Abstract
This study describes the assessment of dyspnea, symptom distress, and quality of life measures in 163 hospice patients with cancer who reported dyspnea. Mean age of the hospice patient sample was 70.22 years and 61.86 for caregivers (65% were spouses). The majority of patients and caregivers were white: 87%, 63% of the patients were male while 78% of caregivers were female. Mean dyspnea intensity as reported by patients was 4.52 (SD 2.29) and caregivers, 4.39 (SD 2.93). Patients' and caregivers' ratings of the patient's dyspnea intensity revealed no significant differences in ratings thus verifying that caregivers can assess dyspnea severity accurately. Patients' perceived quality of life ratings were not significantly correlated with ratings of their caregivers' perceived quality of life. For patients, symptom distress and education were significant predictors of variance in quality of life (R2 = .35, p = .04). However, mastery, symptom distress, age, and education were found to be significant predictors of variance in quality of life of caregivers (R2 = .40, p = .02).
The Problem of Dyspnea and Related Symptoms in Hospice Patients
Respiratory symptoms are often more difficult to treat in hospice patients with irreversible, end-stage cancer for a number of reasons. Patients often express that these symptoms, dyspnea, pleural pain, and panic, are ones that they fear the most. The National Hospice Study showed that for patients in the last six weeks of life, dyspnea occurred in 70% of patients.[1] Other research indicates that dyspnea is the fourth most common symptom of patients who present to the emergency department with advanced cancer. Dyspnea is thought to be a clinical marker for the terminal phase of their disease.[2] When dyspnea becomes emergent in advanced cancer patients, it may indicate a phase in their illness in which resources should be shifted from acute intervention to palliative and supportive care measures.[3]
The problems of dyspnea assessment and management are also of clinical importance for the quality of life of hospice patients and their families. Strategies to reduce and manage dyspnea in hospice patients have been tested only minimally and almost all of the studies have had inadequate sample sizes. Because so little is known about the treatment of severe dyspnea, patients, health care providers, and caregivers are frustrated and left with feelings of helplessness. It is estimated that only 20% of those suffering from chronic dyspnea obtain relief through treatment.[3] Moody, McCormick, and Williams found that in patients with chronic lung disease, dyspnea severity directly affects functional status and impaired functional status subsequently diminishes perceived quality of life.[4]
A study by Webb, Moody, and Mason from two large hospices in Florida revealed nurses' initial and ongoing assessments of patients with end-stage-lung disease did not include regular use of any of the preferred dyspnea measurement scales, such as the graphic rating scale or visual analog scale.[5] Nursing staff from the hospice study sites expressed a need for studies that tested which clinical interventions are most useful in alleviating dyspnea. The aims of the present study were to examine hospice patients with cancer who suffer from dyspnea and its relation to other quality of life indicators and to describe their caregivers' symptom-related distress, feelings of mastery, and perceived quality of life as related to being caregivers.
Research Questions
Severe dyspnea, whether acute or chronic, affects patients' functional status and quality of life as well as other psychosocial aspects of the patient's life.[6] Patients with severe dyspnea who are in hospice care most often manifest the associated symptoms and problems of anxiety, fatigue, and sleeplessness which in turn affect functional status and cognitive status, and subsequently decrease quality of life.[4] Based on previous studies,[4,6,7] a specific study aim was to explore the congruence of dyspnea ratings by caregivers with patients' ratings of dyspnea. Another aim was to explore the relation of the patient's dyspnea and related variables to the patient's quality of life as well as the effect on the caregiver's quality of life. The study addressed three specific research questions in hospice patients with end-stage lung cancer and their caregivers.
1. Are there significant differences in the intensity ratings of the patients' dyspnea between patients and caregivers?
2. To what extent do these patient-variables, age, educational level, dyspnea intensity, symptom distress, and functional status, explain variance in the patient's perceived quality of life?
3. To what extent do these caregiver-variables, age, educational level, dyspnea intensity, symptom distress, and mastery, explain variance in the caregiver's perceived quality of life.
Conceptual Framework
The conceptual framework for this study was adapted from the previous work of Moody, McCormick, and Williams who that found mastery was associated with improved symptom management which leads to improved functional status and perceived quality of life.[4] Another underlying assumption is that accurate assessment of symptoms leads to improved management, an assumption that has yet to be tested but will be the focus of a future study. In the present study, the perceived mastery level of the patient was not measured but it was assessed for the caregiver.
Nurses and physicians often fail to assess dyspnea accurately, as they tend to assess only observable signs of dyspnea. Breathlessness, the subjective component of dyspnea, is often different from what is directly observable.[5] Since the early 1980s, there have been a number of tools available to measure dyspnea in acute and chronic illness. Studies, which have measured dyspnea appropriately, generally use a quantitative method of self-assessment such as the visual analog scale or a graphic rating scale. The graphic rating scale is a bipolar scale with "0" for "No dyspnea" and "10" for Worst Dyspnea Ever." In general, a graphic rating scale is preferred for older people, hospice patients, and those who are apt to be easily fatigued. The researchers' previous experience with the instrument has shown the graphic rating scale to be the best choice for caregivers in terms of ease of use and accuracy.[4,6]
Dyspnea and Hospice Care
In hospice patients, it is not uncommon to find acute and chronic dyspnea because most patients have one of these primary diagnoses: end-stage lung disease (COPD or interstitial fibrosis), lung cancer, or end-stage heart disease.[7] Aggressive treatment of distressing symptomatology contributes to overall quality of life and restores to the patient some of the freedom and autonomy usurped by the disease process.
Dyspnea is often associated with other common symptoms: fatigue, cough, anxiety, and pain.[5] This cluster of symptoms is usually studied together, and caregivers are often asked to assess all of them using various types of analog or graphic rating scales. In planning interventions for dyspnea, it is important to assess the degree of distress the dyspnea causes for the patient and in turn the caregiver. Moody and other researchers have found, that the onset of acute dyspnea in patients who have not experienced dyspnea before, may cause severe anxiety in both the patient and the caregiver.[7] In order to improve symptom management in hospice care, it is important to study whether caregivers are able to use the assessment tools and achieve ratings congruent with the patient's ratings.
Caregivers in Hospice Care
Until recently, most research in psychosocial oncology and palliative care focused exclusively on the patient. However, terminal diseases are a major stressor for both the patient and the family caregiver, causing a new set of challenges for both.[8] At the very least, the routine of daily life is altered and both patients and family members must struggle to adjust and respond to new demands.[9] Recent research suggests that family caregivers experience depression and anxiety, psychosomatic symptoms, restrictions of roles and activities, strain in marital relationships, and diminished physical health.[10] Indeed, research suggests that spouses experience as much, if not more, distress than patients. [11] This emotional distress may affect role functioning, both within the family as well as in society including the ability to care for the patient.[12] Caregiver distress is an important concern because family caregivers are assuming more responsibility for care as treatment moves increasingly to the outpatient arena. It is increasingly important to evaluate quality of life (QOL) of family caregivers because of their increased responsibility and contribution to the care of the cancer patient. However, most studies to date have focused on specific outcomes, such as needs, burden, and emotional distress rather than on a more comprehensive evaluation of QOL outcomes.
Methods
This descriptive, cross-sectional study was a substudy of a larger NCI-funded randomized clinical trial to test an intervention designed to assist caregivers in coping with symptoms near the end of life. Baseline data from matched hospice patients with cancer and their caregivers are included in this analysis.
Setting and Sample
A large hospice provider in SW Florida was the site for the overall study of 300 patient/caregiver dyads that had been accrued at the time of this secondary analysis. This substudy was focused on the 162 dyads (54 percent) from the sample, which were identified through the patient's self-report of dyspnea at the time of admission. To be eligible, patients had to be adults (+18 years) with a diagnosis of cancer and an identified family caregiver who was a spouse or adult child, and both had to consent to participate. Both had to have at least a sixth grade education, be able to read and understand English, and pass cognitive screening with the Short Portable Mini-mental Status Exam [13] (Pfeiffer, 1974).
Study Instruments
Dyspnea Intensity
For hospice patients with severe dyspnea and fatigue, a bipolar 11-point Dyspnea Graphic Rating Intensity Scale (DGRIS) was used.[4] This global scale, which assesses only the perceived severity of the dyspnea, was used due to ease of administration and accuracy. Reliability and validity of the one-item graphic rating scales have been supported by a number of studies. Scores range from 0 to 10. Test-retest reliability has ranged from .89 to .92 and concurrent validity with other measures .88 to .94.[5] Although this scale was designed for patient self-report, the caregiver also was asked to estimate the severity of the patient's dyspnea, using this one-item scale.
Memorial Symptom Assessment Scale (MSAS)
The Memorial Symptom Assessment Scale [14] was administered to both patients and caregivers to measure both the frequency and distress of common symptoms experienced by patients. For patients, it was used to measure distress caused by 24 symptoms commonly seen in persons with advanced cancer, including shortness of breath. The MSAS is a self-report scale that provides data about the occurrence of each symptom and the distress associated with these symptoms. Distress is measured on a 5 point summated rating scale with scores that may range from 0 (no distress) to 96 (very much distress). Validity was supported by high correlations with clinical status and quality of life. Internal consistency was assessed using Cronbach's alpha and found to be high, ranging from r = .83 to .88.[15,16]
The MSAS scale was administered to all caregivers. It was altered slightly by rewording items to determine the degree to which each symptom experienced by the patient caused distress for the caregiver. These scores also ranged from 0 to 96. This approach has been used previously in studies with caregivers who have dementia. [15]
Hospice Quality of Life Index
The Hospice Quality of Life Index (HQLI) is a 28-item self-report tool that includes three aspects of overall quality of life: Psychophysiologic Well-being; Functional Well-being; and Social and Spiritual Well-being.[16] Total scores may range from a low of zero to a high of 280. Evidence of validity and reliability of the HQLI was generated by a recently completed study.[16] Evidence of validity was provided by the ability of the HQLI to differentiate between hospice patients and apparently healthy controls using both discriminate analysis (p = .00) and comparison of means (p = .00). Previously, the HQLI has been validated for use with hospice patients with cancer. The finding that HQLI scores correlated at the expected level (r = .26; p = .00) with functional status scores provides further evidence of validity. Finally, factor analysis confirmed the factor structure of the HQLI. Reliability of the HQLI was provided by generation of coefficient alphas for both total scale scores and subscale scores. Subscale alphas all were .84 and the total scale alpha was high for both cancer (r = .88) and AIDS (r = .93) patients. For this paper, the item asking the patient how breathless he or she feels (0–10) was analyzed separately.
Mastery (Self-Efficacy) was assessed by a six-item summated rating scale with a five-point scale ranging from "None (0)" to "A Great Deal (5)". Scores may range from 0 to 30. It was modified for the study to include content-specific stems based on the context of caregiving and the caregiver's perceived ability to cope with caregiving tasks and manage day-to-day problems. The mastery scale has been used by Moody, et al. in a number of dyspnea and quality of life studies.[4,5] Reliability of the scale has ranged from .88 to .94. Content validity and concurrent validity have ranged from .94 to.96.[4,5]
Palliative Performance Status (PPS) Scale
The purpose of the Palliative Performance Scale (PPS) is to assess the physical condition and functional status of persons receiving palliative care.[17] Scores may range from 0 (dead) to 100 (normal functioning). It is a relatively new tool based on the Karnofsky Performance Scale and is purported to provide a framework for measuring the progressive decline in palliative care patients. The PPS measures three broad areas of function: intake, level of consciousness, and mobility. The PPS is scored from 0–100% at 10% increments. The PPS level for a given patient is determined by reading across the table at each 10% decrement to find the overall best fit. 'Stronger' performance factors are noted to be located on the left of the instrument 'softer' ones on the right. Judgment is required if one of the five factors observed in a particular patient does not fit with the others. The usual approach to decide PPS is to first determine whether the disease has limited the ability to work or ambulate (left side), and then work one's way across the table. Patients who have a lower PPS generally are more functionally impaired than those with higher scores. Prognosis is generally related to functional status in most palliative care patients. Those patients with higher PPS scores may have longer length of stays, although all patients may not be comparable. Progression of disease can generally be noted with declining PPS levels in a given patient. Interpretation of the instrument may have implications about patient acuity and need for amount of services.
The initial validity study assessed 119 patients at home and 213 patients admitted to a hospice unit. Validity of this instrument was assessed comparing the PPS score with length of survival. The average period until death for 129 patients who died on the unit was 1.88 days at 10% PPS on admission, 2.62 days at 20%, 6.7 days at 30%, 10.3 at 40%, 13.87 at 50%. Only two patients at 60% or higher died in the unit. As part of an earlier project we assessed validity and reliability of the PPS. The predicted strong positive correlations between PPS and KFS (r = .88, p = .01) support construct (convergent) validity.[17] Although no reliability data were reported by the tool developers, the researchers in this substudy found that inter-rater reliability was very strong (r = .95. p = .01, df 162).
Demographic Data
Standard demographic data were collected on dyads to allow description of the sample. Patient data included age, gender, education level, marital status, religion, occupation, cancer diagnosis, and length of time since diagnosis. Data on caregivers were assessed by self-report in a semistructured interview: age, ethnicity, gender, education, marital status, and religion.
Study Procedures
This study was approved by the bioethics committees of the hospice. Following approval by this committee, the proposal was approved by the University of South Florida Institutional Review Board for the Protection of Human Subjects. Eligible dyads that were potential study subjects were initially identified by hospice admission staff and referred to the Research Assistant (RA), a trained nursing data collector, at the beginning of each day. The RA contacted the caregiver to arrange a visit by both the nurse and a Home Health Aide (HHA). During this visit, the study was explained, consent of both patient and caregiver obtained, the mental status of the caregiver assessed, and the functional status of the patient evaluated. Patients and caregivers who met the admission criteria were asked to complete their respective questionnaires independently, in separate rooms when possible. The HHA collected patient data and stayed with the patient to assist as needed. The HHA reassured the caregiver that the patient would not be alone while the RA was collecting data from the patient. Therefore, the caregiver was better able to concentrate on the questionnaires knowing that the HHA was with the patient.
Data Analysis
Data for all demographic variables and study variables were analyzed first using descriptive statistics with SPSS version 11.5.[18] Multiple correlation and paired t-tests were used to analyze significant differences in ratings between caregivers and patients and associations between related symptoms and conditions. Stepwise multiple regression models were used to address research questions two and three.
Results
Profile of Study Patients and Caregivers
Usable data were collected on 163 hospice patients/caregiver-dyads. A profile of the study group is included in Table 1. The mean age of the hospice patient was 70.22 years and 61.86 for caregivers. About 30 % of caregivers were adult children and the majority was spouses. The dyad majority was white, 85%, 11% African-American, 6% Hispanic, and 1% Asian. The majority of patients were male, 63%, while 74% of caregivers were female. There were no mean differences in cognitive status (SPMSQ) scores between caregivers and patients. The education level of patients was 12.1 years compared to 12.8 for caregivers. Eighty percent of dyad-participants had annual incomes of less than $50,000. Of the patient sample, about 40 percent had lung cancer, 14 percent colon cancer, 6 percent breast cancer, 6 percent prostate, and 34% other types of cancer. Of the caregivers, 75% reported one or more medical problems and 15% reported a mental health problem, mostly depression.
Table 1.
Demographic Profile and Baseline Symptom Data for Hospice Patients and Their Caregivers
| Baseline Patient Variables | f | % | Range | Mean | SD |
| Male Female | 102 61 | 63 37 | |||
| White Non-white | 141 22 | 87 13 | |||
| Cough with Dyspnea | 106 | 64 | |||
| Age | 37.79 to 91.79 | 70.22 | 10.96 | ||
| Educational Level | 1 to 25 | 12.15 | 3.36 | ||
| Months Since Diagnosis | 1 to 360 | 27.55 | 50.32 | ||
| Dyspnea Intensity | 3 to 10 | 4.52a | 2.29 | ||
| Symptom Distress (MSAS) | 2 to 76 | 25.60 | 1.52 | ||
| Health Related Quality of Life | 137 to 278 | 208.81 | 35.57 | ||
| Palliative Performance Scale | 30 to 100 | 53.52 | .79 | ||
| Baseline Caregiver Variables | f | % | Range | Mean | SD |
| Female Male | 127 35 | 78 22 | |||
| White Nonwhite | 143 20 | 88 12 | |||
| Age | 26.02 to 91.90 | 61.86 | 14.46 | ||
| Caregiver Variables (con't) | Range | Mean | SD | ||
| Educational Level | 4 to 22 | 12.82 | 2.73 | ||
| Mastery Level | 10 to 42 | 25.02 | 4.84 | ||
| Symptom Distress (MSAS) | 10 to 112 | 25.41 | 12.61 | ||
| Health Related Quality of Life | 11 to 116 | 55.06 | 22.10 | ||
| Rating of Patients' Dyspnea | 2 to 9 | 4.39a | 2.93 |
a: Significant correlation between dyad ratings: Pearson r = .33, p = .000, df 161)
Dyspnea Ratings and Overall Symptom Distress (MSAS)
Mean dyspnea ratings as reported by the patients was 4.52 (SD 2.29) and by the caregivers was 4.39 (SD 2.93). To address research question one, a paired, two-tailed t-test was used to assess if there were significant differences between caregivers-patient dyad ratings of the patient's dyspnea intensity. As shown in the boxplot in Figure 1, results indicated that the distribution of scores and means were almost identical (Mean difference = .-13, t = -.53, df 162, p = .59). Paired, bivariate correlation was used to determine the relationship between patient and caregiver ratings of dyspnea. The correlation between patient and caregiver scores was weak but significant (r = .33; p = .000, df 1, 162).
Figure 1.
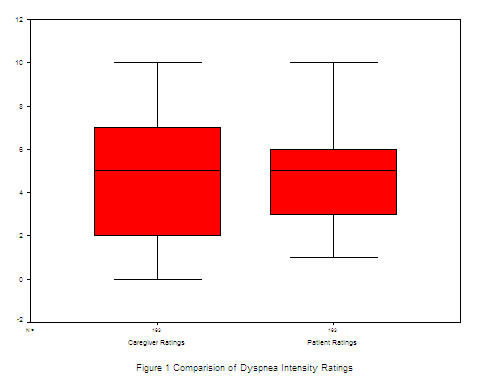
Patients' mean score on the MSAS symptom distress scale (Table 1), which includes 24 common symptoms experienced by hospice patients, including dyspnea were rated at about the same level. Mean score of caregivers was 25.41, compared to that of patients, 25.60.
Factors Influencing Patients' Quality of Life
To address the second research question, a stepwise multiple regression was used to determine if these patient-variables, age, educational level, symptom distress (MSAS), dyspnea intensity, and functional status (PPS), explained variance in the dependent variable, patient's perceived quality of life (HRQL). Variables were entered into the model stepwise with quality of life as the dependent variable (Table 2). All variables were entered into the model except for age, dyspnea intensity, and functional status. Regression results indicated that symptom distress and education were the only significant predictors of the patient's quality of life (R2 = .35, p = .04).
Table 2.
Stepwise Regression Models: Patient and Caregiver Predictors of Quality of Life
| Patient Modela Predictors | B | SE | Beta | Lower CI | Upper CI | t | df | p-value |
| Symptom distress (MSAS) | -1.57 | .17 | -.58 | -1.91 | -1.23 | -9.09 | 2, 160 | .000 |
| Education | -1.32 | .64 | -.13 | -2.58 | -.06 | -2.07 | .040 | |
| Caregiver Modelb Predictors | B | SE | Beta | Lower CI | Upper CI | t | df | p-value |
| Mastery | -2.16 | .30 | -.47 | -2.75 | -1.57 | -7.24 | 3, 156 | .000 |
| Symptom distress (MSAS) | .67 | .15 | .28 | .37 | .98 | 4.34 | .000 | |
| Age | -.32 | .10 | -.20 | -.51 | -.12 | -3.16 | .002 | |
| Education | 1.16 | .51 | .14 | .14 | .16 | 2.16 | .023 |
a: Al lpredictor variables entered stepwise: dyspnea intensity, age, and functional status removed. Dependent variable: Patient Quality of Life b: All predictor variables entered stepwise: dyspnea intensity removed. Dependent variable: Caregiver Quality of Life
Factors Influencing Caregivers' Quality of Life
The third research question was examined using stepwise multiple regression to determine if the caregiver's perceived levels of mastery, symptom distress, age, educational level, and the patient's dyspnea intensity were significantly related to their perceived quality of life. All variables were entered into the model with quality of life as the dependent variable. Results showed that three variables were significant predictors of variance in the caregivers' quality of life (R2 = .40, p = .02): mastery, symptom distress, age, and education (Table 2).
Discussion
Dyad ratings of the patients' dyspnea were almost identical (paired mean difference was -0.13). These findings support other researchers such as those of Sneeuw and colleagues [21] who found that, with few exceptions, mean scores of the proxy raters were equivalent to those of the patient, varying between 0.40 and 0.60, indicating a moderate level of agreement at the individual level. Disagreement was not dependent on the type of proxy rater, or on raters' background characteristics, but was influenced by the QOL dimension under consideration and the clinical status of the patient.[21] Although our findings also confirm that patient-caregiver dyspnea ratings were not significantly different (mean difference -0.19), the correlation between caregiver-patient ratings were significant but relatively not that strong (r .33, p = .000, df 1, 162). Data were checked twice for accuracy, reanalyzed, and the results were identical. Why the correlation is not stronger in view of the negligible mean difference in paired-dyad ratings is puzzling. The results indicate that both significant others and health care providers can be useful sources of information about cancer patients' QOL. Measurement of dyspnea needs to be done frequently using standardized instruments, such as the one-item, graphic rating scale to assess dyspnea and effects of treatments.
Results of the regression analysis revealed that the patient's perceived symptom distress and educational level are the most important variables that explain variance in their quality of life. This finding is similar to a previous study by Moody, McCormick, and Williams.[4] These results confirm the importance of assessing and managing the symptoms to improve or maintain quality of life.
Conclusions
From the results, it is clear that caregivers can obtain ratings of dyspnea that are congruent with the patient's perceived symptoms. This is especially important in hospice care as patients may become so debilitated that they need to rely on proxy ratings from their caregivers to assess and attend to their symptom management Caregiver perceived quality of life was related to four variables, perceived mastery, symptom distress, age, and years of education, with mastery being the strongest predictor. Overall symptom distress and education were found to be the best predictors of the patient's quality of life. In the current study, mastery was tested only in the caregivers. However, Moody and colleagues found mastery to be an important predictor of the patient's quality of life and recommend it be assessed in both patient and caregiver.[4]
Future research in the form of clinical trials is needed to test interventions to manage the severe dyspnea and related symptoms that occur in patients with cancer and end-stage lung disease. Therapies for dyspnea in the hospice setting have been subjected to only minimal scientific study, especially for end-stage chronic obstructive lung disease and lung cancer. Evidence-based clinical guidelines such as those developed for pain management are needed for dyspnea assessment and improved management of symptoms.
Authors' contributions
LM carried out the data analysis and drafted the manuscript. SM is PI and LM is co-investigator on the primary study of the NIH-funded grant from the National Cancer Institute. SM has read, approved, and contributed to the manuscript.
Acknowledgments
Acknowledgements
We would like to thank the Lifepath Hospice and the staff for facilitating the implementation of the study.
Contributor Information
Linda E Moody, Email: lmoody@hsc.usf.edu.
Susan McMillan, Email: smcmilla@hsc.usf.edu.
References
- Cleary JF, Carbone PP. Palliative medicine in the elderly. Cancer. 1997;80:1335–47. doi: 10.1002/(SICI)1097-0142(19971001)80:7<1335::AID-CNCR21>3.3.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stein WM, Min YK. Nebulized morphine for paroxysmal cough and dyspnea in a nursing home resident with metastatic cancer. Am J Hospice & Palliative Care. 1997;14:52–56. doi: 10.1177/104990919701400201. [DOI] [PubMed] [Google Scholar]
- Escalante C, Martin C, Elting L, Cantor S, Harle T, Price K, Kish S, Manzullo E, Rubinstein E. Dyspnea in cancer patients. Cancer. 1996;78:1314–1319. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1314::AID-CNCR21>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Moody L, McCormick K, Williams A. Disease and symptom severity, functional status, and quality of life in chronic bronchitis and emphysema. J Behavioral Medicine. 1990;13:297–304. doi: 10.1007/BF00846836. [DOI] [PubMed] [Google Scholar]
- Webb M, Moody L E, Mason A. Dyspnea assessment and management in hospice patients with pulmonary disorders. Am J Hosp Palliat Care. 2000;17:259–264. doi: 10.1177/104990910001700412. [DOI] [PubMed] [Google Scholar]
- Moody LE, Fraser M, Yarandi H. Effects of guided imagery in patients with chronic bronchitis and emphysema. Clinical Nursing Research. 1993;2:478–486. doi: 10.1177/105477389300200409. [DOI] [PubMed] [Google Scholar]
- Weitzner MA, Moody LE, McMillan S. Symptom management issues in hospice care. Am J Hospice Palliat Care. 1997;14:190–195. doi: 10.1177/104990919701400501. [DOI] [PubMed] [Google Scholar]
- Sabo D. Men, death and anxiety: Critical feminist interpretations of adjustment to mastectomy. In: Clark, Fritz, Rieder, editor. In Clinical Sociological Perspectives on Illness and Loss. Philadelphia: Charles Press; 1990. pp. 71–84. [Google Scholar]
- Northouse L, Peters-Golden H. Cancer and family: Strategies to assist spouses. Seminars in Oncology Nursing. 1993;9:74–82. doi: 10.1016/s0749-2081(05)80102-0. [DOI] [PubMed] [Google Scholar]
- Toseland RW, Blanchard CG, McCallion P. A problem solving intervention for caregivers of cancer patients. Social Sciences and Medicine. 1995;40:517–528. doi: 10.1016/0277-9536(94)E0093-8. [DOI] [PubMed] [Google Scholar]
- Baider L, Kaplan-DeNour A. Adjustment to cancer: Who is the patient – the husband or the wife. Israel Journal of Medical Science. 1988;24:631–636. [PubMed] [Google Scholar]
- Given CW, Stommel M, Given B, Osuch J, Kurtz ME. The influence of cancer patients' symptoms and functional states on patients' depression and family caregivers' reaction and depression. Health Psych. 1993;12:277–285. doi: 10.1037//0278-6133.12.4.277. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatrics Soc. pp. 433–441. [DOI] [PubMed]
- Portenoy RK, Thaler HT, Kornblith AB, et al. The memorial symptom assessment scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Europ J of Cancer. pp. 1326–1336. [DOI] [PubMed]
- Haley W. The family caregiver's role in Alzheimer's disease. Neurology. 1997;48:S25–29. doi: 10.1212/wnl.48.5_suppl_6.25s. [DOI] [PubMed] [Google Scholar]
- McMillan SC, Weitzner M. Quality of life in cancer patients: Use of a revised hospice index. Cancer Practice. 1998;6:282–288. doi: 10.1046/j.1523-5394.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative Performance Scale (PPS): A new tool. J of Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- SPSS SPSS 11.5 Brief Guide. Statistical Processing for the Social Sciences. New York, Prentice Hall, Inc. 2002.
- Zeppetella G. The palliation of dyspnea in terminal disease. Am J Hosp Palliat Care. 1998;15:332–30. doi: 10.1177/104990919801500606. [DOI] [PubMed] [Google Scholar]
- Tarzian AJ. Caring for dying patients who have air hunger. J Nurs Scholarsh. 2000;32:137–43. doi: 10.1111/j.1547-5069.2000.00137.x. [DOI] [PubMed] [Google Scholar]
- Sneeuw KC, Aaronson NK, Sprangers MA, Detmar SB, Wever LD, Schornagel JH. Comparison of patient and proxy EORTC QLQ-C30 ratings in assessing the quality of life of cancer patients. Journal of Clinical Epidemiology. 1998;51:617–31. doi: 10.1016/S0895-4356(98)00040-7. [DOI] [PubMed] [Google Scholar]


