mice as observed for the mosaic embryos (ref. 31, and U.A.K.B., unpublished results). Although we did not detect gross histological abnormalities in brain sections from such embryos, this observation may imply that an impaired brain development ultimately leads to the death of the mutant embryos.
Since components of the NHEJ pathway of DNA repair are recruited during V(D)J recombination, the recent observation that the yeast homologue of polβ works during NHEJ (3) raises the possibility that polβ mutant cells may show deficiencies in the V(D)J recombination. However, FLC derived from day 18.5 p.c. embryos are able to reconstitute the lymphocyte compartment of lethally irradiated hosts to an almost normal extent. Therefore, while there seems to be a competitive disadvantage of polβ-deficient compared with wild-type cells, as observed in thymocytes, T cells (30), and mosaic embryos (31), the results of the present study show that a normal hematopoietic system can be established in the absence of polβ, and that lymphoid cells are able to proliferate and undergo V(D)J recombination under this condition, the latter in agreement with the original observation by Gu et al. (30). Moreover, polβ-deficient B cells are able to mount an efficient T-dependent immune response, in the course of which they hypermutate their rearranged antibody genes normally. This demonstrates that the error-prone DNA polymerase β is not critically involved in somatic hypermutation.
Still, the hypothesis that an error-prone DNA polymerase might be a major player in the somatic hypermutation mechanism remains attractive. In this regard, the recently described polymerase ζ represents a strong candidate (49, 50).
Acknowledgments
We thank C. Göttlinger and C. Uthoff-Hachenberg for technical assistance; S. Willms and J. Jesdinsky for help with the sequencing; A.Egert and A. Roth for help with animal work, Udo Ringeisen for graphical work; and M. Alimzanov for critically reading the manuscript. This work was supported by the European Union (B104-CT96-0077), the Deutsche Forschungsgemeinschaft through SFB 243, the Land Nordrhein-Westfalen, and the Human Frontiers Science Program. G.E. was the recipient of fellowships from the European Community Training and Mobility in Research and the Alexander von Humboldt-Stiftung. G.T. was supported by fellowships from the European Commission (Human Capital and Mobility) and Kölner Verein zur Förderung der Immunologie.
Abbreviations
- polβ
DNA polymerase β
- IgV
Ig variable
- FLC
fetal liver cells
- NP
(4-hydroxy-3-nitrophenyl) acetyl
- NP-CG
NP coupled to chicken γ globulin
- NHEJ
nonhomologous end joining
- GC
germinal center
- p.c.
postcoitum
- PE
phycoerythrin
- CyC
CyChrome
- PNA
peanut agglutinin
References
- 1.Sobol R W, Horton J K, Kühn R, Gu H, Singhal R K, Prasad R, Rajewsky K, Wilson S H. Nature (London) 1996;379:183–186. doi: 10.1038/379183a0. [DOI] [PubMed] [Google Scholar]
- 2.Plug A W, Clairmont C A, Sapi E, Ashley T, Sweasy J B. Proc Natl Acad Sci USA. 1997;94:1327–1331. doi: 10.1073/pnas.94.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson T E, Lieber M R. Proc Natl Acad Sci USA. 1999;33:23599–23609. [Google Scholar]
- 4.Wiebauer K, Jiricny J. Proc Natl Acad Sci USA. 1990;87:5842–5845. doi: 10.1073/pnas.87.15.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunkel T A, Alexander P S. J Biol Chem. 1986;261:160–166. [PubMed] [Google Scholar]
- 6.Clairmont C A, Narayanan L, Sun K-W, Glazer P M, Sweasy J B. Proc Natl Acad Sci USA. 1999;96:9580–9585. doi: 10.1073/pnas.96.17.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacLennan I C M. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 8.Rajewsky K. Nature (London) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 9.Goossens T, Klein U, Küppers R. Proc Natl Acad Sci USA. 1998;95:2463–2468. doi: 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson P C, de Boutellier O, Liu Y-J, Potter K, Banchereau J, Capra J D, Pascual V. J Exp Med. 1998;187:59–70. doi: 10.1084/jem.187.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKean D, Hüppi K, Bell M, Staudt L, Gerhard W, Weigert M. Proc Natl Acad Sci USA. 1984;81:3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocks C, Rajewsky K. Proc Natl Acad Sci USA. 1988;85:8206–8210. doi: 10.1073/pnas.85.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner S D, Neuberger M S. Annu Rev Immunol. 1996;14:441–457. doi: 10.1146/annurev.immunol.14.1.441. [DOI] [PubMed] [Google Scholar]
- 14.Storb U, Peters A, Klotz E, Kim N, Shen H M, Hackett J, Rogerson B, Martin T E. Immunol Rev. 1998;162:153–160. doi: 10.1111/j.1600-065x.1998.tb01438.x. [DOI] [PubMed] [Google Scholar]
- 15.Neuberger M S, Ehrenstein M R, Klix N, Jolly C J, Yelamos J, Rada C, Milstein C. Immunol Rev. 1998;162:107–116. doi: 10.1111/j.1600-065x.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 16.Steele E J, Rothenfluh H S, Blanden R V. Immunol Cell Biol. 1997;75:82–95. doi: 10.1038/icb.1997.12. [DOI] [PubMed] [Google Scholar]
- 17.Storb U, editor. Immunol Rev. 1998;162:5–11. doi: 10.1111/j.1600-065x.1998.tb01424.x. [DOI] [PubMed] [Google Scholar]
- 18.Brenner S, Milstein C. Nature (London) 1966;211:242–243. [Google Scholar]
- 19.Cascalho M, Wong J, Steinberg C, Wabl M. Science. 1998;279:1207–1210. doi: 10.1126/science.279.5354.1207. [DOI] [PubMed] [Google Scholar]
- 20.Phung Q H, Winter D B, Cranston A, Tarone R E, Bohr V A, Fishel R, Gearhart P J. J Exp Med. 1998;187:1745–1751. doi: 10.1084/jem.187.11.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winter D B, Phung Q H, Umar A, Baker S M, Tarone R E, Tanaka K, Liskay R M, Kunkel T A, Bohr V A, Gearhart P J. Proc Natl Acad Sci USA. 1998;95:6953–6958. doi: 10.1073/pnas.95.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frey S, Bertocci B, Delbos F, Quint L, Weill J-C, Reynaud C-A. Immunity. 1998;9:127–134. doi: 10.1016/s1074-7613(00)80594-4. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs H, Fukita Y, van der Horst G T J, de Boer J, Weeda G, Essers J, de Wind N, Engelward B P, Samson L, Verbeek S, et al. J Exp Med. 1998;187:1735–1743. doi: 10.1084/jem.187.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rada C, Ehrenstein M R, Neuberger M S, Milstein C. Immunity. 1998;9:135–141. doi: 10.1016/s1074-7613(00)80595-6. [DOI] [PubMed] [Google Scholar]
- 25.Vora K A, Tumas-Brundage K M, Lentz V M, Cranston A, Fishel R, Manser T. J Exp Med. 1999;189:471–482. doi: 10.1084/jem.189.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Q, Maizels N. Mol Immunol. 1999;36:83–91. doi: 10.1016/s0161-5890(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 27.Reynaud A-C, Bertocci B, Frey S, Delbos F, Quint L, Weill J-C. Immunol Today. 1999;20:522–527. doi: 10.1016/s0167-5699(99)01540-6. [DOI] [PubMed] [Google Scholar]
- 28.Bertocci B, Quint L, Delbos F, Reynaud C-A, Weill J-C. Immunity. 1998;9:257–265. doi: 10.1016/s1074-7613(00)80608-1. [DOI] [PubMed] [Google Scholar]
- 29.Sale J E, Neuberger M S. Immunity. 1998;9:859–869. doi: 10.1016/s1074-7613(00)80651-2. [DOI] [PubMed] [Google Scholar]
- 30.Gu H, Marth J D, Orban P C, Mossman H, Rajewsky K. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 31.Betz U A K, Voβhenrich C A J, Rajewsky K, Müller W. Curr Biol. 1996;6:1307–1316. doi: 10.1016/s0960-9822(02)70717-3. [DOI] [PubMed] [Google Scholar]
- 32.Coffman R L, Weissman I L. Nature (London) 1981;289:681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- 33.Zou Y-R, Takeda S, Rajewsky K. EMBO J. 1993;12:811–820. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roes J, Müller W, Rajewsky K. J Immunol Meth. 1995;183:231–237. doi: 10.1016/0022-1759(95)00059-j. [DOI] [PubMed] [Google Scholar]
- 35.Schüppel R, Wilke J, Weiler E. Eur J Immunol. 1987;17:739–741. doi: 10.1002/eji.1830170527. [DOI] [PubMed] [Google Scholar]
- 36.Nishikawa S-I, Sasaki Y, Kina T, Amagai T, Katsura Y. Immunogenetics. 1986;23:137–139. doi: 10.1007/BF00377976. [DOI] [PubMed] [Google Scholar]
- 37.Dialynas D P, Quan Z S, Wall K A, Pierres A, Quintans J, Loken M R, Pierres M, Fitch F W. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 38.Ledbetter J A, Herzenberg L A. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 39.Oi V T, Herzenberg L A. Mol Immunol. 1979;16:1005–1017. doi: 10.1016/0161-5890(79)90034-8. [DOI] [PubMed] [Google Scholar]
- 40.Gu H, Tarlinton D, Müller W, Rajewsky K, Förster I. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dzierzak E, Medvinsky A. Trends Genet. 1995;11:359–366. doi: 10.1016/s0168-9525(00)89107-6. [DOI] [PubMed] [Google Scholar]
- 42.Cumano A, Rajewsky K. EMBO J. 1986;5:2459–2468. doi: 10.1002/j.1460-2075.1986.tb04522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blier P R, Bothwell A. J Immunol. 1987;139:3996–4006. [PubMed] [Google Scholar]
- 44.Weiss U, Rajewsky K. J Exp Med. 1990;172:1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss U, Zoebelein R, Rajewsky K. Eur J Immunol. 1992;22:511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- 46.Allen D, Simon T, Sablitzky F, Rajewsky K, Cumano A. EMBO J. 1988;7:1995–2001. doi: 10.1002/j.1460-2075.1988.tb03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Texido G, Jacobs H, Meiering M, Kühn R, Roes J, Müller W, Gilfillan S, Fujiwara H, Kikutani H, Yoshida N, et al. Eur J Immunol. 1996;26:380–382. doi: 10.1002/eji.1830260843. [DOI] [PubMed] [Google Scholar]
- 48.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Nature (London) 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 49.Holbeck S L, Strathern J N. Genetics. 1997;147:1017–1024. doi: 10.1093/genetics/147.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibbs P E, McGregor W G, Maher V M, Nisson P, Lawrence C W. Proc Natl Acad Sci USA. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]