Abstract
Members of the muscarinic acetylcholine receptor family (M1–M5) are known to be involved in a great number of important central and peripheral physiological and pathophysiological processes. Because of the overlapping expression patterns of the M1–M5 muscarinic receptor subtypes and the lack of ligands endowed with sufficient subtype selectivity, the precise physiological functions of the individual receptor subtypes remain to be elucidated. To explore the physiological roles of the M2 muscarinic receptor, we have generated mice lacking functional M2 receptors by using targeted mutagenesis in mouse embryonic stem cells. The resulting mutant mice were analyzed in several behavioral and pharmacologic tests. These studies showed that the M2 muscarinic receptor subtype, besides its well documented involvement in the regulation of heart rate, plays a key role in mediating muscarinic receptor-dependent movement and temperature control as well as antinociceptive responses, three of the most prominent central muscarinic effects. These results offer a rational basis for the development of novel muscarinic drugs.
Muscarinic acetylcholine receptors are known to regulate numerous fundamental physiological processes, including the muscarinic actions of acetylcholine on peripheral effector tissues and a multitude of central sensory, vegetative, and motor functions (1–4). In addition, disturbances in central muscarinic neurotransmission have been implicated in a variety of pathophysiological conditions, including Alzheimer’s and Parkinson’s diseases (1–4).
Molecular cloning studies have revealed the existence of five molecularly distinct muscarinic receptor subtypes referred to as M1–M5 (5–7). The M1–M5 receptors are prototypical members of the superfamily of G protein-coupled receptors. Although the odd-numbered muscarinic receptor subtypes (M1, M3, and M5) are selectively linked to Gq/11 proteins, the even-numbered receptors (M2 and M4) are preferentially coupled to G proteins of the Gi/o family (5–7).
The M1–M4 receptors are widely expressed throughout the central nervous system and the body periphery (6, 8–10). Studies with subtype-selective antibodies and in situ mRNA hybridization experiments have shown that most brain regions express several different muscarinic receptor subtypes (8–10). Based on this observation, it has been extremely difficult to assign specific central functions to individual muscarinic receptor subtypes.
In addition, the lack of muscarinic agonists and antagonists with pronounced subtype selectivity also has represented a major limitation in studying the physiological roles of the M1–M5 receptors (5–7). This problem is accentuated further in the case of in vivo studies in which the actual concentrations of drugs at their sites of action are difficult to determine because of pharmacokinetic factors.
In the body periphery, muscarinic receptors mediate the well known functions of acetylcholine at parasympathetically innervated effector organs, including contraction of smooth muscle, stimulation of glandular secretion, and reduction in heart rate (1, 2). The major receptor subtypes involved in these functions have been identified (1–6), primarily because of the availability of functional in vitro preparations and the fact that the expression pattern of muscarinic receptors in peripheral organs is less complex than that observed in the central nervous system.
The availability of the cloned muscarinic receptor genes and recent progress in gene knockout methodologies have provided the opportunity to examine the physiological roles of the individual muscarinic receptors in an unambiguous fashion. We therefore decided to examine the functions of the two Gi/o-coupled muscarinic receptor subtypes, M2 and M4, by using gene ablation technology in mice.
In this study, we report the a pharmacologic characterization of a mouse line lacking functional M2 receptors. The M2 receptor is the predominant muscarinic receptor subtype in the heart, where it serves to mediate a decrease in cardiac beating frequency and a reduction in atrial contractility (1, 2, 5, 6). However, much less is known about its possible role in the many central processes in which muscarinic receptors are known to be involved. Thus, the primary focus of this study was to investigate the potential involvement of the M2 subtype in central muscarinic functions. Most experiments were carried out with mice that had been injected with the centrally acting, nonselective muscarinic agonist oxotremorine (OXO). Our results suggest that the M2 receptor subtype plays a critical role in the regulation of movement control, body temperature, and attenuation of pain responses.
MATERIALS AND METHODS
Generation of M2 Muscarinic Receptor −/− Mice.
A murine M2 muscarinic receptor clone was isolated from a 129 mouse genomic library (Genome Systems, St. Louis) by using a PCR fragment (corresponding in sequence to the central portion of the third intracellular loop) as a probe. The M2 targeting vector was derived from pPN2T (11) and was constructed by replacing a 0.67-kilobase NheI-NsiI genomic fragment (which encodes the region between the third transmembrane domain and the C terminus of the third intracellular loop) with the PGK-neomycin resistance gene (PGK-neo). Two copies of the herpes simplex virus thymidine kinase gene (HSV-TK) were attached 5′ of the regions of homology. The targeting vector was linearized with NotI and was introduced into J1 129 mouse embryonic stem (ES) cells by electroporation. G418- and gancyclovir-resistant clones were isolated and screened by Southern blotting for homologous recombination.
Several properly targeted ES cell clones were obtained (7 of a total of 151 screened clones). Positive ES cell clones were microinjected into C57BL/6 blastocysts to generate male chimeric offspring, which in turn were mated with female CF-1 mice (Charles River Breeding Laboratories) to generate F1 offspring. Mice homozygous for the M2 receptor mutation were produced by crossing F1 heterozygotes. All mice used in the studies reported here were F2 hybrids.
In Situ Hybridization Experiments.
Cryostat sections (12 μm thick) were prepared and hybridized with a [35S]-labeled ribonucleotide antisense probe as described (15). The probe was synthesized from a 0.67-kilobase NheI-NsiI genomic fragment cloned into pBluescript and corresponded in sequence to the region that was deleted during the construction of the M2 targeting vector (Fig. 1a).
Figure 1.
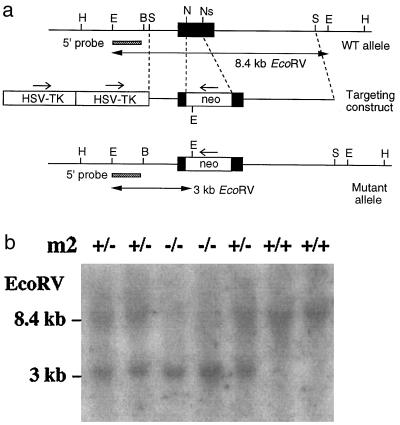
Targeted disruption of the mouse M2 muscarinic receptor gene. (a) Restriction maps of wild-type receptor locus, targeting vector, and targeted allele. Only relevant restriction sites are shown. The receptor coding region is represented by a filled bar. The probe used for Southern blot analysis and the sizes of the restriction fragments detected with this probe are indicated. H, HindIII; E, EcoRV; B, BamHI; S, StuI; N, NheI; Ns, NsiI. (b) Genotyping of offspring from M2+/− × M2+/− matings by Southern blot analysis of mouse tail DNA digested with EcoRV. The 8.4- and 3-kilobase bands represent the wild-type and mutant M2 receptor alleles, respectively.
Ligand Binding Studies.
Mouse brains or hearts were removed, dissected, frozen immediately on dry ice, and stored at −70°C until use. Tissues were homogenized by hand with 20 strokes of a Dounce tissue grinder in 0.32 M sucrose, 5 mM Tris⋅HCl (pH 7.5), and 1 mM phenylmethylsulfonyl fluoride. Membranes were prepared, and ligand binding experiments were carried out by using 2 nM of the nonselective muscarinic antagonist [3H]quinuclidinyl benzilate ([3H]QNB), essentially as described (12). Binding reactions were carried out for 1 hr at room temperature (22°C). Nonspecific binding was determined in the presence of 10 μM atropine.
Immunoprecipitation Assays.
For immunoprecipitation studies, M2 and M4 receptor-specific rabbit polyclonal antisera were raised against nonconserved regions of the third cytoplasmic loops of the mouse M2 and M4 receptor proteins according to Levey et al. (13). Membranes derived from different mouse brain regions or heart were prepared as described above, were incubated with 2 nM [3H]QNB, were washed thoroughly, and were solubilized with 1% digitonin, followed by immunoprecipitation of solubilized [3H]QNB-labeled receptors by using a protocol essentially similar to that described by Yasuda et al. (14).
Pharmacologic and Behavioral Studies.
Mice used for pharmacologic behavioral studies were between 3 and 6 months old and were housed in a temperature- and humidity-controlled vivarium that was kept on a 12-hr dark–light cycle. No significant differences in responses were found between male and female animals. Thus, data were generated with mice of either sex and were pooled subsequently.
To generate OXO dose-response curves, mice from each genotype were injected s.c. with vehicle (distilled water) or different doses of the nonselective muscarinic agonist OXO. Body temperature, salivation, tremor, and analgesia were assessed before and 30 min after injections. Core body temperature was measured by a rectal thermometer (model BAT 8, Bailey Instruments, Saddle Brook, NJ).
Salivation and tremor were scored by a trained observer on a scale of 0 (no salivation or tremor), 1 [moderate salivation (moisture on face only) or tremor (intermittent head and body tremor)] and 2 [marked salivation (moisture on face and chest) or tremor (nearly continuous whole body tremor)]. The data were expressed as percent effect, where the score for each mouse was expressed as a percent of the maximum possible score (i.e., 2).
The tail-flick test was carried out by immersing mouse tails in a 55°C water bath. Withdrawal latency was measured immediately before (baseline) and 30 min after drug or vehicle injection; a 10-sec maximum cut-off value was imposed to prevent tissue damage. Data were expressed as percent maximum possible effect (MPE), where percent MPE = 100 × [(postdrug latency − baseline)/(10 − baseline)]. Tail-flick, temperature, salivation, and tremor responses to a given OXO dose were studied in the same group of mice.
The hot plate test was performed by using an electronically controlled hot plate analgesia meter (model HP/fj, Omnitech Electronics, Columbus, OH). Response latency to lick the front or hind paws after being placed on a 55°C hot plate was measured before (baseline) and 30 min after OXO or vehicle injection; the cut-off time was 30 sec. Data were expressed as percent MPE = 100 × [(postdrug latency − baseline)/(30 − baseline)].
To determine whether the M2 receptor mutant mice had gross motor impairments, performance on a rotarod was examined. Mice were placed on a rotarod apparatus (model 7600, Ugo Basile, Varese, Italy) with the rod (3 cm in diameter) rotating at 14 revolutions per min during 60-sec trials. After two training trials, the number of seconds until the first fall from the rotarod during a 60-sec test trial was recorded. To assess spontaneous locomotor activity, mice from each genotype were placed in polypropylene cages (24 × 45 × 15 cm), and the number of photocell beam interruptions were counted for 1 hr (Photobeam Activity System, San Diego Instruments, San Diego).
RESULTS
Inactivation of the M2 Muscarinic Receptor Gene.
To generate mice lacking functional M2 muscarinic receptors, we inactivated the M2 gene in mouse ES cells via homologous recombination. As shown in Fig. 1a, a segment of the receptor coding sequence (encoding the region ranging from the N terminus of the third transmembrane domain to the C terminus of the third intracellular loop) was replaced with a neomycin-resistance cassette, which is predicted to disrupt M2 receptor function.
ES cells harboring the desired mutation were used to generate chimeric mice. These animals then were crossed with CF-1 mice, and the resulting heterozygous mice were intercrossed to obtain wild-type (+/+) as well as heterozygous (+/−) and homozygous (−/−) mutant mice. Mouse genotyping was carried out by Southern blot analysis of mouse tail DNA, as shown in Fig. 1b.
Homozygous M2−/− mice (n = 102) were obtained with the expected Mendelian frequency, indicating that there was no increase in embryonic or postnatal mortality in the mutant mice. Moreover, the mutant mice did not show any obvious morphological abnormalities and did not differ from their wild-type littermates in overall health, fertility, and longevity. We noted, however, that adult M2−/− mutant mice weighed ≈5% (1.5–2.5 g) less than their control littermates (P < 0.05).
In Situ Hybridization Experiments.
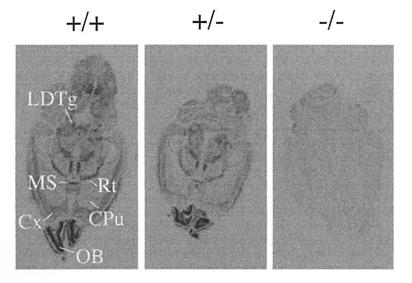
To study the expression pattern of M2 receptor mRNA and to confirm the absence of functional M2 transcripts in the M2−/− mutant animals, mRNA in situ hybridization experiments were carried out by using brain tissue prepared from wild-type and mutant mice (Fig. 2). For these experiments, a [35S]-labeled antisense riboprobe corresponding in sequence to the receptor coding region deleted in the M2 mutant mice was used as a probe. Consistent with previous studies on rat brain (8, 9, 16), M2 receptor mRNA was found to be widely distributed throughout the brain. As expected, only background staining was found in the case of M2−/− mutant mice (Fig. 2).
Figure 2.
In situ mRNA hybridization analysis of wild-type and M2 receptor mutant mice. The distribution of M2 receptor mRNA was studied in horizontal brain sections using an antisense riboprobe corresponding in sequence to the receptor region deleted in the M2 knockout mice (Fig. 1a). CPu, caudate-putamen; Cx, cerebral cortex; LDTg, laterodorsal tegmental nucleus; MS, medial septal nucleus; OB, olfactory bulb; Rt, reticular thalamic nucleus.
Radioligand Binding Studies.
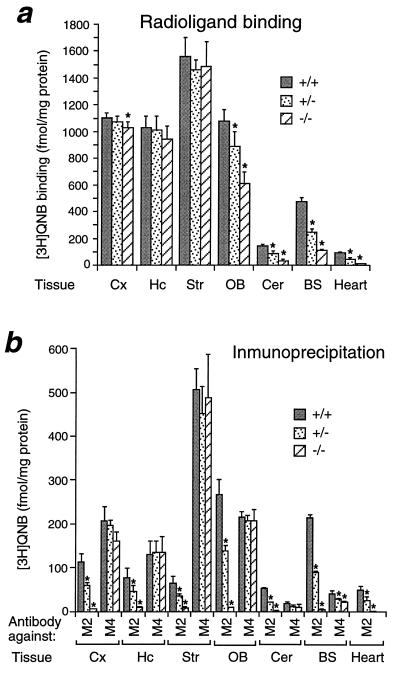
To examine muscarinic receptor expression more directly, radioligand binding studies were carried out using the nonselective muscarinic antagonist [3H]QNB. For these studies, a saturating concentration (2 nM) of [3H]QNB was incubated with membrane homogenates prepared from various mouse brain regions and heart. [3H]QNB binding activity was virtually abolished in the heart of M2−/− mice and was significantly reduced in the olfactory bulb, cerebellum, and brainstem (pons-medulla) (Fig. 3a). These brain regions are known to be particularly rich in M2 receptors as compared with other members of the muscarinic receptor family (8–10).
Figure 3.
Expression of muscarinic receptor proteins in different regions of mouse brain and heart. (a) Radioligand binding studies. Membranes were prepared from the indicated tissues of wild-type and M2 receptor mutant mice. Muscarinic receptor densities were determined in radioligand binding studies by using a saturating concentration (2 nM) of the nonselective muscarinic antagonist [3H]QNB. (b) Immunoprecipitation studies. Membranes were prepared from the indicated tissues of wild-type and M2 receptor mutant mice, followed by labeling of muscarinic receptors with 2 nM [3H]QNB and solubilization of labeled receptors with 1% digitonin. [3H]QNB-labeled M2 or M4 muscarinic receptors were immunoprecipitated with subtype-specific rabbit antisera. Cx, cerebral cortex; Hc, hippocampus; Str, striatum; OB, olfactory bulb; Cer, cerebellum; BS; brain stem. Data are given as means ± SD (n = 3–4 for each dose and genotype). ∗, P < 0.001 (Student’s t test).
Immunoprecipitation Studies.
To study the expression of M2 receptor protein in a more direct fashion, an M2 receptor-selective antiserum was used for immunoprecipitation studies. For control purposes, we also used an antiserum directed against the functionally closely related M4 receptor subtype. The specificity of the antisera (which were raised in rabbits against nonconserved regions of the third cytoplasmic loops of the mouse M2 and M4 receptor proteins) was verified by using Chinese hamster ovary cell lines transfected with the M1–M5 receptor subtypes (data not shown). Use of the M2 receptor antiserum showed that wild-type mice express M2 receptors in the heart as well as in all examined brain regions, though at relatively low density (Fig. 3b). Expectedly, the M2 receptor antiserum was unable to immunoprecipitate significant amounts of radioactivity ([3H]QNB-labeled receptors) from tissues derived from M2−/− mice, confirming the lack of functional M2 receptors in these animals. Importantly, in all tissues examined, the M4 receptor antiserum detected similar numbers of M4 receptors in M2−/− mutant mice and their wild-type littermates, indicating that disruption of the M2 receptor gene did not lead to a compensatory overexpression of M4 receptor protein.
Isolated Atria.
Further evidence for the lack of functional M2 receptors in M2−/− mutant mice was derived from in vitro studies using spontaneously beating right atria. Incubation of atria derived from M2−/− mice with the nonselective muscarinic agonist carbachol (1 μM) had no significant effect on atrial beating frequencies (n = 4). In contrast, carbachol (1 μM) produced a marked bradycardia in atrial preparations derived from wild-type control littermates (reduction in atrial rate: 56 ± 3%; n = 7) (P. W. Stengel and M. L. Cohen, personal communication).
Locomotor Activity.
Spontaneous locomotor activity and motor coordination of M2 receptor mutant mice were assayed in an “open-field” and the rotarod test, respectively. Recording of the total number of consecutive photobeams broken in 1 hr in the open-field test revealed no significant differences in locomotor activity between mutant mice (+/− and −/−) and their wild-type littermates (n = 5 for each genotype). Similarly, M2 receptor mutant mice performed equally as well as their control littermates on the rotarod in that they did not fall during a 60-sec test (n = 5 for each genotype) (data not shown).
Muscarinic Receptor-Mediated Tremor.
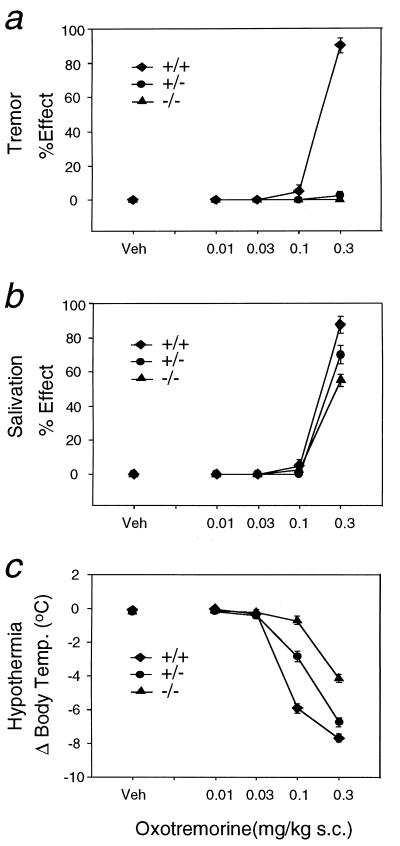
Injection of increasing doses of the centrally acting, nonselective muscarinic agonist OXO (0.01–0.3 mg/kg, injected s.c.) into wild-type control mice reproducibly resulted in massive whole-body tremor and akinesia at the highest dose tested (0.3 mg/kg; doses >0.3 mg/kg resulted in a pronounced increase in lethality) (Fig. 4a). Strikingly, the tremorogenic effects of OXO were absent in M2−/− mutant mice (Fig. 4a), even when the OXO dose was increased to 1 mg/kg (data not shown). Interestingly, heterozygous M2+/− mutant mice also did not display the OXO-induced tremor response (Fig. 4a).
Figure 4.
Tremorogenic, sialagogic, and hypothermic responses to OXO administration in wild-type and M2 receptor mutant mice. (a) Tremor. (b) Salivation. (c) Reduction in core body temperature. Mice of the indicated genotypes were injected s.c. with varying doses of the nonselective muscarinic agonist OXO. Vehicle-treated (Veh) mice of the same genotype (littermates) served as controls. Antinociceptive effects were measured as described in Materials and Methods. Data are given as means ± SEM (n = 16–20 for each dose and genotype). Hypothermia data were analyzed by a two-way ANOVA with a significant effect for the M2 genotype (F2, 283 = 111.9, P < 0.0001). Orthogonal comparisons revealed that the dose-response curve for M2−/− mice was significantly different from the curves for M2+/− and M2+/+ mice (t = −13.5, P < 0.00001) and that the curve for M2+/− mice was significantly different from that for M2+/+ mice (t = −6.4, P < 0.00001).
Muscarinic Receptor-Mediated Salivation and Hypothermia.
As shown in Fig. 4b, OXO also induced a dose-dependent increase in salivary secretion in wild-type control mice. This response remained largely unaffected in the M2 receptor mutant mice (Fig. 4b). In wild-type mice, OXO administration also produced a pronounced, dose-dependent decrease in core body temperature (Fig. 4c). OXO dose-response curves were shifted to the right (by a factor of ≈3) in heterozygous M2+/− mice. In M2−/− knockout mice, OXO-induced hypothermia was impaired to an even greater extent (Fig. 4c). In these animals, no change in body temperature was seen after injection of 0.1 mg/kg OXO (as compared with a decrease of ≈6°C seen with wild-type littermates), and the hypothermic response induced by 0.3 mg/kg OXO was reduced by ≈50%.
Muscarinic Receptor-Mediated Analgesia.
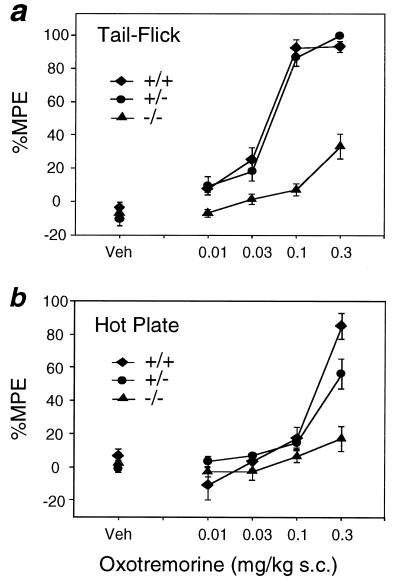
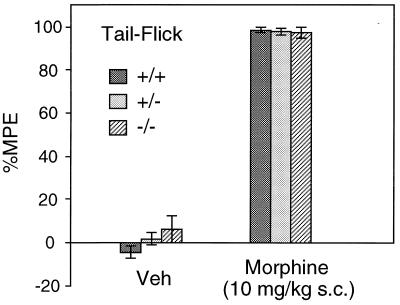
To study the potential involvement of the M2 receptor subtype in muscarinic receptor-dependent analgesia, OXO-induced antinociceptive effects were studied by using the tail-flick and hot plate tests (Fig. 5). Although the tail-flick method assesses pain sensitivity primarily at the spinal level, the hot plate test measures pain responses and analgesia mediated predominantly by supraspinal mechanisms (17). OXO administration induced strong dose-dependent analgesic effects in wild-type as well as in heterozygous M2+/− mutant mice in both tests (Fig. 5). Strikingly, OXO-dependent antinociceptive responses were found to be drastically reduced in homozygous M2−/− knockout mice in both assays. However, as shown in Fig. 6 (tail-flick test), M2−/− mutant mice did not differ significantly from wild-type littermates in their responsiveness to the opioid analgesic morphine (10 mg/kg, injected s.c.). Similar results were obtained when morphine-induced analgesic effects were studied in the hot plate test (data not shown).
Figure 5.
Antinociceptive responses to OXO administration in wild-type and M2 receptor mutant mice. (a) Tail-flick test. (b) Hot plate assay. Mice of the indicated genotypes were injected s.c. with varying doses of the nonselective muscarinic agonist OXO. Vehicle-treated (Veh) mice of the same genotype (littermates) served as controls. Data are presented as means ± SEM (n = 18–20 for each dose and genotype) and are expressed as the percent MPE, where percent MPE = 100 × [(postdrug latency − baseline)/(10 or 30 − baseline)]. Two-way ANOVA revealed a significant main effect for genotype in the M2 receptor mutant mice for both the tail-flick (F2, 283 = 99.7, P < 0.0001) and hot plate (F2, 285 = 15.6, P < 0.0001) tests. Orthogonal comparisons revealed that, in the tail-flick test, the dose-response curve for M2−/− mice was significantly different from that for M2+/− and M2+/+ mice (t = −14.1, P < 0.0001) but that the curve for M2+/− mice was not significantly different from that for M2+/+ mice (t = 0.8, P > 0.05). In the hot plate test, the dose-response curve for M2−/− mice was significantly different from that for M2+/− and M2+/+ mice (t = −5.2, P < 0.00001), and the curve for M2+/− mice was significantly different from that for M2+/+ mice (t = −2.0, P = 0.045).
Figure 6.
Analgesic responses to morphine administration in wild-type and M2 receptor mutant mice. Mice of the indicated genotypes were injected with morphine (10 mg/kg, injected s. c.), and antinociceptive responses were measured in the tail-flick test as described in Materials and Methods. Vehicle-treated (Veh) mice of the same genotype (littermates) served as controls. Data are presented as means ± SEM (n = 19 for each genotype) and are expressed as the percent MPE, where percent MPE = 100 × [(postdrug latency − baseline)/(10 − baseline)].
DISCUSSION
Previous studies have shown that cardiac muscarinic receptors almost exclusively consist of the M2 subtype (1, 2, 5, 6). In agreement with this finding, hearts derived from M2−/− mutant mice were unable to bind significant amounts of the muscarinic radioligand [3H]QNB. Moreover, muscarinic agonist-induced bradycardia was no longer observed in M2−/− mutant mice.
To explore the potential involvement of the M2 receptor subtype in central muscarinic mechanisms, wild-type and M2 mutant mice were injected with OXO, a nonselective muscarinic agonist that easily penetrates the blood–brain barrier (18). Administration of OXO to wild-type mice produces a number of striking pharmacologic effects (including stimulation of salivation, tremor and akinesia, hypothermia, and analgesia), most of which are mediated by central muscarinic receptors (see below). The increase in salivary secretion caused by OXO administration, a response thought to be mediated primarily by peripheral muscarinic receptors (1, 2), was similar in wild-type and M2 mutant mice. This observation is in agreement with previous pharmacologic studies indicating that glandular secretion is mediated predominantly by glandular M3 muscarinic receptors (1, 2, 5, 6).
Central muscarinic receptors are known to play key roles in the regulation of extrapyramidal movement control, and an imbalance between muscarinic cholinergic and dopaminergic neurotransmission in the striatum is considered a hallmark of Parkinson’s disease (19). Administration of OXO or other centrally acting muscarinic agonists to experimental animals causes several of the key symptoms of Parkinson’s disease, such as akinesia and tremor (18, 20, 21). These responses can be elicited by direct intrastriatal injection of muscarinic agonists (22, 23) and can be suppressed by pretreatment of animals with widely used anti-Parkinson drugs, such as muscarinic antagonists and L-DOPA (24–26). For these reasons, OXO-induced tremor serves as a routine model system for the screening of new anti-Parkinson drugs. Consequently, identification of the muscarinic receptor subtype mediating the tremorogenic actions of muscarinic agonists should be of great therapeutic interest.
To address this question, OXO-dependent tremor was studied in wild-type and M2 receptor mutant mice. Strikingly, OXO was unable to induce tremor responses in M2+/− and M2−/− mutant mice. The observed lack of a tremor response in animals heterozygous for the M2 receptor mutation was somewhat surprising but may indicate that OXO-induced tremor is characterized by a small receptor reserve. This latter notion is supported by previous pharmacologic studies using OXO derivatives capable of irreversible alkylating central muscarinic receptors (27). Our results indicate that the ability of therapeutically useful muscarinic antagonists to inhibit OXO-mediated tremor responses is attributable to the blockade of central M2 muscarinic receptors.
The molecular mechanisms by which M2 receptor activation can induce Parkinson-like symptoms remain unknown at present. Detailed studies by Hersch et al. (28) have shown that striatal M2 receptors are present presynaptically (as auto- or heteroreceptors) as well as postsynaptically. The potential involvement of these receptors in mediating movement control should be addressed in future studies.
It should be noted that Hamilton et al. (29) recently reported an initial pharmacologic analysis of a mouse line lacking functional M1 muscarinic receptors. Although agonist-dependent salivation and tremor still were observed in these animals, homozygous and heterozygous M1 receptor mutant mice were found to be highly resistant to seizures produced by administration of the muscarinic agonist, pilocarpine (“pilocarpine model of epilepsy”).
Administration of muscarinic agonists also produces pronounced analgesic effects (30–34). Muscarinic receptor-mediated analgesia depends on both spinal and supraspinal mechanisms (30–34), is similar in magnitude to morphine-induced analgesia (32, 34), and is less likely to lead to tolerance and addiction associated with opioid analgesics (34, 35). In keeping with these findings, muscarinic receptors are abundantly expressed in the dorsal horn of the spinal cord (36) as well as the thalamus (8–10), two areas of the central nervous system known to be intimately involved in pain transmission. Identification of the receptor subtype involved in muscarinic receptor-dependent antinociception is therefore critical for the rational design of novel analgesic drugs with improved pharmacologic properties.
We found that OXO-injected M2−/− mutant mice showed greatly reduced analgesic effects, as assessed in the hot plate and tail-flick tests. This observation is consistent with previous findings (37) that muscarinic agonist-induced antinociceptive responses can be prevented by pretreatment of animals with pertussis toxin, an agent that selectively inactivates G proteins of the Gi/o family. Preliminary studies have shown that OXO-induced analgesic effects are largely preserved in M4 receptor knockout mice (J.G., H.S., E.K., C.F., L.Z., J.B., C. Deng, and J.W., unpublished results). Taken together, these findings indicate that muscarinic analgesia is mediated predominantly (but not exclusively) by the M2 receptor subtype, at least in the mouse.
Many studies have shown that central muscarinic receptors also play an important role in the regulation of body temperature (38). Consistent with this notion, injection of OXO into wild-type mice produces pronounced hypothermic effects that are thought to be mediated largely by muscarinic receptors located in thermoregulatory centers of the hypothalamus (38). OXO-induced hypothermia was found to be significantly reduced in M2 receptor mutant mice, indicating that the M2 subtype plays a role in muscarinic receptor-mediated temperature regulation. However, because OXO-induced hypothermia was reduced but not completely abolished in M2−/− mutant mice, other muscarinic receptor subtypes also appear to be involved in this response. In heterozygous M2+/− mice, OXO dose-response curves were shifted to the right by a factor of ≈3, suggesting that efficient M2 receptor-induced hypothermia requires a relatively high fractional receptor occupancy.
In conclusion, we have demonstrated that the M2 muscarinic receptor subtype, besides its well documented involvement in controlling cardiac function, plays a key role in mediating muscarinic receptor-dependent movement and temperature control as well as analgesia, three of the most prominent central muscarinic effects. These findings shed light on the central nervous system functions of the M2 muscarinic receptor and should provide a rational basis for the development of novel muscarinic drugs.
Acknowledgments
We thank G. Aronson, A. Cummins, and C. R. Gerfen for carrying out the in situ hybridization studies; P. W. Stengel and M. L. Cohen for performing the experiments on isolated atria; S. C. Peters, B. Xia, and C. Li for expert technical assistance; C. Paszty for providing the pPN2T targeting vector; M. Weinstein, D. Accili, and H. Westphal for advice and helpful discussions; and A. M. Spiegel for generous support of this work.
ABBREVIATIONS
- ES cells
embryonic stem cells
- MPE
maximum possible effect
- OXO
oxotremorine
- QNB
quinuclidinyl benzilate
References
- 1.Brown J H, Taylor P. In: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. pp. 141–160. [Google Scholar]
- 2.Wess J, Buhl T, Lambrecht G, Mutschler E. In: Comprehensive Medicinal Chemistry. Emmett J C, editor. Vol. 3. Oxford: Pergamon Press; 1990. pp. 423–491. [Google Scholar]
- 3.Levine, R. R. & Birdsall, N. J. M., eds. (1995) Life Sci.56, 801–1050.
- 4.Levine, R. R. & Birdsall, N. J. M., eds. (1997) Life Sci.60, 963–1207.
- 5.Hulme E C, Birdsall N J, Buckley N J. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- 6.Caulfield M P. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 7.Wess J. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 8.Levey A I. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 9.Vilaro M T, Mengod G, Palacios J M. Prog Brain Res. 1993;98:95–101. doi: 10.1016/s0079-6123(08)62385-7. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe B B, Yasuda R P. Ann NY Acad Sci. 1995;757:186–193. doi: 10.1111/j.1749-6632.1995.tb17474.x. [DOI] [PubMed] [Google Scholar]
- 11.Paszty C, Mohandas N, Stevens M E, Loring J F, Liebhaber S A, Brion C M, Rubin E M. Nat Genet. 1995;11:33–39. doi: 10.1038/ng0995-33. [DOI] [PubMed] [Google Scholar]
- 12.Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann M R. J Pharmacol Exp Ther. 1991;256:727–733. [PubMed] [Google Scholar]
- 13.Levey A I, Kitt C A, Simonds W F, Price D L, Brann M R. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasuda R P, Ciesla W, Flores L R, Wall S J, Li M, Satkus S A, Weisstein J S, Spagnola B V, Wolfe B B. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- 15.Gerfen C R, Keefe K A, Gauda E B. J Neurosci. 1995;15:8167–8176. doi: 10.1523/JNEUROSCI.15-12-08167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley N J, Bonner T I, Brann M R. J Neurosci. 1989;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.König M, Zimmer A M, Steiner H, Holmes P V, Crawley J N, Brownstein M J, Zimmer A. Nature (London) 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 18.George R, Haslett W L, Jenden D J. Life Sci. 1962;1:361–363. doi: 10.1016/0024-3205(62)90060-7. [DOI] [PubMed] [Google Scholar]
- 19.Standaert D G, Young A B. In: The Pharmacological Basis of Therapeutics. Hardman J G, Limbird L E, editors. New York: McGraw–Hill; 1996. pp. 503–519. [Google Scholar]
- 20.Ringdahl B, Roch M, Jenden D J. J Med Chem. 1988;31:160–164. doi: 10.1021/jm00396a024. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez C, Meier E. Pharmacol Toxicol. 1993;72:262–267. doi: 10.1111/j.1600-0773.1993.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 22.Connor J D, Rossi G V, Baker W W. Int J Neuropharmacol. 1966;5:207–216. doi: 10.1016/0028-3908(66)90025-6. [DOI] [PubMed] [Google Scholar]
- 23.Connor J D, Rossi G V, Baker W W. Exp Neurol. 1966;14:371–382. doi: 10.1016/0014-4886(66)90121-x. [DOI] [PubMed] [Google Scholar]
- 24.Horst W D, Pool W R, Spiegel H E. Eur J Pharmacol. 1973;21:337–342. doi: 10.1016/0014-2999(73)90136-2. [DOI] [PubMed] [Google Scholar]
- 25.Korczyn A D, Eshel Y. Neuropharmacology. 1979;18:601–603. doi: 10.1016/0028-3908(79)90111-4. [DOI] [PubMed] [Google Scholar]
- 26.Quock R M, Lucas T S. Eur J Pharmacol. 1983;95:193–198. doi: 10.1016/0014-2999(83)90634-9. [DOI] [PubMed] [Google Scholar]
- 27.Ringdahl R, Jenden D J. J Pharmacol Exp Ther. 1987;240:370–375. [PubMed] [Google Scholar]
- 28.Hersch S M, Gutekunst C-A, Rees H D, Heilman C J, Levey A I. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton S E, Loose M D, Qi M, Levey A I, Hille B, McKnight G S, Idzerda R L, Nathanson N M. Proc Natl Acad Sci USA. 1997;94:13311–13316. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaksh T L, Dirksen R, Harty G J. Eur J Pharmacol. 1985;117:81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- 31.Green P G, Kitchen I. Prog Neurobiol. 1986;26:119–146. doi: 10.1016/0301-0082(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 32.Hartvig, P., Gillberg, P. G., Gordh, T., Jr. & Post, C. (1989) Trends Pharmacol. Sci.10, Suppl., 75–79. [PubMed]
- 33.Iwamoto E T, Marion L. J Pharmacol Exp Ther. 1993;266:329–338. [PubMed] [Google Scholar]
- 34.Swedberg M D, Sheardown M J, Sauerberg P, Olesen P H, Suzdak P D, Hansen K T, Bymaster F P, Ward J S, Mitch C H, Calligaro D O, et al. J Pharmacol Exp Ther. 1997;281:876–883. [PubMed] [Google Scholar]
- 35.Widman M, Tucker S, Brase D A, Dewey W L. Life Sci. 1985;36:2007–2015. doi: 10.1016/0024-3205(85)90450-3. [DOI] [PubMed] [Google Scholar]
- 36.Höglund A U, Baghdoyan H A. J Pharmacol Exp Ther. 1997;281:470–477. [PubMed] [Google Scholar]
- 37.Shannon H E, Womer D E, Bymaster F P, Calligaro D O, Delapp N W, Mitch C H, Ward J S, Whitesitt C A, Swedberg M D, Sheardown M J, et al. Life Sci. 1997;60:969–976. doi: 10.1016/s0024-3205(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 38.Myers R D. In: Handbook of the Hypothalamus. Morgane P J, Panksepp J, editors. 3, Part A. New York: Dekker; 1980. pp. 83–210. [Google Scholar]