Abstract
Individuals with systemic lupus erythematosus and rheumatoid arthritis are characterized by the presence of high levels of circulating IgM and IgG autoantibodies. Although IgG autoantibodies often are pathogenic, the role of IgM autoantibodies in autoimmune disease is not clear. Using mice that are unable to secrete IgM but are able to express surface IgM and IgD and to secrete other classes of immunoglobulins, we examined the effect of the absence of secreted IgM in the development of IgG autoantibodies and autoimmune disease in lupus-prone lymphoproliferative (lpr) mice. Compared with regular lpr mice, lpr mice that lack secreted IgM developed elevated levels of IgG autoantibodies to double-stranded DNA and histones and had more abundant deposits of immune complexes in the glomeruli; they also suffered more severe glomerulonephritis and succumbed to the disease at an earlier age. Similarly, the absence of secreted IgM also resulted in an accelerated development of IgG autoantibodies in normal mice. These findings suggest that secreted IgM, including IgM autoantibodies produced naturally or as part of an autoimmune response, may lessen the severity of autoimmune pathology associated with IgG autoantibodies.
High levels of IgM and IgG autoantibodies are associated with many autoimmune diseases (1–3). In systemic lupus erythematosus (SLE), a large proportion of the autoantibodies are specific to chromatin and its component DNA and histones (4). In rheumatoid arthritis, more than 70% of the patients develop high titers of serum IgM and IgG, referred to as rheumatoid factors (RF), specific to the Fc portion of their own IgG molecules (2). Evidence suggests that IgG autoantibodies are usually pathogenic whereas IgM autoantibodies are not. Introduction of monoclonal IgG antibody specific to DNA into normal mice, either by direct injection of purified antibodies, implantation of antibody-secreting hybridomas, or expression from Ig transgenes, induces lupus-like glomerulonephritis (5–8). Similarly, transfer of IgG from T cell receptor transgenic mice that spontaneously develop rheumatoid arthritis causes the disease in immunodeficient mice (9). In contrast, injection of DNA-specific IgM or IgM RF rarely causes the autoimmune lesions in normal mice (9, 10). The role of IgM autoantibodies in autoimmune responses and the associated organ damage remains to be elucidated.
To investigate the role of secreted IgM in various immunological processes, we previously have constructed a mutant mouse strain in which B cells are incapable of secreting IgM but still capable of expressing surface IgM and IgD and secreting IgG antibodies. We showed that in the absence of secreted IgM, mutant mice had an impaired IgG antibody response to a suboptimal dose of a T cell-dependent antigen (11). An identical result also was obtained by another group by using an independently constructed mutant mouse strain that carried the same mutation (12). The impaired IgG antibody response to suboptimal doses of antigen also was observed in mice deficient in complement component C3 or C4 or complement receptor CD21/CD35 (13, 14). Together, these findings suggest that secreted IgM and complement components normally can augment IgG antibody responses to foreign antigens, probably through formation of immune complexes of antigen, IgM, and activated complement component C3 (15, 16). The resulting immune complexes are more effective in activating B cells by crosslinking B cell antigen receptor and CD19/CD21/CD81 coreceptors and/or in promoting germinal center reactions through more efficient antigen trapping on follicular dendritic cells (17, 18).
To examine the role of secreted IgM in IgG autoantibody response and autoimmune disease, we introduced the secreted IgM mutation into the lupus-prone lymphoproliferative (lpr) mice. Lpr mice spontaneously develop high levels of anti-DNA autoantibodies and glomerulonephritis (1) because a defective fas (CD95) gene impairs lpr T cells to undergo activation-induced cell death and the maintenance of peripheral tolerance (19–21). Surprisingly, in the absence of secreted IgM, lpr mice developed significantly increased levels of IgG autoantibodies specific for double-stranded DNA (dsDNA) and histones, suffered from more severe glomerulonephritis, and had a shortened life span. Thus, secreted IgM appears to suppress the development of IgG autoantibodies and autoimmune disease under physiological conditions.
Materials and Methods
Mice.
Mice were maintained in a specific pathogen-free facility. Mice deficient in secreted IgM (sIgM−/−) on the mixed 129 × C57BL/6 background have been described (11). They were bred with MRL-lpr/lpr mice (abbreviated MRL/lpr), which were kindly provided by Suzanne Marusic of the Massachusetts Institute of Technology. Six pairs of heterozygous double mutant mice were bred to obtain lpr mice that were heterozygous (lpr/lpr/sIgM+/−) or homozygous (lpr/lpr/sIgM−/−) for secreted IgM mutation. Thirteen breeding pairs of lpr/lpr/sIgM+/− × lpr/lpr/sIgM−/− mice were used to generate sufficient numbers of female littermates of lpr/lpr/sIgM+/− and lpr/lpr/sIgM−/− mice for various analyses. For simplicity, lpr/lpr/sIgM+/− and lpr/lpr/sIgM−/− mice are referred to as lpr and lpr/sIgM−/− mice, respectively. As shown previously, there is no difference in IgG response to a T cell-dependent antigen between wild-type and heterozygous mutant mice (11). Neither are there any differences in the levels of IgG autoantibodies between lpr/sIgM+/+ and lpr/sIgM+/− mice (data not shown).
Differences in background genes are known to affect the expression of the autoimmune phenotype (22). Ideally, lpr and sIgM mutations should be backcrossed onto a homogenous background such as C57BL/6 or MRL mice. Although lpr and lpr/sIgM−/− mice used in our analyses were not backcrossed, they should be very similar in the background genes among themselves because littermates were used and large numbers of breeding pairs were used for their generation. Indeed, no significant difference in autoimmune phenotype was detected among mice that were the same genotype but from different litters (data not shown). Two major loci that affect the renal disease in MRL/lpr mice have been mapped (23); one of them is linked to IgH locus on chromosome 12. It is not clear whether there is any difference at this locus among different strains of mice. Because lpr mutation on MRL background results in the most severe autoimmune disease, the allele from MRL strain may be more potent in promoting autoimmune disease than the one from other strains including 129 strain. In control lpr/sIgM+/− mice, the mutant allele was from 129 strain. If the 129 allele promoted rapid development of autoimmune disease, one would expect that allele is dominant and, therefore, may not have detected the observed differences in the autoimmune response between lpr/sIgM+/− and lpr/sIgM−/− mice. In addition, 129 mice or sIgM−/− mice on 129 background were not more prone to autoimmune disease compared with their respective controls.
ELISA.
For measuring the levels of serum IgG isotypes, ELISA plates were coated with goat–anti-mouse Ig(M+G+A), incubated with serially diluted sera (1:50, 1:150, 1:450, 1:1,350, 1:4,050, 1:12,150, and 1:36,450), and developed with horseradish peroxidase (HRP)-conjugated goat Ab specific for each mouse IgG isotype (Southern Biotechnology Associates). For measuring autoantibodies specific for dsDNA and histones, ELISA plates (Nunc) were coated with dsDNA (4 μg/ml in PBS) from calf thymus that was sonicated to approximately 350 bp or with a mixture of H1, H2a, H2b, H3, and H4 histones (2 μg/ml in PBS; Boehringer Mannheim). Plates were developed with HRP-conjugated goat Abs specific for mouse IgM and IgG isotypes and Dako TMB One-Step substrate. Antibody concentrations were calculated by using the linear ranges of the dilution and standard curves generated with purified mouse monoclonal IgG antibodies. The sensitivity of ELISA for dsDNA and histones was approximately 0.0025 μg/ml and 0.01 μg/ml, respectively.
Antinuclear Antibody (ANA) Staining.
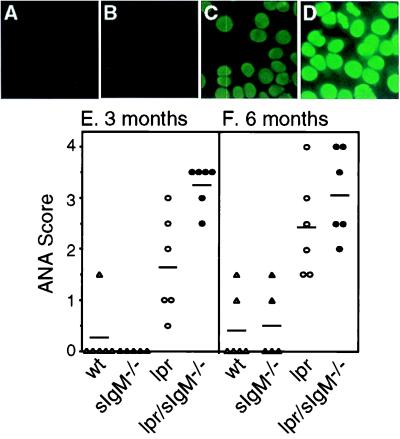
ANAs were measured by immunofluorescent staining of HEp2 cells (Antibodies, Inc.). Serial dilutions of sera (1:50, 1:150, 1:450, and 1:1,350) were made in 0.2% BSA/PBS and added onto HEp2-coated glass slides, followed by a FITC-conjugated goat–anti-mouse IgG Ab (Southern Biotechnology Associates). Brightness of staining was compared by using the same dilution (1:150) of each serum and scored by two observers in double-blinded fashion by using a scale from 0 to 4, with the latter being the brightest. As an example, ANA scores of Fig. 2 A–D are 0, 0, 2.0, and 3.5, respectively.
Figure 2.
Comparison of the levels of ANA among various types of mice. (A–D) Representative ANA stains of fixed HEp-2 cells with sera (1:150 dilution) from 3-month-old wild-type (wt), sIgM−/−, lpr, and lpr/sIgM−/− females (×40). (E and F) ANA scores of wt, sIgM−/−, lpr, and lpr/sIgM−/− mice at 3 and 6 months of age, respectively.
Kidney Analysis.
One kidney of each mouse was fixed in buffered formalin, embedded in paraffin, and stained with hematoxylin/eosin. To quantify the degree of glomerulonephritis, diameters of 25 randomly selected glomeruli from each mouse were measured by using a computer-based imaging technique (scion image 1.62a; Scion, Frederick, MD). The other kidney was frozen in OCT compound, and the deposition of immune complexes in glomeruli was assayed by staining cryosections with FITC-conjugated goat–anti-mouse IgG diluted in 1% BSA/PBS (1:250).
IgM Purification and Reconstitution.
Hybridoma 3H9/Vλ1 no. 29 secreting IgM reactive to dsDNA was from Jan Erikson (the Wistar Institute) (24), and hybridoma MRB2 secreting IgM specific for histones was from Marc Monestier (Temple University School of Medicine, Philadelphia) (25). IgM antibody was isolated from hybridoma supernatant by an anti-IgM Sepharose affinity column (Zymed). Bound protein was eluted with 0.1 M glycine/0.15 M NaCl, pH 2.5, and neutralized with 1 M Tris, pH 8.0. Protein content in each fraction was determined by spectrophotometry, and positive fractions were pooled, dialyzed, concentrated, and sterile-filtered. Purity of IgM was close to 100% as indicated by SDS/PAGE followed by Coomassie blue staining. IgM degradation was undetectable as determined by Western blot using goat Abs specific for mouse IgM. Lpr/sIgM−/− mice were reconstituted with 150, 45, or 15 μg of purified IgM in 0.5 ml of PBS i.p. three times a week for 10 weeks, starting at 2 weeks of age. Control lpr/sIgM−/− littermates were injected with equal volumes of PBS. Sera were collected once a month during the reconstitution and at the end of the reconstitution.
Statistical Analysis.
Two-tailed Mann–Whitney test was used for statistical analysis.
Results
Lpr/sIgM−/− Mice Developed Elevated Levels of IgG to dsDNA and Histones.
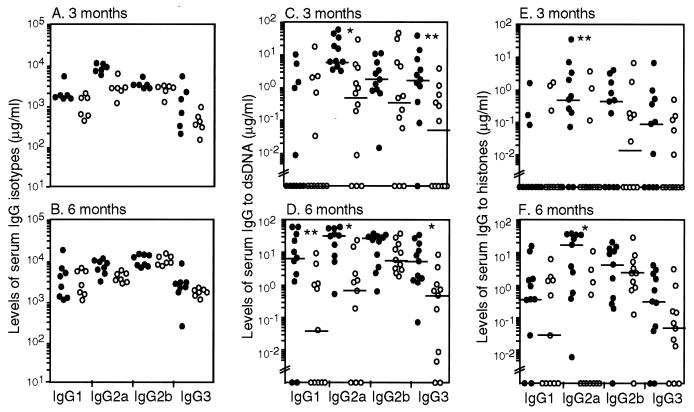
The sIgM−/− mutation was introduced into lupus-prone lpr mice. Serum levels of IgG isotypes were assayed in lpr and lpr/sIgM−/− females by ELISA. At 3 months of age, the levels of IgG1, IgG2b, and IgG3 were similar in both types of mice, but the levels of IgG2a were approximately 3-fold higher in lpr/IgM−/− mice than in lpr mice (Fig. 1A). At 6 months of age, the overall IgG levels had increased in both lpr and lpr/sIgM−/− mice, but the difference in the levels of IgG2a isotype between the two types of mice had diminished (Fig. 1B). Compared with those of the wild-type and sIgM−/− mice, serum levels of IgGs were about 3–10 times higher in lpr and lpr/sIgM−/− mice at 3 months of age (11). Both lpr and lpr/sIgM−/− mice had similarly enlarged lymph nodes and showed no significant differences in the numbers of CD4−CD8− T cells in the lymph nodes (data not shown), indicating that secreted IgM is not required for the development of lymphadenopathy.
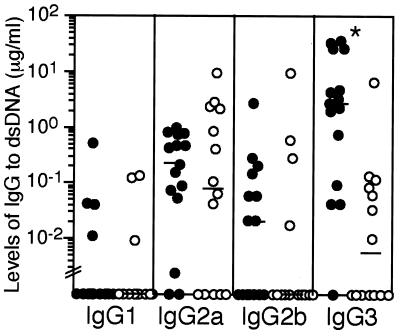
Figure 1.
Lpr/sIgM−/− mice developed elevated levels of IgG to dsDNA and histones. Serially diluted sera from 3- and 6-month-old lpr and lpr/sIgM−/− female littermates were assayed by ELISA for the levels of each IgG isotype (A and B) and the levels of IgG specific to dsDNA (C and D) or to histones (E and F). ●, lpr/sIgM−/− mice; ○, lpr mice. Horizontal bars indicate the median levels of IgG antibodies. * and **, P values of <0.005 and <0.05, respectively, between lpr and lpr/sIgM−/− mice. Values below “//” are considered below the detection of the assay.
The levels of IgGs specific for dsDNA and histones then were measured in sera from lpr and lpr/sIgM−/− females. At 3 months of age, the median levels of IgG2a and IgG3 specific to dsDNA were significantly higher in lpr/sIgM−/− mice than in lpr littermates (Fig. 1C, P < 0.05 or 0.005). By 6 months of age, the median level of IgG1 to dsDNA also was elevated more significantly in lpr/sIgM−/− mice than in lpr mice (Fig. 1D). Higher levels of IgG2a to histones were also evident in lpr/sIgM−/− mice at both 3 and 6 months of age (Fig. 1 E and F). As mice became older, the overall levels of IgG autoantibodies increased, but the levels in lpr/sIgM−/− mice were consistently higher.
To determine the overall levels of ANA in the absence of secreted IgM, we assayed the reactivity of serially diluted sera from 3- and 6-month-old lpr and lpr/sIgM−/− females to fixed HEp-2 cells by immunofluorescence. Compared with the sera from wild-type and sIgM−/− mice, which gave minimal ANA staining (Fig. 2 A, B, and E), sera from 3-month-old lpr mice gave clearly positive staining (Fig. 2 C and E). At the same dilution, however, much brighter staining was observed with sera from lpr/sIgM−/− littermates (Fig. 2 D and E), indicating higher levels of ANA. The intensities of ANA staining were increased in 6-month-old mice (Fig. 2F and data not shown), consistent with increased levels of autoantibodies in older mice. However, the difference between lpr and lpr/sIgM−/− mice was no longer distinguishable by the assay. Together, these data show that the development of IgG autoantibodies is accelerated in lpr mice in the absence of secreted IgM.
Lpr/sIgM−/− Mice Developed More Severe Glomerulonephritis.
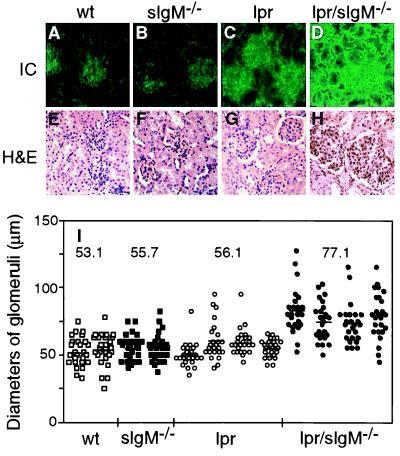
To determine whether the increased levels of serum IgG autoantibodies were associated with more abundant deposits of immune complexes in glomeruli, cryosections of kidneys were stained with FITC-labeled goat anti-mouse IgG antibody. In 3-month-old wild-type and sIgM−/− mice, only background levels of immunofluorescence were detected in the glomeruli (Fig. 3 A and B). In contrast, positive staining was clearly observed in glomeruli of both lpr and lpr/sIgM−/− mice at 3 months of age (Fig. 3 C and D). On average, much brighter staining, indicating more deposits of immune complexes, was detected in glomeruli of lpr/sIgM−/− mice than in lpr littermates. In addition, renal tubules often stained positive in kidneys of lpr/sIgM−/− mice but not in lpr controls at 3 months of age.
Figure 3.
Lpr/sIgM−/− mice developed more severe glomerulonephritis. (A–D) Representative immunofluorescence stains for IgG-containing immune complexes in glomeruli of wild-type (wt), sIgM−/−, lpr, and lpr/sIgM−/− females at 3 months of age. Kidney sections were stained with a FITC-labeled goat anti-mouse IgG Ab (×40). (E–H) Representative histological stains of kidney sections of wt, sIgM−/−, lpr, and lpr/sIgM−/− females at 6 months of age. Kidney sections were stained with hematoxylin/eosin (×40). (I) Comparison of the diameters of glomeruli in wt, sIgM−/−, lpr, and lpr/sIgM−/− females at 6 months of age. The diameters of 25 randomly selected glomeruli were measured for two wt mice, two sIgM−/− mice, four lpr mice, and four lpr/sIgM−/− mice. Because glomeruli were not perfectly round, each given diameter was an average of the smallest and largest measurements. Each dot represents one glomerulus. The numbers represent the average diameters of glomeruli of specific genotypes.
To examine the occurrence and severity of glomerulonephritis, kidney sections of 6-month-old lpr and lpr/sIgM−/− littermates and the wild-type and sIgM−/− controls were stained with hematoxylin/eosin. Glomeruli of the wild-type and sIgM−/− mice had no obvious pathological changes at 6 months of age (Fig. 3 E and F). In contrast, glomeruli of lpr/sIgM−/− mice and, to a lesser extent, lpr mice showed characteristics of a diffused proliferative glomerulonephritis, including hypercellularity, missing patent capillary loops, and enlarged Bowmans space (Fig. 3 G and H). In addition, mononuclear cell infiltrates were observed frequently in kidney sections of both lpr and lpr/sIgM−/− mice (data not shown). To quantify the differences in glomerulonephritis between lpr and lpr/sIgM−/− mice, diameters of 25 randomly selected glomeruli were measured for each mouse. As shown in Fig. 3I, the average diameter of glomeruli of lpr/sIgM−/− mice was 77.1 μm, 46%, 39%, and 38% larger than that of wild-type, sIgM−/−, and lpr mice, respectively (P < 0.0001). Thus, the absence of secreted IgM leads to more severe glomerulonephritis in lpr mice.
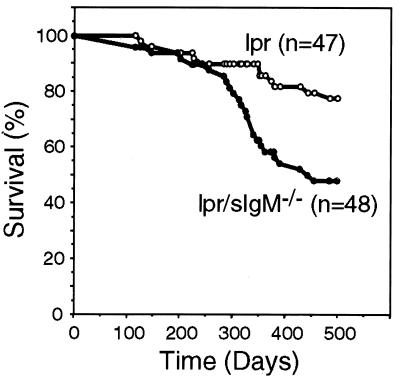
Lpr/sIgM−/− Mice Had a Shortened Life Span.
Lpr mice generally die from kidney failure resulting from glomerulonephritis. Male lpr and lpr/sIgM−/− mice were monitored weekly for body conditions, signs of infection, and survival. By 500 days, 78% of lpr mice were alive whereas only 48% of lpr/sIgM−/− mice were still living (Fig. 4, P < 0.0003). The appearance of signs of morbidity, including loss of body weight and ruffled furs, occurred earlier in lpr/sIgM−/− mice than in lpr mice in the absence of any obvious infection (data not shown). Compared with males, female lpr mice are known to develop higher levels of IgG autoantibodies and a more severe glomerulonephritis (1). Accordingly, only 32% of female lpr/sIgM−/− mice survived for 400 days (n = 34, data not shown); however, we did not have sufficient numbers of female lpr mice for a statistically significant estimation of survival rate. The longer life span of lpr/sIgM−/− mice than that of MRL/lpr mice (1) probably reflects that our mutant mice were not on the MRL background. Nevertheless, these data clearly show that lpr mice had a shortened life span in the absence of secreted IgM.
Figure 4.
Lpr/sIgM−/− mice had a shortened life span. Survival of 47 lpr males and 48 lpr/sIgM−/− males were followed for 500 days and were plotted vs. time.
Spontaneous Development of IgG Autoantibodies in sIgM−/− Mice.
Lpr mice are predisposed to the development of IgG autoantibodies and autoimmune disease because of the fas gene mutation. To investigate whether the absence of secreted IgM also leads to the development of IgG autoantibodies in normal (non-lpr) mice, we assayed serum levels of IgGs to dsDNA and histones in littermates of wild-type and sIgM−/− mice at different ages. There was no detectable IgGs specific to dsDNA at 3 months of age or to histones at even 12 months of age in both types of mice (data not shown). By 12 months of age, some sIgM−/− mice, as well as the wild-type mice, developed low levels of anti-DNA IgG2a and IgG2b (Fig. 5). However, almost all sIgM−/− mice analyzed had elevated levels of IgG3 specific to dsDNA, and the median was significantly higher than that in wild-type mice (P < 0.0006). Thus, the development of IgG autoantibodies also may be accelerated in normal mice in the absence of secreted IgM.
Figure 5.
sIgM−/− mice spontaneously developed elevated levels of IgG3 specific to dsDNA. Sera from 12-month-old wild-type (○) and sIgM−/− (●) females were assayed by ELISA for the levels of IgG specific to dsDNA. *, P value of <0.0006 between wt and sIgM−/− mice. Values below “//” are considered below the detection of the assay.
Reconstitution of lpr/sIgM−/− Mice with Monoclonal IgM Autoantibodies.
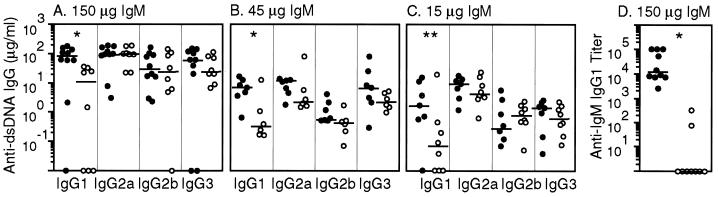
Secreted IgM is a collection of IgM molecules with diverse specificity, including IgM autoantibodies produced naturally or produced as part of an autoimmune response. To determine whether the accelerated development of IgG autoantibodies is caused by the absence of autoreactive IgM, we tested whether reconstitution of lpr/sIgM−/− mice with monoclonal IgM specific to dsDNA would suppress the IgG response to dsDNA. To maintain a relatively stable level of the injected IgM, lpr/sIgM−/− mice were injected i.p. with 150, 45, or 15 μg of purified IgM three times a week for 10 weeks. Reconstitution started at 2 weeks of age and continued until mice reached 12 weeks of age, when the difference in the levels of anti-DNA IgG was clearly detectable between lpr and lpr/sIgM−/− mice. Despite reconstitution with 150 μg of IgM three times a week, ELISA assays showed that serum levels of anti-DNA IgM 3 days after injection were generally undetectable (data not shown), probably because the half-life of the injected IgM was very short when the total level of IgM was low (26). Unexpectedly, by the end of IgM reconstitution, there were significantly higher levels of IgG1, but not other IgG isotypes, specific for dsDNA in mice that were injected with IgM (at all three doses) than in littermates that were injected with saline (Fig. 6 A–C). IgM-reconstituted mice also had larger glomeruli and more deposits of IgG-containing immune complexes in glomeruli (data not shown). Further analysis revealed that there were also significant levels of IgG1, but not other IgG isotypes, specific to the injected IgM at all three doses (Fig. 6D and data not shown). Similar results also were obtained when an antihistone monoclonal IgM was injected (data not shown).
Figure 6.
Reconstitution of lpr/sIgM−/− mice with monoclonal autoreactive IgM specific to DNA. Littermates of lpr/sIgM−/− mice were injected with either 150, 45, or 15 μg monoclonal IgM (●) or saline (○) three times a week for 10 weeks, starting at 2 weeks of age. At the end of the reconstitution (week 12), serum levels of IgG to dsDNA (A–C) and to the injected IgM (D) were assayed. The titers of IgG1 specific for IgM represent folds of serum dilutions required to give an OD450 of 1.0. Data shown were from mice injected with 150 μg of either IgM or saline. * and **, P values of < 0.005 and < 0.05, respectively, between IgM and saline-reconstituted mice.
Discussion
In this report, we show that in the absence of secreted IgM, normal mice spontaneously developed autoreactive IgG specific for dsDNA. In lupus-prone lpr mice, the absence of secreted IgM accelerated the development of IgG autoantibodies and glomerulonephritis, and the mice succumbed to the disease at an earlier age. These findings demonstrate that secreted IgM can suppress the development of IgG autoantibodies and autoimmune disease under physiological conditions.
The accelerated autoantibody responses in mice deficient in secreted IgM resemble the effect of complement deficiency on the development of autoimmune disease. In humans, deficiencies in the early components of the complement cascade, including C1q, C1r, C1s, C2, and C4, are associated with a high incidence of SLE (27–29). Similarly, mice deficient in C1q, C4, or complement receptor CD21/CD35 are prone to the development of ANA and glomerulonephritis (30–32). Two different mechanisms appear to be involved in the complement-mediated regulation of autoantibody response. First, complement promotes the removal of autoantigens and, hence, could reduce the chance that autoreactive B cells are activated. In human SLE and lpr mice, a large fraction of IgG autoantibodies are specific to DNA and histones. Intravenous injection of syngeneic apoptotic cells into normal mice induces a rapid ANA response (33), suggesting that the source of autoantigens for the autoantibody response may be the apoptotic cells (34). C1q can bind apoptotic blebs directly (35), and the activation of complement is required for the clearance of apoptotic cells by macrophages (36). In addition, large numbers of apoptotic bodies are present in glomeruli of C1q-deficient mice (30), suggesting a critical role of complement in the clearance of apoptotic cells. Second, developing B cells that express IgM specific for DNA are either deleted or inactivated (24, 37, 38). In the absence of complement receptors (CD21/CD35), autoreactive B cells are not anergized (31), indicating that complement components also promote induction of B cell tolerance and, therefore, reduce autoantibody responses (39).
Secreted IgM is a diverse collection of IgM molecules of both auto- and nonautoreactivity (40–42). Autoreactive IgM is part of the natural antibody repertoire (43, 44) and also is produced during autoimmune responses. It is most probable that the absence of autoreactive IgM rather than the nonautoreactive IgM resulted in the accelerated development of autoantibody responses in both normal and lpr mice. IgM is a potent activator of complement. Deficiency in secreted IgM has the same effect on IgG antibody responses to both foreign antigens and self-antigens as deficiency in complement components (11, 13, 14, 30–32), indicating that secreted IgM may affect the development of IgG autoantibodies and autoimmune disease through the same pathways as complement components.
Autoreactive IgM may suppress autoimmunity by inducing B cell tolerance. Theoretically, natural autoreactive IgM could participate in the negative selection of autoreactive B cells that develop in the adult bone marrow. The absence of secreted IgM may impair the selection process, resulting in the emergence of more autoreactive B cells in the periphery. However, enzyme-linked immunospot analysis failed to detect any significant differences in the number of B cells that are reactive to dsDNA or to histone between lpr and lpr/sIgM−/− mice (data not shown). More likely, IgM autoantibodies produced either naturally or during an autoimmune response may bind to autoantigens and promote their removal through complement activation. Tens of millions of apoptotic cells, including dying lymphocytes and myeloid cells, must be cleared daily (45). In the course of apoptosis, nuclear components are translocated into membranous blebs (34). Some of the IgM autoantibodies, such as those reactive to DNA, histones, and phosphatidylserine, may bind to self-antigens in the blebs, activate complement, and, therefore, promote their removal. In the absence of secreted IgM, apoptotic blebs may not be removed efficiently and the more abundant autoantigens may lead to an increased frequency of autoantibody response (46) and, therefore, an accelerated development of autoimmune disease.
Our attempt to demonstrate this mechanism directly by reconstituting lpr/sIgM−/− mice with monoclonal autoreactive IgM was unsuccessful because of the induction of IgG antibody response to the injected IgM. Although mice were given IgM in saline i.p. starting at 2 weeks of age, IgG1 antibody responses were induced at all three doses of IgM used. This is probably because the injected IgM differs from the endogenous membrane-bound IgM by having the secreted portion of the IgM molecule and purified IgM tended to aggregate and was injected repeatedly. Unexpectedly, IgM-reconstituted lpr/sIgM−/− mice also produced significantly higher levels of IgG1 specific for dsDNA than littermates that were injected with saline. One explanation of IgG1 antibody responses to both DNA and DNA-specific IgM is that injected IgM binds to DNA and activates complement in the reconstituted mice. The resulting immune complexes of DNA-IgM-C′ were able to activate both DNA- and IgM-specific B cells through crosslinking the antigen receptors and CD19/CD21/CD81 coreceptors, promoting IgG1 responses to both the endogenous DNA and the injected IgM.
The apparently contradicting results from lpr mice that lack secreted IgM and from the same mice reconstituted with monoclonal autoreactive IgM may reflect the two different pathways that the immune complexes of Ag-IgM-C′ can follow. On one hand, the immune complexes can be cleared more efficiently through complement-mediated processes. On the other hand, the immune complexes are more effective in activating B cells by crosslinking BCR and coreceptors. Perhaps, the overall levels of autoreactive IgM and/or its local concentration may determine which pathway the immune complexes may follow, analogous to the antigen dose-dependent enhancing effect that IgM and complement have on IgG antibody response (11, 13, 14). When there is too much autoreactive IgM, autoantibody response may be dominant, such as in the advanced stage of SLE. However, in the absence of autoreactive IgM, autoantibody response also may be promoted because autoantigens are not cleared efficiently, such as in the secreted IgM-deficient mice.
Acknowledgments
We thank Dr. Suzanne Marusic for MRL/lpr mice; Drs. Jan Erikson and Marc Monestier for IgM-secreting hybridomas; Dr. Ian Rifkin for evaluating histological stains; Drs. Michael Carroll, Herman Eisen, and Ronald Corley for discussion and critical reading of the manuscript; and members of the Chen laboratory for help and discussion. This work was supported in part by National Institutes of Health Grants AI41762 (to J.C.) and AR-35230 (to A.M.R.) and a predoctoral fellowship from Boehringer Ingelheim (to M.B.).
Abbreviations
- dsDNA
double-stranded DNA
- lpr
lymphoproliferative
- SLE
systemic lupus erythematosus
- ANA
antinuclear antibody
- sIgM
secreted IgM
References
- 1.Theofilopoulos A N, Dixon F J. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Brennan F M, Maini R N. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 3.Kotzin B L. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 4.Hahn B H. N Eng J Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi K, Ebling F M, Mitchell B, Singh R R, Hahn B H, Tsao B P. Int Immunol. 1994;6:817–830. doi: 10.1093/intimm/6.6.817. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenstein M R, Katz D R, Griffiths M H, Papadaki L, Winkler T H, Kalden J R, Isenberg D A. Kidney Int. 1995;48:705–711. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 7.Tsao B P, Ohnishi K, Cheroutre H, Mitchell B, Teitell M, Mixter P, Kronenberg M, Hahn B H. J Immunol. 1992;149:350–358. [PubMed] [Google Scholar]
- 8.Radic M Z, Ibrahim S M, Rauch J, Camper S A, Weigert M. J Immunol. 1995;155:3213–3222. [PubMed] [Google Scholar]
- 9.Korganow A-S, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali J-L, et al. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 10.Vaughan J H. Arthritis Rheum. 1993;36:1–6. doi: 10.1002/art.1780360102. [DOI] [PubMed] [Google Scholar]
- 11.Boes M, Esau C, Fischer M B, Schmidt T, Carroll M, Chen J. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 12.Ehrenstein M R, O'Keefe T L, Davies S L, Neuberger M S. Proc Natl Acad Sci USA. 1998;95:10089–10093. doi: 10.1073/pnas.95.17.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahearn J M, Fisher M, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard R G, Rothstein T L, Caroll M C. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M B, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard R G, Rothstein T L, et al. J Immunol. 1996;157:549–556. [PubMed] [Google Scholar]
- 15.Heyman B. Immunol Today. 1990;11:310–313. doi: 10.1016/0167-5699(90)90126-t. [DOI] [PubMed] [Google Scholar]
- 16.Carroll M C. Annu Rev Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 17.Dempsey P W, Allison M E D, Akkaraju S, Goodnow C C, Fearon D T. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 18.Fischer M B, Goerg S, Shen L, Prodeus A P, Goodnow C C, Kelsoe G, Carroll M C. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe-Fukunaga R, Brannan C I, Copeland N G, Jenkins N A, Nagata S. Nature (London) 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 20.Singer G G, Abbas A K. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 21.Elkon K B, Marshak-Rothstein A. Curr Opin Immunol. 1996;8:852–859. doi: 10.1016/s0952-7915(96)80015-x. [DOI] [PubMed] [Google Scholar]
- 22.Winchester R J, Lahita R G. In: Systemic Lupus Erythematosus. Lahita R G, editor. New York: Wiley; 1987. pp. 81–118. [Google Scholar]
- 23.Watson M L, Rao J K, Gilkeson G S, Ruiz P, Eicher E M, Pisetsky D S, Matsuzawa A, Rochelle J M, Seldin M F. J Exp Med. 1992;176:1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandik-Nayak L, Bui A, Noorchashm H, Eaton A, Erikson J. J Exp Med. 1997;186:1257–1267. doi: 10.1084/jem.186.8.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novick K E, Fasy T M, Losman M J, Monestier M. Int Immunol. 1992;4:1103–1111. doi: 10.1093/intimm/4.10.1103. [DOI] [PubMed] [Google Scholar]
- 26.Waldmann T A, Stober W, Blaese R M. In: Immunoglobulins. Merler E, editor. Washington, DC: Natl. Acad. Press; 1970. pp. 33–51. [Google Scholar]
- 27.Schifferli J A, Peters D K. Lancet. 1983;2:957–959. doi: 10.1016/s0140-6736(83)90464-6. [DOI] [PubMed] [Google Scholar]
- 28.Morgan B P, Walport M J. Immunol Today. 1991;12:301–309. doi: 10.1016/0167-5699(91)90003-C. [DOI] [PubMed] [Google Scholar]
- 29.Davies K A, Schifferli J A, Walport M J. Springer Semin Immunopathol. 1994;15:397–416. doi: 10.1007/BF01837367. [DOI] [PubMed] [Google Scholar]
- 30.Botto M, Dell'Agnola C, Bygrave A E, Thompson E M, Cook H T, Petry F, Loos M, Pandolfi P P, Walport M J. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 31.Prodeus A P, Goerg S, Shen L M, Pozdnyakova O O, Chu L, Alicot E M, Goodnow C C, Carroll M C. Immunity. 1998;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 32.Quigg R J, Lim A, Haas M, Alexander J J, He C, Carroll M C. Kidney Int. 1998;53:320–330. doi: 10.1046/j.1523-1755.1998.00723.x. [DOI] [PubMed] [Google Scholar]
- 33.Mevorach D, Zjou J L, Song X, Elkon K B. J Exp Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casciola-Rosen L A, Anhalt G, Rosen A. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korb L C, Ahearn J M. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 36.Mevorach D, Mascarenhas J O, Gershov D, Elkon K B. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C, Nagy Z, Radic M Z, Hardy R R, Huszar D, Camper S A, Welgert M. Nature (London) 1995;373:252–255. doi: 10.1038/373252a0. [DOI] [PubMed] [Google Scholar]
- 38.Tsao B P, Chow A, Cheroutre H, Song Y W, McGrath M E, Kronenberg M. Eur J Immunol. 1993;23:2332–2339. doi: 10.1002/eji.1830230942. [DOI] [PubMed] [Google Scholar]
- 39.Carroll M C. Nat Genet. 1998;19:3–4. doi: 10.1038/ng0598-3. [DOI] [PubMed] [Google Scholar]
- 40.Tlaskalová-Hogenová H, Mandel L, Stepánková R, Bártová J, Barot R, Leclerc M, Kováru F, Trebichavsky I. Folia Bioligica. 1992;38:202–215. [PubMed] [Google Scholar]
- 41.Hardy R R, Hayakawa K. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 42.Casali P, Kasaian M T, Haughton G. In: Autoimmunity, Physiology and Disease. Coutinho A, Kazatchkine M D, editors. New York: Wiley–Liss; 1994. pp. 57–88. [Google Scholar]
- 43.Avrameas S. Immunol Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 44.Coutinho A, Kazatchkine M D, Avrameas S. Curr Opin Immunol. 1995;7:812–818. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 45.Vaishnaw A K, McNally J D, Elkon K B. Arthritis Rheum. 1997;40:1917–1927. doi: 10.1002/art.1780401102. [DOI] [PubMed] [Google Scholar]
- 46.Bell D A, Morrison B. Clin Immunol Immunopathol. 1991;60:13–26. doi: 10.1016/0090-1229(91)90108-m. [DOI] [PubMed] [Google Scholar]