Abstract
We have shown previously that either Grb2- or Shc-mediated signaling from the oncogenic Met receptor Tpr-Met is sufficient to trigger cell cycle progression in Xenopus oocytes. However, direct binding of these adaptors to Tpr-Met is dispensable, implying that another Met binding partner mediates these responses. In this study, we show that overexpression of Grb2-associated binder 1 (Gab1) promotes cell cycle progression when Tpr-Met is expressed at suboptimal levels. This response requires that Gab1 possess an intact Met-binding motif, the pleckstrin homology domain, and the binding sites for phosphatidylinositol 3-kinase and tyrosine phosphatase SHP-2, but not the Grb2 and CrkII/phospholipase Cγ binding sites. Importantly, we establish that Gab1-mediated signals are critical for cell cycle transition promoted by the oncogenic Met and fibroblast growth factor receptors, but not by progesterone, the natural inducer of cell cycle transition in Xenopus oocytes. Moreover, Gab1 is essential for Tpr-Met–mediated morphological transformation and proliferation of fibroblasts. This study provides the first evidence that Gab1 is a key binding partner of the Met receptor for induction of cell cycle progression, proliferation, and oncogenic morphological transformation. This study identifies Gab1 and its associated signaling partners as potential therapeutic targets to impair proliferation or transformation of cancer cells in human malignancies harboring a deregulated Met receptor.
INTRODUCTION
The hepatocyte growth factor (HGF)/scatter factor/Met receptor tyrosine kinase (RTK) is a cell surface receptor that was first identified as an oncoprotein, Tpr-Met (Cooper et al., 1984). This oncoprotein is the product of a chromosomal rearrangement fusing a leucine zipper dimerization domain to the Met cytoplasmic domain, resulting in a constitutively active cytosolic kinase in the absence of ligand (Park et al., 1986; Rodrigues and Park, 1993). Deregulation of Met receptor signaling has been implicated in a variety of human malignancies. For example, the Met receptor is overexpressed in many different types of human tumors, including breast, gastric, thyroid, and colorectal carcinomas and sarcomas, and point mutations have been identified in the Met receptor in both hereditary and sporadic papillary renal carcinomas as well as lung cancers (Jeffers et al., 1997; Birchmeier et al., 2003; Ma et al., 2003; Zhang and Vande Woude, 2003).
From studies in mice, the Met receptor has been implicated in several facets of embryogenesis, such as the growth and survival of epithelial cells, migration of myogenic and neuronal precursor cells, development of the kidney and mammary glands as well as liver and placenta formation (Bladt et al., 1995; Schmidt et al., 1995; Uehara et al., 1995). Moreover, the activation of the Met receptor promotes angiogenesis and wound healing in mice (Toyoda et al., 2001; Horiguchi et al., 2002), and evidence indicates a role in primitive hematopoiesis during early Xenopus development (Koibuchi et al., 2004). In cell culture systems, the HGF/Met receptor has been shown to affect many cellular functions, including mitogenesis of hepatocytes and myoblasts, cell scattering, invasion and branching tubulogenesis of epithelial cells as well as chemoattraction of motor neurons (for reviews, see Birchmeier et al., 2003; Zhang and Vande Woude, 2003).
The recruitment of signaling proteins and the biological activities of both the Met receptor and Tpr-Met oncoprotein are dependent on only two phosphotyrosine residues outside of the catalytic domain (pTyr-482 and -489 in Tpr-Met and pTyr-1349 and -1356 in Met). When phosphorylated, Tyr-1356 (Tyr-489 in Tpr-Met) provides a direct binding site for the Grb2 and Shc proteins (Fixman et al., 1996; Ponzetto et al., 1996). In turn, Grb2 acts to indirectly recruit the c-Cbl ubiquitin ligase and docking protein Gab1 (Weidner et al., 1996; Bardelli et al., 1997; Fixman et al., 1997; Nguyen et al., 1997). In addition, the docking protein Gab1 associates directly with the Met receptor through an interaction between the Met-binding motif (MBM) within Gab1 (13-amino acid proline-rich motif), and pTyr-1349 along with upstream residues of the Met receptor (pTyr-482 in Tpr-Met) (Lock et al., 2003). Importantly, regardless of its mode of association, Gab1 becomes phosphorylated on multiple tyrosine residues upon its recruitment by the Met receptor, providing binding sites for multiple signaling proteins, including the p85 subunit of phosphatidylinositol 3-kinase (PI3K), phospholipase Cγ (PLCγ), the Crk adaptor protein, and the tyrosine phosphatase SHP-2 (Garcia-Guzman et al., 1999; Maroun et al., 1999a; Gual et al., 2000; Lamorte et al., 2000).
Structure–function studies have revealed key roles for Grb2, Shc, and Gab1 adaptor proteins downstream of the Met receptor in various biological events, which, if not strictly coordinated, may contribute to the development and progression of cancers. Recruitment of the Grb2 or Shc adaptor proteins is required and sufficient for oncogenic transformation, anchorage-independent growth, and experimental metastasis by the Met receptor oncoprotein (Fixman et al., 1996; Ponzetto et al., 1996; Bardelli et al., 1999; Saucier et al., 2002). Shc also plays a critical role in promoting the production of vascular endothelial growth factor and early onset of tumor angiogenesis by the Met receptor (Saucier et al., 2004). Alternatively, the recruitment of the docking protein Gab1 is essential for Met receptor-mediated invasive branching morphogenesis of epithelial cells (Weidner et al., 1996; Nguyen et al., 1997; Maroun et al., 1999a).
Although the mitogenic effect of HGF is well documented (Birchmeier et al., 2003), little is known about the signals involved in the induction of cell cycle progression by the HGF/Met receptor. The Xenopus system is an extremely tractable system for dissecting the signaling networks implicated in cell cycle progression induced by proto-oncogenes and kinases. Significantly, many of the signaling networks implicated in cell cycle progression of Xenopus oocytes have been shown to be shared with those of somatic cell division (Ferrell, 1999; Nebreda and Ferby, 2000). In a recent study, we exploited this system to define which one of the many signaling pathways engaged downstream of the oncogenic Met receptor Tpr-Met is involved in cell cycle progression (Mood et al., 2006). Similar to the signal requirements for Met receptor-mediated cell transformation, we have shown that cell cycle progression as well as activation of MAPK and c-Jun NH2-terminal kinase (JNK) required both the kinase activity of Tpr-Met and an intact multidocking protein site (phosphotyrosine residues Y482 and Y489). Moreover, using Met oncoproteins engineered to recruit a signaling protein of choice (Saucier et al., 2002), we have established that although the recruitment of Grb2 or Shc is sufficient to induce cell cycle progression and the activation of MAPK and JNK, their recruitment to the oncogenic Met receptor is not essential for cell cycle progression (Mood et al., 2006). This study suggested that, in addition to Grb2 and Shc adaptor proteins, another proximal-binding protein of the Met receptor contributes to its ability to induce cell cycle progression and cell transformation.
In the present study, we establish for the first time a critical contribution of the Gab1-docking protein downstream of the oncogenic Met receptor Tpr-Met in cell cycle progression of Xenopus oocytes as well as proliferation and cellular transformation of fibroblast cells. By performing structure–function studies, we show that the direct interaction of Gab1 protein with the Tpr-Met oncoprotein is necessary and sufficient to promote cell cycle progression, and associated MAPK and JNK activation, whereas the recruitment of Gab1 via Grb2 is dispensable. Furthermore, we show that the Gab1 pleckstrin homology (PH) domain and the PI3K and SHP-2 binding sites in Gab1 are crucial for cell cycle progression mediated by Tpr-Met.
MATERIALS AND METHODS
Tpr-Met and Gab1 cDNA Constructs
Generation and characterization of the wild-type (wt) or mutants of Tpr-Met, signaling-specific variants as well as XFGFR1 and XFRS2 cDNA constructs were described previously (Fixman et al., 1996; Mood et al., 2002, 2006; Saucier et al., 2002). For expression in Xenopus oocytes, mouse Gab1 cDNAs were subcloned into the BamH1/EcoR1 site of the pCS2+ vector. For retroviral infection of mammalian cells, Gab1 C-terminal hemagglutinin (HA)-tagged cDNAs were produced by PCR, using as template the pCDNA 1.1 construct encompassing wt or mutant Gab1 mouse cDNAs (Maroun et al., 1999a, b, 2000; Lock et al., 2000, 2003; Lamorte et al., 2002a), and PCR products were subcloned into the BamH1/Ecor1 site of the pBabe retroviral vector. Primer sequences used were as follows: full-length Gab1 constructs: sense, 5′-GCTGGGATCCCCATGAGCGGCGG-3′ and antisense, 5′-ACTGGAATTCAGGGCCCAGCGTAATCTGGAACATCGTATGGGTACTTCACA-3′; Gab1ΔPH: sense, 5′-GCTGGGATCCACCATGGGATTCAATCCCACAGAAGAAGATCCTTCTT GGTGGGTGTCTCGG-3′ and antisense, same as above; and Met-binding domain (MBD) domain constructs: sense, 5′-GCTGGGATCCACCATGGACGAGAACTTCGTTCCCATGAACCCCAACTC-3′ and antisense, 5′-ACTGGAATTCTCAGGGCCCAGCGTAATCTGGAACATCGTATGGGTAGTC TAA AGGTGCCGGCTTGACTT-3′. The putative full-length Gab1 cDNA was cloned by PCR from a Xenopus oocyte cDNA library (a gift of Dr. Alan Wolfe, National Institutes of Health, Bethesda, MD) using the following primers: sense, 5′-ACGACGAGGAGCAG GATGATGAGC-3′ and antisense, 5′-TCATTTGATATTCTTG GTTGGAG-3′. Sequence analyses were performed using the MacVector 7.2 program (Accelrys, San Diego, CA).
RNA Injections in Frog Oocytes
Xenopus laevis females were purchased from Nasco (Fort Atkinson, WI) and prepared as described previously (Fabian et al., 1993). All capped mRNA was made using the SP6 mMessage mMachine kit as specified by the manufacturer (Ambion, Austin, TX). Microinjections of various RNAs, at the amounts indicated in the text or figures, were performed 18 h after oocyte isolation with a volume of 10–30 nl (Harvard Apparatus, Holliston, MA). Experiments using Gab1 mutants were performed by microinjecting mutant RNAs and culturing oocytes 4–18 h in 50% L-15 media with 1% bovine serum albumin before microinjection with Tpr-Met construct RNAs. For experiments using the wt fibroblast growth factor receptor (FGFR)1, oocytes were microinjected with mutant Gab1 RNAs, cultured 4 h, coinjected with wt FGFR1 and fibroblast receptor substrate-2 (FRS2) RNAs, and then cultured an additional 4 h before the addition of 200 ng/ml basic FGF. Oocytes were scored 16–20 h later for cell cycle progression as evidenced by the appearance of a white spot at the animal pole, a process know as germinal vesicle breakdown (GVBD), and this was verified in many cases by manual dissection of oocytes after fixation in 8% trichloroacetic acid.
Western Blot Analysis and Antibodies
The methodology for the preparation of lysates, SDS-PAGE electrophoresis, and Western blot analyses were described previously (Lock et al., 2003; Mood et al., 2006). Primary antibodies were used at the following dilutions: Met rabbit polyclonal at 1:2000 (Rodrigues et al., 1991); phospho-Met polyclonal to Y1234 and Y1235 (Upstate Biotechnology, Lake Placid, NY) at 1:2000; Gab1 (Upstate Biotechnology) at 1:1000; phospho-Gab1-Tyr627 (Abcam, Cambridge, MA) at 1:1000, phospho-Cdc2-Tyr15 (Upstate Biotechnology) at 1:2000; phospho-MAP kinase (Sigma-Aldrich, St. Louis, MO) at 1:5000; MAPK (extracellular signal-regulated kinase [ERK]2; Upstate Biotechnology) at 1:2000; phospho-JNK-Thr183/Tyr185 (Upstate Biotechnology) at 1:1000; JNK2 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:2000; horseradish-conjugated HA antibody (Roche Diagnostics, Indianapolis, IN) at 1:1000; α-tubulin (Sigma-Aldrich) at 1:5000; and phospho-Tyr (Upstate Biotechnology or BD Biosciences Transduction Laboratories, Lexington, KY) at 1:1000.
Cell Culture, Transfection, and Retroviral Infection
Fisher Rat 3T3 fibroblast (Fr3T3) and human epithelial kidney (HEK) 293 cells were cultured in DMEM containing 10% fetal bovine serum and 50 μg/ml gentamicin (Invitrogen, Carlsbad, CA). Transient transfections in HEK 293 cells were performed using Lipofectamine Plus reagent according to the manufacturer’s instructions (Invitrogen). Retroviral infection of Fr3T3 cells were performed as described previously (Fixman et al., 1996). After selection with 2 μg/ml puromycin for at least 10 d, >100 colonies were pooled to generate cell populations, or alternatively, individual resistant colonies were picked and expanded to establish stable cell lines. Phase contrast images were taken with a Zeiss Axiovision 135 microscope with a 10× objective (Carl Zeiss Canada, Toronto, Ontario, Canada) using Northern Eclipse version 6.0 (Empix Imaging, Mississauga, Ontario, Canada).
Proliferation Assays
Cells were seeded in triplicate at a density of 5 × 104/well in six-well plates, and the number of cells was subsequently counted daily. Growth curve analysis was performed using Prism version 3.0c (GraphPad Software, San Diego, CA).
RESULTS
Xenopus, Mouse, and Human Gab1 Proteins Are Structurally Conserved
To better understand the individual contributions to cell cycle progression that are mediated by signaling proteins recruited to the Met receptor tyrosine kinase, we previously used Tpr-Met variants lacking the ability to bind a specific associated protein or capable of directly recruiting one particular signaling protein (Saucier et al., 2002). This approach exploits the fact that recruitment of effectors by the Tpr-Met oncoprotein depends on Y482 and Y489. Tpr-Met mutants were generated where phenylalanine is substituted for Tyr-482 (Y482F), which is known to disrupt the direct recruitment of Gab1 (Lock et al., 2003), or by substitution of asparagine for histidine within the Grb2 binding site (YVNV; N491H; Figure 1) that causes the selective loss of direct Grb2 binding (Fixman et al., 1996; Ponzetto et al., 1996). Docking-specific variants were generated from the Y482F Tpr-Met mutant in which tyrosine Y489 along with surrounding amino acids (486-494) were replaced with amino acids corresponding to binding motifs specific for Shc or Grb2 adaptor proteins (Figure 1; Saucier et al., 2002).
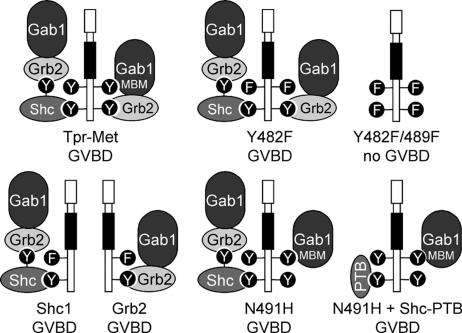
Figure 1.
Tpr-Met mutants that induce GVBD have the capacity to recruit Gab1. Diagram displays the mechanism by which each mutant of the Tpr-Met oncoprotein is predicted to recruit the Gab1 protein and whether these Tpr-Met oncoproteins induce GVBD (Mood et al., 2006): Tpr-Met, wt Tpr-Met oncoprotein, which interacts with Gab1 via direct and indirect Grb2-dependent mechanisms (Lock et al., 2003); Y482F, mutant of Tpr-Met where Tyr-482 is replaced with Phe (Fixman et al., 1996) and cannot bind directly to Gab1 but retains a Grb2-dependent means to associate with Gab1 (Lock et al., 2003); Y482/489F, mutant of Tpr-Met where Tyr-482 and Tyr-489 are replaced with Phe (Fixman et al., 1996), preventing direct and indirect interaction with Gab1; Shc1, Shc-specific docking Tpr-Met variant that interacts with Gab1 via a Grb2-dependent mechanism (Saucier et al., 2002); Grb2, Grb2-specific docking Tpr-Met variant that interacts indirectly with Gab1 (Saucier et al., 2002); N491H, mutant of Tpr-Met where Gln-491 is replaced with His, preventing direct binding of Grb2 (Fixman et al., 1996), but it can interact directly with Gab1 via phosphotyrosine Y482 (Lock et al., 2003); PTB, PTB domain of Shc, which when expressed in cells, blocks the association of Tpr-Met with the adaptor protein Shc (Mood et al., 2006); and Gab1-Grb2–associated binder 1 and MBM-Met receptor binding motif of Gab1 (Lock et al., 2003).
We have previously shown that the activation of either Grb2- or Shc-dependent signaling pathways by the oncogenic Met receptor Tpr-Met is sufficient to mediate cell cycle progression and concomitant activation of MAPK and JNK in Xenopus oocytes (Mood et al., 2006). However, the recruitment of Grb2 and Shc adaptor proteins to the oncogenic Met receptor is not essential for this process (Mood et al., 2006). This implies that another proximal binding partner of the Met receptor contributes to cell cycle progression.
The adaptor protein Gab1 has binding motifs for the recruitment of multiple signal transduction proteins, including the Crk adaptor, PLCγ, PI3K, and the SHP-2 tyrosine phosphatase (Gu and Neel, 2003). Thus, Gab1 amplifies and diversifies the signals downstream from receptors by assembling multiprotein complexes. We have previously shown that all Tpr-Met mutants and docking-specific variants able to induce oocyte maturation (Mood et al., 2006) have in common the capacity to recruit Gab1 (Figure 1), suggesting that a Gab1-like scaffolding protein is a potential key player in Tpr-Met–mediated cell cycle progression in Xenopus oocytes.
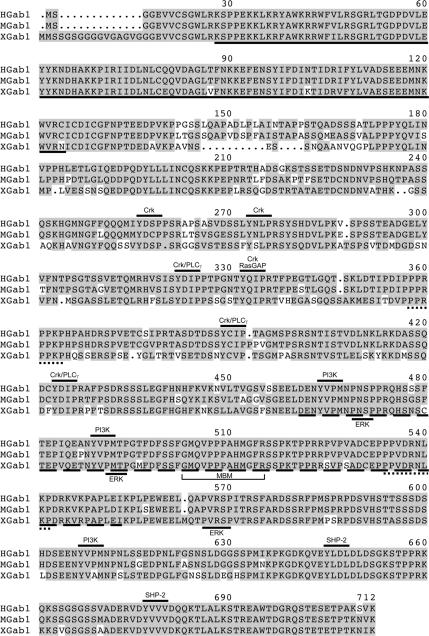
A Xenopus Gab1 cDNA clone (EMBL accession no. Q6AZI1) with a high degree of homology to the human and mouse Gab1 sequences has been previously identified (Klein et al., 2002), and we have isolated full-length Gab1 cDNA from a Xenopus oocyte library. Xenopus Gab1 (XGab1) displays 75 and 73% identity to the human and murine Gab1 protein sequences, respectively (Holgado-Madruga et al., 1996; Weidner et al., 1996) (Figure 2). Significantly, all of the functionally relevant tyrosine phosphorylation sites identified previously in mammalian Gab1 proteins (Gu and Neel, 2003) are conserved within the XGab1 protein (Figure 2), including those responsible for the binding of PI3K (Y443, Y468, and Y586), Crk (Y239, Y255, Y303, Y313, Y369, and Y402), PLCγ (Y303, Y369, and Y402), and SHP-2 (Y624 and Y656). Moreover, like the mammalian Gab1 protein, XGab1 contains an N-terminal PH domain (Maroun et al., 1999a), the two Grb2 carboxy-terminal SH3 domain binding sites (Lock et al., 2000) as well as the MBD (aa 440-532) that encompasses the 13-amino acid sequence corresponding to the MBD (aa 482-494) responsible for the direct recruitment of Gab1 to the Met receptor (Lock et al., 2003). Notably, the mammalian Gab1 antibody detects a protein of appropriate size in uninjected Xenopus oocyte extracts (Figure 3A). These data suggest that Xenopus oocytes express a form of Gab1 that possesses all of the appropriate structural features for signaling downstream of the oncogenic Met receptor.
Figure 2.
Xenopus, mouse, and human Gab1 proteins are structurally conserved. The putative full-length Gab1 cDNA was cloned by PCR from a Xenopus oocyte cDNA library. The alignment of XGab1 amino acid sequences with those of the human and mouse Gab1 proteins is shown (Holgado-Madruga et al., 1996; Weidner et al., 1996). Conserved residues are highlighted in gray; the PH domain and MBD are denoted by a full or dashed underline, respectively. Lines above the sequences indicate the conserved PI3K, Crk, PLCγ, RasGAP, or SHP-2 binding sites. Potential ERK phosphorylation sites are denoted by lines below the sequences. The Grb2 carboxy-terminal SH3 domain binding sites and the MBM are marked by underbracket and a dotted underline, respectively.
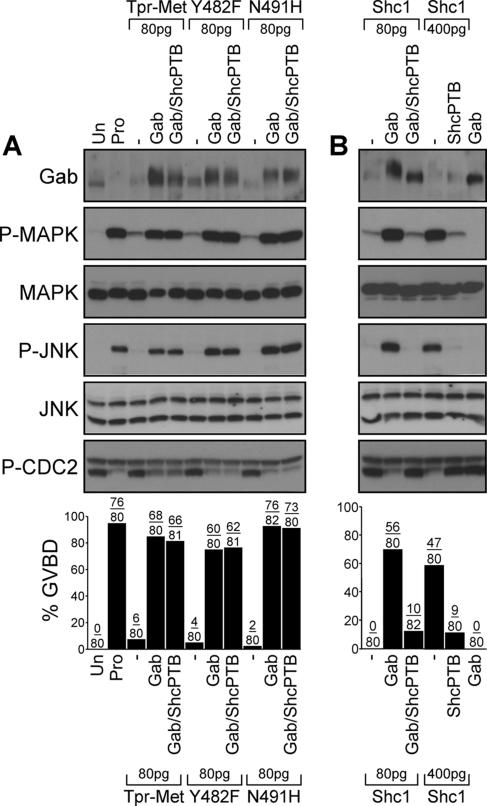
Figure 3.
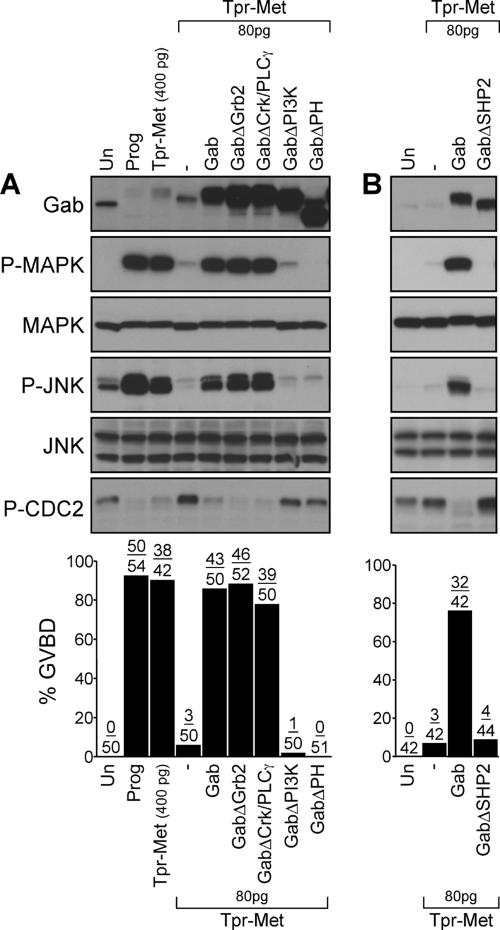
Gab1 is sufficient to mediate cell cycle progression and activation of MAPK and JNK by low levels of the Tpr-Met oncoprotein. (A) Oocytes were injected with suboptimal levels of Tpr-Met, Y482F, or N491H RNA (80 pg/oocyte) in the presence or absence of Gab1 (400 pg/oocyte) and ShcPTB (400 pg/oocyte). Oocytes left uninjected or treated with 5 μM progesterone were used, respectively, as negative and positive controls for induction of GVBD. (B) Oocytes were injected with low (80 pg/oocyte) or high (400 pg/oocyte) concentrations of the Tpr-Met Shc-specific docking variant RNA in the presence or absence of Gab1 (400 pg/oocyte), or ShcPTB (400 pg/oocyte) RNA. Histograms show the percentage of oocytes displaying GVBD, and the number of oocytes with GVBD over the number of oocytes injected is indicated above bars. Lysates prepared from oocytes described in A and B were analyzed by immunoblot conducted with specific antibodies raised against Gab1, phospho-MAPK, MAPK, phospho-JNK, JNK1/2, or phospho-tyrosine15-Cdc2. These data are representative of three independent experiments. Un, uninjected; Pro, treated with 5 μM progesterone; Gab, mouse Gab1; Shc1, the Tpr-Met Shc-specific docking variant (Saucier et al., 2002); and ShcPTB, Myc-tagged Shc PTB domain (Mood et al., 2006).
Overexpression of Gab1 Promotes Cell Cycle Progression as Well as Activation of MAPK and JNK in the Presence of Suboptimal Levels of the Tpr-Met Oncoprotein
We have shown previously in stage VI oocytes that expression of the Met receptor oncoprotein, Tpr-Met, induces cell cycle progression, as evidenced by a dose-dependent induction of GVBD (Mood et al., 2006). To define whether Gab1-dependent signals may lie downstream of Tpr-Met for cell cycle progression, we designed a signal amplification strategy where the ability of exogenous mouse Gab1 to induce cell cycle progression was evaluated under conditions where Tpr-Met was expressed at suboptimal levels for induction of cell cycle progression (Mood et al., 2006). Only 8% of oocytes injected with 80 pg of Tpr-Met RNA underwent GVBD, whereas none of the oocytes injected with 14 ng of Gab1 RNA progressed through the cell cycle (Figure 3, A and B). However, GVBD was observed in 85% of the oocytes coinjected with Gab1 and Tpr-Met RNA (14 ng and 80 pg, respectively; Figure 3A). Similarly, GVBD was induced when Gab1 was coexpressed (Figure 3A) with low levels of the Tpr-Met mutants that either associate with Gab1 indirectly through Grb2 (Y482F; Figure 1) or directly via Y482 (N491H; Figure 1).
The Shc adaptor protein binds through its PTB domain to Tyr-489 of Tpr-Met (Saucier et al., 2002), an association that is not abrogated in the context of the Y482F or N491H Tpr-Met mutants (Fournier et al., 1996; Saucier et al., 2002), which may lead to the engagement of Gab1 (Figure 1). To determine whether the association of Shc with Tpr-Met contributes to the induction of GVBD mediated by Gab1, we tested the effect of the ShcPTB domain that binds Tyr-489 and blocks the direct interaction of Shc with Tpr-Met (Saucier et al., 2002; Mood et al., 2006). In oocytes injected with an optimal level of the Shc-specific docking variant of Tpr-Met (400 pg/oocyte), GVBD was efficiently suppressed in the presence of the ShcPTB domain (Figure 3B). Consistent with Gab1 being recruited through Shc, the expression of Gab1 mediates cell cycle progression in the presence of low levels of the Shc specific docking variant of Tpr-Met, and this event is blocked in the presence of the ShcPTB domain (Figure 3B). However, abrogating Shc recruitment had no effect on the ability of Gab1 to restore Tpr-Met-induced cell cycle progression as well as GVBD mediated by the Y482F or N491H Tpr-Met mutants (Figure 3A).
The induction of GVBD was confirmed by detection of tyrosine phosphorylation of Cdc2 at position 15 (Figure 3, A and B), an inhibitory phosphorylation site that displays an inverse correlation with GVBD (Pickham et al., 1992). Moreover, when Gab1 was expressed in oocytes in the presence of low levels of Tpr-Met, Y482F, or N491H as well as the Shc- specific docking oncoprotein, a corresponding activation of MAPK and JNK was observed (Figure 3, A and B). As expected, the ShcPTB domain blocked MAPK and JNK phosphorylation in the presence of high levels of the Shc-specific docking mutant (400 pg; Figure 3B), but not in the presence of Tpr-Met, Y482F, or N491H (Figure 3A). Together, these data support the role of Gab1 as a downstream mediator of the oncogenic Met receptor for induction of cell cycle progression, and activation of MAPK and JNK, and that neither the engagement of Shc-dependent signals nor the Grb2-dependent recruitment of Gab1 protein to the Met receptor is essential for the induction of these responses.
Direct Recruitment of Gab1 Is Required for Induction of Cell Cycle Progression in the Presence of Suboptimal Levels of the Tpr-Met Oncoprotein
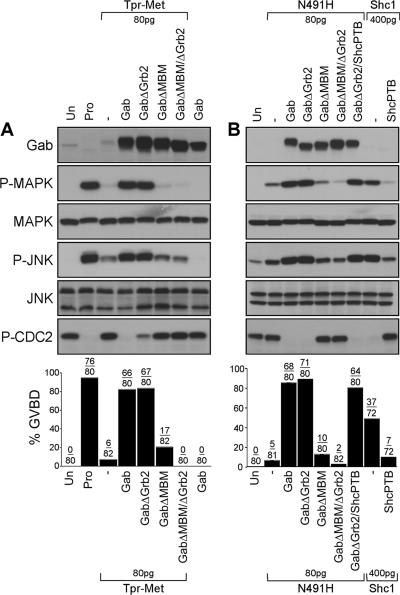
To formally assess the role of the direct or indirect interaction of Gab1 with the Met receptor for cell cycle progression, we tested the ability of Gab1 mutants either lacking the Grb2-binding sites (GabΔGrb2) or the MBM (GabΔMBM), or both (GabΔGrb2ΔMBM) to induce GVBD in the presence of suboptimal levels of either the wt Tpr-Met (Figure 4A) or the Tpr-Met N491H mutant specifically devoid of its Grb2 binding site (Figure 4B). We observed that the Gab1ΔGrb2 mutant efficiently promotes cell cycle progression in the presence of either wt Tpr-Met or the Tpr-Met N491H mutant (Figure 4, A and B). Furthermore, the ability of the Gab1ΔGrb2 mutant to mediate Tpr-Met N491H-induced GVBD was not impaired in the presence of the Shc-PTB domain (Figure 4B), supporting the dispensability of Shc-dependent signals for the induction of these responses. In contrast, Gab1-mediation of GVBD responses by wt Tpr-Met or the Tpr-Met N491H mutant, was severely impaired with the Gab1ΔMBM mutant and completely abrogated for the mutant of Gab1 lacking both the Grb2 and MBM binding motifs (Figure 4, A and B). In a similar manner to wt Gab1, the coexpression of Gab1ΔGrb2 with either the wt Tpr-Met or N491H mutant enhances Cdc2 dephosphorylation, and MAPK and JNK activation (Figure 4, A and B). In contrast, this activation was considerably reduced with Gab1 mutants lacking the MBM, or lacking both the MBM and Grb2 binding sites (Figure 4, A and B). These data establish that Grb2-dependent recruitment of Gab1 to the Met receptor complex is dispensable for the induction of cell cycle progression in Xenopus oocytes and that the direct interaction of Gab1 with the Met receptor is both necessary and sufficient for the induction of cell cycle progression as well as activation of MAPK and JNK.
Figure 4.
The Grb2 binding site of Gab1, but not the MBM, is dispensable for the induction of cell cycle progression and activation of MAPK and JNK by the Tpr-Met oncoprotein. (A) Suboptimal levels of Tpr-Met RNA (80 pg/oocyte) were injected in oocytes in the presence or absence of RNAs encoding wt or mutant forms of Gab1 (400 pg/oocyte). Oocytes left uninjected or treated with 5 μM progesterone were used, respectively, as negative and positive controls for GVBD. (B) Oocytes were injected with low levels of the N491H Tpr-Met mutant RNA (80 pg/oocyte) in the presence or absence of the wt or mutant forms of Gab1 RNAs (400 pg/oocyte), or they were singly injected with optimal concentrations of the Tpr-Met Shc-specific docking variant RNA (400 pg/oocyte), in the presence or absence of the dominant-negative ShcPTB RNA (400 pg/oocyte). These data are representative of three independent experiments, and the number of oocytes displaying GVBD over the number of oocytes injected is shown as a percentage in histograms. Lysates prepared from oocytes described above were analyzed by immunoblot conducted with specific antibodies raised against Gab1, phospho-MAPK, MAPK, phospho-JNK, or JNK1/2 or phospho-tyrosine15-Cdc2. GabΔGrb2, mutant of Gab1 with deletion of proline-rich domains (Pro4, aa 337-346/Pro5, aa 517-522) required for Grb2 interaction (Lock et al., 2000); GabΔMBM, mutant of Gab1 where Arg-491 is substituted with Ala, disrupting the MBM essential for direct interaction with the Met receptor (Lock et al., 2003); and GabΔMBMΔGrb2, mutant of Gab1 lacking both the Grb2 binding sites and MBM (Lock et al., 2003).
Gab1 Requires an Intact PH Domain and PI3K and SHP-2 Binding Sites to Rescue Cell Cycle Progression in the Presence of Suboptimal Levels of Tpr-Met
To define which of the Gab1-dependent signals are required for Tpr-Met–induced cell cycle progression, and activation of MAPK and JNK, we tested the ability of various Gab1 mutants to mediate these responses in the presence of suboptimal levels of Tpr-Met (Figure 5). GVBD is induced by coexpression of Tpr-Met with Gab1 mutants lacking the interaction sites for either Grb2 or Crk/PLCγ (Figure 5A). Consistent with this, a decrease in the level of Cdc2 phosphorylation was detected by Western analysis, and MAPK and JNK phosphorylation was induced to levels comparable with that observed with wt Gab1 (Figure 5A). In contrast, a Gab1 mutant lacking the PH domain, or the PI3K (Figure 5A) or SHP-2 interaction sites (Figure 5B), fails to promote Tpr-Met–mediated cell cycle progression as well as MAPK and JNK activation. These data demonstrate that the PH domain and the PI3K and SHP-2 binding sites in Gab1 are essential for the ability of Gab1 to rescue cell cycle progression and for activation of MAPK and JNK mediated by Tpr-Met.
Figure 5.
Cell cycle progression mediated by Gab1 requires an intact PH domain, PI3K and SHP-2 binding sites, but not the Grb2 or Crk/PLCγ binding sites. (A and B) Suboptimal levels of Tpr-Met RNA (80 pg/oocyte) were injected in oocytes in the presence or absence of wt or mutant forms of Gab1 RNAs (400 pg/oocyte). Oocytes left uninjected or treated with 5 μM progesterone were used as negative and positive controls for GVBD, respectively. The histograms represent the number of oocytes displaying GVBD over the number of oocytes injected. The data are representative of three independent experiments. Oocyte lysates were analyzed by immunoblot conducted with specific antibodies raised against Gab1, phospho-MAPK, MAPK, phospho-JNK, JNK1/2 or phospho-tyrosine15-Cdc2. GabΔCrkΔPLCγ, mutant of Gab1 where Tyr-242, -265, -307, -317, -373, and -404 are replaced with Phe, preventing the binding of Crk and PLCγ (Lamorte et al., 2000); GabΔPH, mutant of Gab1 deleted of amino acids 1-115 encoding the PH domain (Maroun et al., 1999a); GabΔPI3K, mutant of Gab1 where Tyr-449, -474, and -591 are replaced with Phe, preventing the recruitment of PI3K (Holgado-Madruga et al., 1997); and GabΔSHP2, mutant of Gab1 where Tyr-659 is substituted with Phe, disrupting the binding of the tyrosine SHP-2 (Maroun et al., 2000).
Gab1-dependent Signals Are Required for Cell Cycle Progression and Activation of MAPK and JNK Mediated by Optimal Levels of the Tpr-Met Oncoprotein
When Tpr-Met is expressed at suboptimal levels in Xenopus oocytes, exogenous mouse Gab1 protein promotes cell cycle progression as well as MAPK and JNK activation, establishing that Gab1 is a downstream effector of the oncogenic Met receptor for induction of these responses. However, it was still unclear whether signals engaged by the endogenous XGab1 protein were essential for the induction of cell cycle progression promoted by robust expression of Tpr-Met oncoprotein.
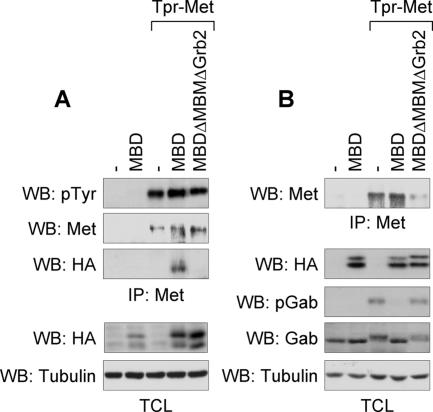
Overexpression of the MBD of Gab1 in epithelial cells inhibits HGF-induced scattering and branching morphogenesis (Weidner et al., 1996). Given that the MBD encompasses one Grb2 SH3 binding site and the MBM responsible for the direct interaction of Gab1 with the Met receptor (Lock et al., 2000, 2003), the dominant-negative effect of the MBD of Gab1 on Met-induced responses may reflect a block in the engagement of endogenous Gab1 by the Met receptor. Consistent with this, Tpr-Met and HA-tagged MBD of the Gab1 protein are present in an immune complex when coexpressed in HEK 293 cells, but not when Tpr-Met was cotransfected with an MBD of Gab1 lacking both the MBM and Grb2 interaction site (Figure 6A). Significantly, Tpr-Met–induced Gab1 phosphorylation, which is associated with Gab1 signaling functions, was potently inhibited by expression of the MBD of Gab1 (Figure 6B).
Figure 6.
The Gab1 MBD interacts with Tpr-Met and impairs Gab1 phosphorylation. (A and B) Independent transient transfection of HEK 293 cells with Tpr-Met or/and HA-tagged wt MBD of Gab1, or a mutant of lacking the Grb2- and Met-binding motifs. (A) Top, Tpr-Met proteins were immunoprecipitated and subsequently blotted with phosphotyrosine antibody to detect phosphorylated Tpr-Met and then stripped and reblotted for detection of Tpr-Met using antibody 144, or blotted with HA for detection of the MBD proteins. Bottom, Western analyses of total cell lysates (TCL) show the levels of HA-tagged MBD or tubulin expression. (B) Top, Tpr-Met proteins were immunoprecipitated and blotted with Tpr-Met–specific antibody. Bottom, Western analyses of TCL show the expression level of HA-tagged MBD, phospho-Gab1, Gab1 proteins, or tubulin.
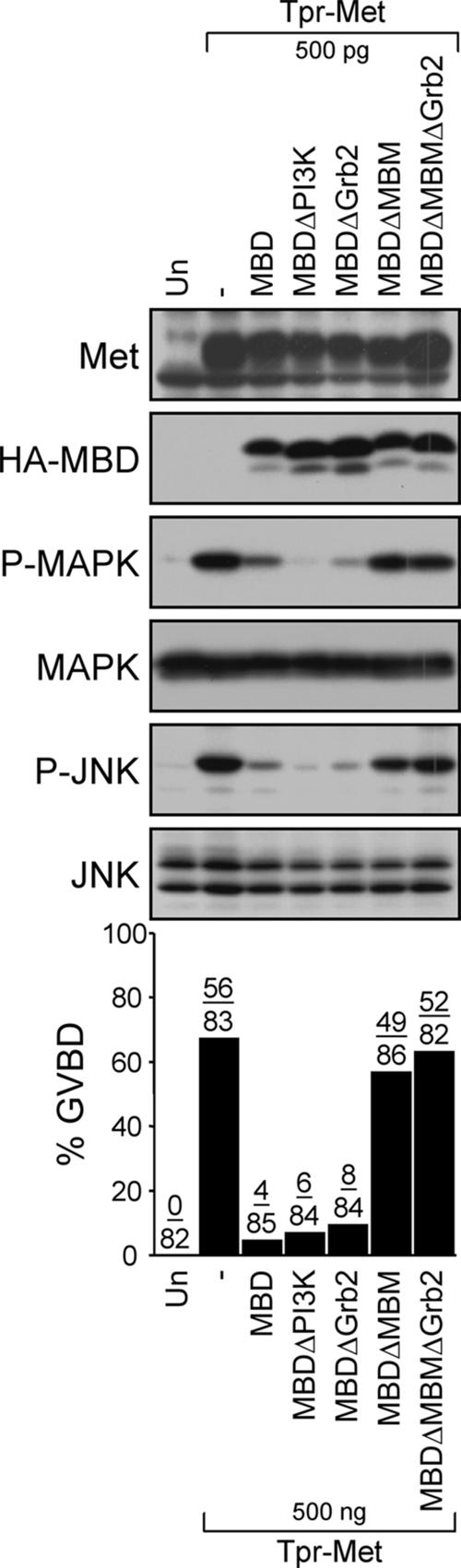
Therefore, to define whether Gab1 is required for Tpr-Met–induced cell cycle progression, and activation of MAPK and JNK in Xenopus oocytes, we examined whether expression of the Gab1 MBD can block these Tpr-Met–mediated responses. Expression of the HA-tagged MBD of Gab1 considerably represses GVBD induced by optimal expression of the Tpr-Met oncoprotein in oocytes (500 pg of RNA; Figure 7). Similar repression of GVBD is observed when a Gab1-MBD mutant lacking the PI3K or the Grb2 binding sites (MBDΔPI3K or MBDΔGrb2; Figure 7) is expressed, suggesting that the dominant-negative effect is independent of PI3K or Grb2 binding. Importantly, expression of a Gab1-MBD in which the MBM is deleted (MBDΔMBM or MBDΔMBMΔGrb2) is unable to block GVBD promoted by Tpr-Met (Figure 7). A strong correlation between GVBD and the activation of MAPK and JNK was also observed (Figure 7). These data demonstrate that cell cycle progression and activation of MAPK and JNK induced by the oncogenic Met receptor require the engagement of Gab1-dependent signaling pathways.
Figure 7.
The Gab1 MBD represses cell cycle progression mediated by the oncogenic Met receptor. Oocytes were injected with optimal levels of Tpr-Met RNA (500 pg/oocyte) in the presence or absence of RNAs encoding wt or mutants forms of the Gab1 MBD (14 ng/oocyte). Histograms show the percentage of oocytes displaying GVBD, and the number of oocytes with visible GVBD over the number of oocytes injected is indicated above histogram bars. The data are representative of three independent experiments. Lysates prepared from oocytes were analyzed by immunoblot conducted with antibodies raised against the Met receptor, HA to detect HA-tagged MBD, phospho-MAPK, MAPK, phospho-JNK, or JNK1/2. MBD, wt, MBD of Gab1; MBDΔPI3K, mutant of the Gab1 MBD lacking the PI3K binding sites; MBDΔGrb2, mutant of the Gab1 MBD lacking Grb2 interaction site; MBDΔMBM, mutant of the Gab1 MBD lacking the MBM; and MBDΔMBMΔGrb2, mutant of the Gab1 MBD lacking the Grb2 and Met receptor binding sites.
Gab1 MBD Represses GVBD Induced by the FGF Receptor, but Not by Progesterone
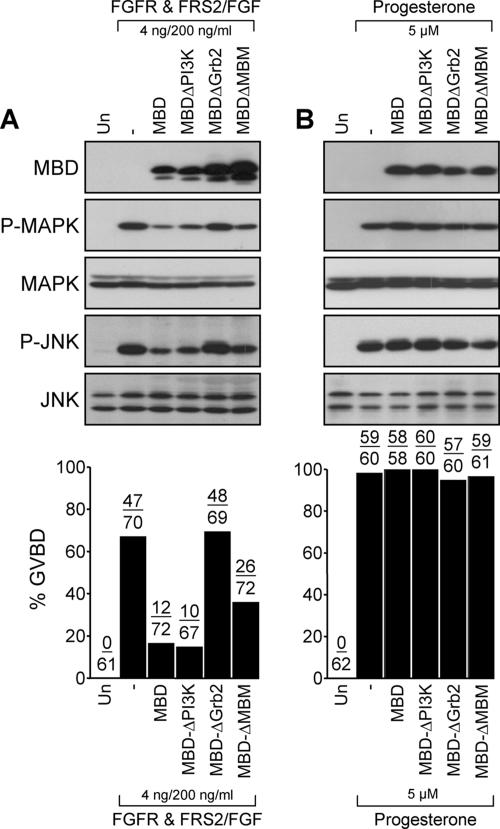
As demonstrated for the Met receptor, the docking protein Gab1 is also an essential functional component of the FGFR signaling cascade that serves in the assembly of multiprotein complexes with the receptor (Lamothe et al., 2004). In contrast to the Met receptor, which can directly interact with Gab1 (Lock et al., 2003), the FGFR recruits Gab1 indirectly, via the binding of Grb2 to another scaffold protein known as FRS2 (Lamothe et al., 2004). Activation of the FGFR1 induces cell cycle progression, and activation of MAPK and JNK in Xenopus oocytes, which has been shown to be dependent on FRS2 and Grb2 (Browaeys-Poly et al., 2000; Carballada et al., 2001; Mood et al., 2002). To define the requirement of Gab1-dependent signals in the FGFR1-induced cell cycle transition, RNAs encoding FGFR1 and FRS2 were injected into oocytes in the presence or absence of the MBD of Gab1, and these oocytes were subsequently stimulated with 200 ng/ml basic FGF. Similar to Tpr-Met–induced responses (Figure 7), expression of the wt MBD of Gab1, or a mutant lacking the two PI3K binding sites, greatly represses FGFR-mediated GVBD and activation of MAPK and JNK (Figure 8A). The expression of an MBD mutant lacking the Grb2 binding site does not inhibit FGFR-mediated GVBD or activation of MAPK and JNK (Figure 8A), consistent with the Gab1 and FGFR interaction being mediated by Grb2-dependent mechanisms (Lamothe et al., 2004). Unexpectedly, the Gab1 MBDΔMBM only partially prevents oocytes from undergoing GVBD upon activation of FGFR (Figure 8A). These results show that Gab1 plays an important role in FGFR-induced cell cycle progression and support a major contribution for the Grb2-dependent mechanism for the engagement of Gab1 downstream of the FGFR in these processes. Importantly, both cell cycle progression and activation of MAPK or JNK triggered by treatment of the oocytes with the natural inducer progesterone (5 μM) are not effected by the expressing the wt MBD of Gab1 or any of the MBD mutants (Figure 8B). These data support the specificity of the MBD domain toward the Met and FGF receptors and show that Gab1-dependent signals are not required for progesterone-mediated cell cycle progression, and for activation of MAPK or JNK.
Figure 8.
The MBD of Gab1 represses FGFR1-mediated cell cycle, but not GVBD induced by progesterone. (A) Oocytes injected with FGFR1 and FRS2 mRNAs (4 ng each/oocyte) in the presence or absence of wt or mutant of MBD of Gab1 mRNAs (14 ng/oocyte) were stimulated with 200 ng/ml human recombinant basic FGF. (B) Oocytes left uninjected or injected with wt or mutant MBD mRNAs (14 ng/oocyte) were stimulated with 5 μM progesterone. Histograms represent the number of oocytes with visible GVBD over the number of oocytes injected and shown as percentage of GVBD. The data are representative of three independent experiments. Lysates prepared from oocytes were analyzed by immunoblot conducted with specific antibodies raised against HA, to detect HA-tagged MBD, phospho-MAPK, MAPK, phospho-JNK, or JNK1/2.
Gab1 MBD Inhibits Cell Transformation of Tpr-Met–expressing Fibroblasts
Oncogenic transformation of fibroblast cells is associated with drastic morphological changes that allow cells to progress through the phases of the cell cycle and to continue to proliferate even upon cell–cell contact. The signaling requirements for Tpr-Met–mediated cell cycle progression in Xenopus oocytes are remarkably similar to those implicated in fibroblast cell transformation. In addition, induction of cell cycle transition and cell transformation by the oncogenic Met receptor is associated with a concomitant activation of PI3K, MAPK, and JNK signaling pathways, all of which can be activated downstream of the Met receptor via Gab1 (Rodrigues et al., 1997; Lamorte et al., 2000; Mood et al., 2006). This suggests that the Tpr-Met oncoprotein may promote oncogenic transformation through the engagement of Gab1.
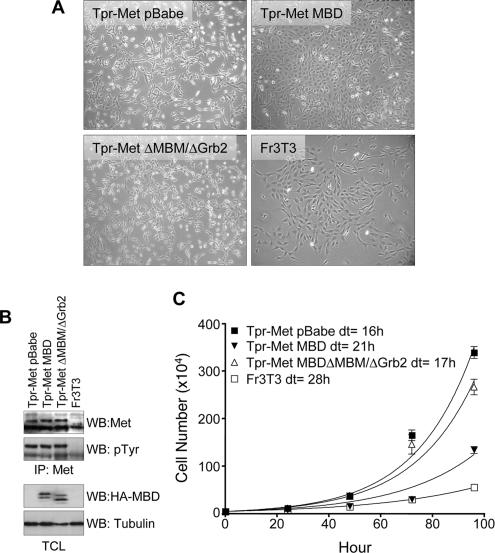
To address whether Gab1 may be essential for Tpr-Met–induced morphological transformation, Tpr-Met–expressing fibroblasts (Fr3T3) were infected with retroviruses encoding the wt MBD of Gab1, with an MBD mutant lacking both MBM and Grb2 binding sites, or with empty pBabe vector. We observed that Tpr-Met–expressing fibroblasts infected with a Gab1 MBD lacking both the MBM and Grb2 motifs (ΔMBM/ΔGrb2) remained refractile, spindle shaped, and grew in a disorganized manner, a typical morphology for Tpr-Met–transformed fibroblasts (Figure 9A). In contrast, the transformed morphology of Tpr-Met–expressing cells was dramatically reversed in cells infected with the wt MBD of Gab1, as evidenced by reduced refractility and a flatter, more organized growth pattern and morphology (Figure 9A). Stable cell lines expressing Tpr-Met and the Gab1 MBD proteins were isolated to perform cell proliferation assays (Figure 9, B and C). We observed that Tpr-Met–transformed cells coexpressing wt MBD of Gab1 grow at a considerably slower rate (doubling time of 21 h), compared with those expressing the empty vector (doubling time of 16 h), or those coexpressing the Gab1 MBD lacking the MBM and Grb2 binding sites (doubling time of 17 h; Figure 9C). These data provide the first evidence that engagement of Gab1-dependent signals is essential for induction of proliferation and oncogenic morphological transformation promoted by the oncogenic Met receptor in fibroblasts.
Figure 9.
The MBD of Gab1 is sufficient to reverse Tpr-Met–mediated cell transformation and proliferation. (A) Representative morphology of parental Fr3T3 or Tpr-Met–expressing Fr3T3 fibroblast cells infected with retroviruses encoding the indicated Gab1 MBD constructs. Photographs were taken at a 10× magnification after retroviral infection of Tpr-Met–expressing cells with the Gab1 MBD, the MBDΔMBMΔGrb2, or empty vector, 2 wk after selection with 2 μg/ml puromycin. (B) Expression levels of the MBD or Tpr-Met protein in Fr3T3 stable cell lines. Top, Tpr-Met proteins were immunoprecipitated from lysates of Fr3T3 stable cell lines expressing the HA-tagged MBD and Tpr-Met. The expression of Tpr-Met protein and its level of phosphorylation on tyrosine residues are shown. Bottom, level of MBD and tubulin proteins expressed in each cell line by Western analyses conducted with HA or tubulin-specific antibodies. (C) Cell proliferation assays were performed with the indicated stable cell lines. Data on the graph represents the number of cells ± the mean SD (n = 3) over time (hour), and the doubling time was estimated from the curves. Similar results were obtained with other independent cell lines.
DISCUSSION
In this study, we identify for the first time a critical role for Gab1 in cell cycle progression, proliferation, and cell transformation downstream of the Met receptor. We show that overexpression of Gab1 in Xenopus oocytes dramatically enhances the ability of suboptimal levels of Tpr-Met to induce cell cycle progression and the concomitant activation of MAPK and JNK (Figure 3). In contrast, the expression of the MBD of Gab1 acts in a dominant-negative manner and blocks Tpr-Met–induced cell cycle progression (Figure 7). We demonstrate that the engagement of Gab1-dependent signals is essential for cell cycle transition and the activation of MAPK and JNK promoted by the Met and FGF receptors, but not upon activation of the progesterone receptor, the natural mediator of GVBD in Xenopus oocytes (Figures 7 and 8). Importantly, we show that Gab1-dependent signals are required for morphological transformation and proliferation of mammalian cells downstream of the Tpr-Met oncoprotein (Figure 9).
Structure–function analyses show that either the direct binding of Gab1 to the oncogenic Met receptor or the indirect recruitment of Gab1 via the Grb2 adaptor protein is sufficient to induce cell cycle progression in Xenopus oocytes. For example, overexpression of either wt Gab1 or a mutant of Gab1 devoid of Grb2 binding sites (GabΔGrb2), promotes cell cycle progression in the presence of suboptimal levels of the wt Tpr-Met (Figure 4). Similar results are observed with the N491H Tpr-Met mutant that lacks the Grb2 binding site but retains Y482 required for direct recruitment of Gab1 through the Gab1 MBM (Lock et al., 2003), demonstrating that the direct recruitment of Gab1 through the MBM is sufficient to mediate GVBD. Conversely, we have shown that Tpr-Met docking mutants that specifically bind Grb2 or Shc adaptor proteins, and thus recruit Gab indirectly, promote cell cycle progression (Mood et al., 2006). In agreement with these data, overexpression of Gab1 mediates cell cycle progression in the presence of suboptimal levels of Y482F Tpr-Met (Figure 3), a mutant that lacks the direct Gab1 interaction site (Y482) but retains the ability to recruit Gab1 indirectly via either Grb2 or Shc, binding to Y489 in Tpr-Met (Lock et al., 2003). Interestingly, deletion of the MBM in Gab1 impairs its ability to mediate cell cycle progression downstream of Tpr-Met (Figure 4), under conditions where Gab1 is recruited indirectly to Met via Grb2 (Lock et al., 2003). Considering that deletion of the MBM does not hinder the association of Grb2 with the MBD of Gab1 (our unpublished data), this suggests that the MBM of Gab1 may have additional functions in Gab1 signaling beyond its direct interaction with the Met receptor.
Gab1 is an essential mediator of the Met receptor-induced epithelial morphogenetic program; a biological process that requires coordinated epithelial cell proliferation, adhesion, migration, and invasion (Pollack et al., 1998). Given the importance of cell proliferation during epithelial morphogenesis, our finding that Gab1 plays a critical role in cell cycle progression and proliferation downstream of the Met receptor (Figures 7 and 9) suggests that Gab1 is a key modulator of cell proliferation during Met receptor epithelial morphogenesis. Consistent with this concept, the morphogenetic defect resulting from expression of a Met receptor mutant devoid of the direct Grb2 binding site (N1358H) is rescued by overexpression of wt Gab1 or a Gab1 mutant lacking the Grb2 interaction sites. In contrast, a Gab1 mutant lacking the MBM fails to rescue the defect (Lock et al., 2002, 2003), and an intact MBM in Gab1 is essential for Met-mediated cell cycle progression in Xenopus oocytes (Figure 4). Moreover, overexpression of the MBD domain prevents both oncogenic Met receptor-induced cell cycle progression (Figure 7) and HGF/Met receptor epithelial morphogenesis (Weidner et al., 1996).
By structure–function analyses, we show that the ability of Gab1 to promote cell cycle progression downstream of Tpr-Met is dependent on the Gab1 PH domain, and the association of Gab1 with the tyrosine phosphatase SHP-2 and with PI3K, whereas the recruitment of Crk and PLCγ signaling molecules is dispensable (Figure 5). Thus, many of the identified signaling requirements for induction of cell cycle progression by the oncogenic Met receptor are also necessary for Met-induced cell dispersal or epithelial morphogenesis, including a requirement for the PH domain and SHP-2 binding sites in Gab1 (Figure 5), and activation of MAPK or PI3K (Maroun et al., 1999a, 2000; Yu et al., 2003; Mood et al., 2006).
The association of Gab1 with the tyrosine phosphatase SHP-2 is required for sustained ERK activation and for epithelial morphogenesis downstream from the Met receptor and may act upstream of Ras (Maroun et al., 2000; Cunnick et al., 2002). A Gab1 mutant lacking the ability to bind SHP-2 fails to activate MAPK and cell cycle progression downstream of the Tpr-Met (Figure 5), indicating that activation of MAPK may occur predominantly through the Gab1/SHP-2 protein complex during cell cycle progression in oocytes.
Tpr-Met induces activation of Ras, an upstream activator of Raf1, and we have shown that activation of MAPK and cell cycle progression induced by Tpr-Met are dependent on Raf kinase (Mood et al., 2006). This implies that activation of MAPK and cell cycle progression mediated by Tpr-Met may also require Ras activation, likely via the recruitment of an exchange factor for Ras. This may occur through the recruitment of both Grb2/Sos and Gab1/Crk/C3G proteins to a complex with Tpr-Met, because a Gab1 mutant lacking the Grb2 binding sites maintains the ability to enhance cell cycle transition downstream of a Tpr-Met mutant lacking the direct Grb2 binding site, even in the presence of the Shc-PTB domain (Figure 4B). Moreover, the Crk binding sites in Gab1 are dispensable for the induction of cell cycle progression mediated by the Tpr-Met (Figure 5), suggesting that direct binding of Crk alone to Gab1 is not essential for the process.
The Gab1 PH domain has been shown to bind phosphatidylinositol-3,4,5-trisphosphate (PIP3), the lipid products of PI3K, and to mediate the recruitment Gab1 and Gab1-associated protein complexes to PIP3-rich microdomains (Maroun et al., 1999b). This recruitment may be critical for Met-induced cell cycle progression and for the enhanced activation of PI3K by membrane associated Gab1. PI3K activity is required for cell proliferation induced by a variety of mitogens, but evidence suggests that the activation of PI3K is not sufficient for cell cycle progression (Katso et al., 2001) and as demonstrated for the oncogenic Met receptor (Mood et al., 2006). Indeed, PI3K may act in conjunction with MAPK signaling pathways to stimulate cell cycle progression and proliferation (Katso et al., 2001). The function of the PH domain and PI3K binding of Gab1 therefore may be to localize the Tpr-Met/Gab1/SHP-2 protein complex to a particular membrane compartment, where Gab1-dependent recruitment of SHP-2 leads to the sustained activation of MAPK signaling pathways.
We observed that the binding of Crk/PLCγ to Gab1 is required to rescue Met receptor-induced branching morphogenesis (Lamorte et al., 2002b), and it has been shown that inhibition of PLCγ activity prevents HGF-mediated epithelial branching morphogenesis (Gual et al., 2000), but it does not preclude cell cycle progression induced by Tpr-Met (Mood et al., 2006). The distinct signaling requirement observed downstream of Gab1 for cell cycle progression and branching morphogenesis suggests that Gab1 may serve multiple functions during Met-induced branching morphogenesis. Whereas one function may be in the regulation of cell proliferation through Gab1 PH-dependent mechanism and the activation of SHP-2, PI3K and MAPK signaling pathways, another may involve a key role for the Crk adaptor protein and/or PLCγ, which affect epithelial branching morphogenesis through functions in cell spreading, breakdown of adherens junctions, and migration of epithelial cells (Lamorte et al., 2002b; Rodrigues et al., 2005).
The signaling requirements for Tpr-Met–induced cell cycle progression in Xenopus oocytes are similar to those implicated in fibroblast cell transformation. For example, both processes are dependent on the presence of the twin phosphotyrosine (Y482 and Y489) residues in the C terminus of Tpr-Met and are associated with activation of PI3K, MAPK, and JNK signaling pathways (Kamikura et al., 1996; Rodrigues et al., 1997; Bardelli et al., 1999; Lamorte et al., 2000; Mood et al., 2006). Furthermore, the specific recruitment of Grb2 or Shc adaptor is sufficient to induce these responses, but the direct recruitment of PLCγ or PI3K to Tpr-Met is not sufficient (Saucier et al., 2002; Mood et al., 2006). Importantly, as demonstrated for cell cycle progression in Xenopus oocytes (Figure 7), expression of the MBD impairs proliferation and morphological transformation of Tpr-Met–expressing fibroblasts (Figure 9). This is the first demonstration that engagement of Gab1 signals are required for the transforming activities of the Met receptor oncoprotein.
Bcr-Abl and TEL-TRKC (ETV6-NTRK3) oncoproteins, like Tpr-Met, are cytoplasmic constitutively activated kinases resulting from chromosomal rearrangements that fuse dimerization motifs to their kinase domains. Interestingly, the transforming activity of Bcr-Abl has been shown to be dependent on its ability to associate with Gab2, whereas TEL-TRKC requires association with IRS-1, and to be dependent on activation of PI3K (Sattler et al., 2002; Lannon et al., 2004). In addition, oncogenic transformation induced by the polyoma middle T antigen, a cytoplasmic oncoprotein, tightly correlates with its capacity to interact with and to phosphorylate Gab1, and to activate PI3K (Ong et al., 2001). Given that these are scaffolding proteins that all contain a PH domain, their importance in cell transformation may reflect the property of these proteins to target a multiprotein complex to a specific membrane location, thus allowing for an interaction with appropriate effectors.
Gab1 becomes tyrosine phosphorylated upon the activation of several RTKs that stimulate cell proliferation, and overexpression of Gab1 has been shown to enhance cell growth and transformation of NIH3T3 cells downstream of the epidermal growth factor receptor (Holgado-Madruga et al., 1996). Because we demonstrate that the engagement of Gab1-dependent signals is required for FGFR-mediated cell cycle progression in Xenopus oocytes, and for the oncogenic activity of the Met receptor in fibroblasts (Figures 7–9), we predict that cell proliferation and transformation promoted by several RTKs may also be dependent on the engagement of Gab1-dependent signals. Significantly, our results identify Gab1 and/or Gab1-dependent signals as potential therapeutic targets to impair proliferation and/or transformation of cancer cells harboring a deregulated Met receptor.
ACKNOWLEDGMENTS
We thank members of the Park laboratory for helpful comments. This work was supported by National Cancer Institute of Canada operating grant awarded to M.P. with money from the Canadian Cancer Society, and for I.O.D., this research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Abbreviations used:
- Gab1
Grb2-associated binder 1
- GVBD
germinal vesicle breakdown
- MBD
Met-binding domain
- MBM
Met-binding motif
- RTK
receptor tyrosine kinase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-03-0244) on June 14, 2006.
REFERENCES
- Bardelli A., Basile M. L., Audero E., Giordano S., Wennstrom S., Menard S., Comoglio P. M., Ponzetto C. Concomitant activation of pathways downstream of Grb2 and PI 3-kinase is required for MET-mediated metastasis. Oncogene. 1999;18:1139–1146. doi: 10.1038/sj.onc.1202607. [DOI] [PubMed] [Google Scholar]
- Bardelli A., Longati P., Gramaglia D., Stella M. C., Comoglio P. M. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15:3103–3111. doi: 10.1038/sj.onc.1201561. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G. F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Bladt F., Riethmacher D., Isenmann S., Aguzzi A., Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Browaeys-Poly E., Cailliau K., Vilain J. P. Signal transduction pathways triggered by fibroblast growth factor receptor 1 expressed in Xenopus laevis oocytes after fibroblast growth factor 1 addition. Role of Grb2, phosphatidylinositol 3-kinase, Src tyrosine kinase, and phospholipase Cgamma. Eur. J. Biochem. 2000;267:6256–6263. doi: 10.1046/j.1432-1327.2000.01710.x. [DOI] [PubMed] [Google Scholar]
- Carballada R., Yasuo H., Lemaire P. Phosphatidylinositol-3 kinase acts in parallel to the ERK MAP kinase in the FGF pathway during Xenopus mesoderm induction. Development. 2001;128:35–44. doi: 10.1242/dev.128.1.35. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Park M., Blair D. G., Tainsky M. A., Huebner K., Croce C. M., Vande Woude G. F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Cunnick J. M., Meng S., Ren Y., Desponts C., Wang H. G., Djeu J. Y., Wu J. Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 2002;277:9498–9504. doi: 10.1074/jbc.M110547200. [DOI] [PubMed] [Google Scholar]
- Fabian J. R., Morrison D. K., Daar I. O. Requirement for Raf and MAP kinase function during the meiotic maturation of Xenopus oocytes. J. Cell Biol. 1993;122:645–652. doi: 10.1083/jcb.122.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr Xenopus oocyte maturation: new lessons from a good egg. Bioessays. 1999;21:833–842. doi: 10.1002/(SICI)1521-1878(199910)21:10<833::AID-BIES5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Fixman E. D., Fournier T. M., Kamikura D. M., Naujokas M. A., Park M. Pathways downstream of Shc and Grb2 are required for cell transformation by the Tpr-Met oncoprotein. J. Biol. Chem. 1996;271:13116–13122. doi: 10.1074/jbc.271.22.13116. [DOI] [PubMed] [Google Scholar]
- Fixman E. D., Holgado-Madruga M., Nguyen L., Kamikura D. M., Fournier T. M., Wong A. J., Park M. Efficient cellular transformation by the Met oncoprotein requires a functional Grb2 binding site and correlates with phosphorylation of the Grb2-associated proteins, Cbl and Gab1. J. Biol. Chem. 1997;272:20167–20172. doi: 10.1074/jbc.272.32.20167. [DOI] [PubMed] [Google Scholar]
- Fournier T. M., Kamikura D., Teng K., Park M. Branching tubulogenesis but not scatter of Madin-Darby canine kidney cells requires a functional Grb2 binding site in the Met receptor tyrosine kinase. J. Biol. Chem. 1996;271:22211–22217. doi: 10.1074/jbc.271.36.22211. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M., Dolfi F., Zeh K., Vuor K. Met-induced JNK activation is mediated by the adapter protein Crk and correlates with the Gab1-Crk signaling complex formation. Oncogene. 1999;18:7775–7786. doi: 10.1038/sj.onc.1203198. [DOI] [PubMed] [Google Scholar]
- Gu H., Neel B. G. The “Gab” in signal transduction. Trends Cell Biol. 2003;13:122–130. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- Gual P., Giordano S., Williams T. A., Rocchi S., Van Obberghen E., Comoglio P. M. Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene. 2000;19:1509–1518. doi: 10.1038/sj.onc.1203514. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M., Emlet D. R., Moscatello D. K., Godwin A. K., Wong A. J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M., Moscatello D. K., Emlet D. R., Dieterich R., Wong A. J. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl. Acad. Sci. USA. 1997;94:12419–12424. doi: 10.1073/pnas.94.23.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi N., Takayama H., Toyoda M., Otsuka T., Fukusato T., Merlino G., Takagi H., Mori M. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–1799. doi: 10.1038/sj.onc.1205248. [DOI] [PubMed] [Google Scholar]
- Jeffers M., Schmidt L., Nakaigawa N., Webb C. P., Weirich G., Kishida T., Zbar B., Vande Woude G. F. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc. Natl. Acad. Sci. USA. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikura D. M., Naujokas M. A., Park M. Identification of tyrosine 489 in the carboxy terminus of the Tpr-Met oncoprotein as a major site of autophosphorylation. Biochemistry. 1996;35:1010–1017. doi: 10.1021/bi9514065. [DOI] [PubMed] [Google Scholar]
- Katso R., Okkenhaug K., Ahmadi K., White S., Timms J., Waterfield M. D. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- Klein S. L., Strausberg R. L., Wagner L., Pontius J., Clifton S. W., Richardson P. Genetic and genomic tools for Xenopus research: the NIH Xenopus initiative. Dev. Dyn. 2002;225:384–391. doi: 10.1002/dvdy.10174. [DOI] [PubMed] [Google Scholar]
- Koibuchi N., Kaneda Y., Taniyama Y., Matsumoto K., Nakamura T., Ogihara T., Morishita R. Essential role of HGF (hepatocyte growth factor) in blood formation in Xenopus. Blood. 2004;103:3320–3325. doi: 10.1182/blood-2003-02-0352. [DOI] [PubMed] [Google Scholar]
- Lamorte L., Kamikura D. M., Park M. A switch from p130Cas/Crk to Gab1/Crk signaling correlates with anchorage independent growth and JNK activation in cells transformed by the Met receptor oncoprotein. Oncogene. 2000;19:5973–5981. doi: 10.1038/sj.onc.1203977. [DOI] [PubMed] [Google Scholar]
- Lamorte L., Rodrigues S., Naujokas M., Park M. Crk synergizes with epidermal growth factor for epithelial invasion and morphogenesis and is required for the met morphogenic program. J. Biol. Chem. 2002a;277:37904–37911. doi: 10.1074/jbc.M201743200. [DOI] [PubMed] [Google Scholar]
- Lamorte L., Royal I., Naujokas M., Park M. Crk adapter proteins promote an epithelial-mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol. Biol. Cell. 2002b;13:1449–1461. doi: 10.1091/mbc.01-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe B., Yamada M., Schaeper U., Birchmeier W., Lax I., Schlessinger J. The docking protein Gab1 is an essential component of an indirect mechanism for fibroblast growth factor stimulation of the phosphatidylinositol 3-kinase/Akt antiapoptotic pathway. Mol. Cell. Biol. 2004;24:5657–5666. doi: 10.1128/MCB.24.13.5657-5666.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannon C. L., Martin M. J., Tognon C. E., Jin W., Kim S. J., Sorensen P. H. A highly conserved NTRK3 C-terminal sequence in the ETV6-NTRK3 oncoprotein binds the phosphotyrosine binding domain of insulin receptor substrate- 1, an essential interaction for transformation. J. Biol. Chem. 2004;279:6225–6234. doi: 10.1074/jbc.M307388200. [DOI] [PubMed] [Google Scholar]
- Lock L. S., Frigault M. M., Saucier C., Park M. Grb2-independent recruitment of Gab1 requires the C-terminal lobe and structural integrity of the Met receptor kinase domain. J. Biol. Chem. 2003;278:30083–30090. doi: 10.1074/jbc.M302675200. [DOI] [PubMed] [Google Scholar]
- Lock L. S., Maroun C. R., Naujokas M. A., Park M. Distinct recruitment and function of Gab1 and Gab2 in Met receptor-mediated epithelial morphogenesis. Mol. Biol. Cell. 2002;13:2132–2146. doi: 10.1091/mbc.02-02-0031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. S., Royal I., Naujokas M. A., Park M. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 2000;275:31536–31545. doi: 10.1074/jbc.M003597200. [DOI] [PubMed] [Google Scholar]
- Ma P. C., Kijima T., Maulik G., Fox E. A., Sattler M., Griffin J. D., Johnson B. E., Salgia R. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63:6272–6281. [PubMed] [Google Scholar]
- Maroun C. R., Holgado-Madruga M., Royal I., Naujokas M. A., Fournier T. M., Wong A. J., Park M. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 1999a;19:1784–1799. doi: 10.1128/mcb.19.3.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun C. R., Moscatello D. K., Naujokas M. A., Holgado-Madruga M., Wong A. J., Park M. A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis. J. Biol. Chem. 1999b;274:31719–31726. doi: 10.1074/jbc.274.44.31719. [DOI] [PubMed] [Google Scholar]
- Maroun C. R., Naujokas M. A., Holgado-Madruga M., Wong A. J., Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 2000;20:8513–8525. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mood K., Friesel R., Daar I. O. SNT1/FRS2 mediates germinal vesicle breakdown induced by an activated FGF receptor 1 in Xenopus oocytes. J. Biol. Chem. 2002;277:33196–33204. doi: 10.1074/jbc.M203894200. [DOI] [PubMed] [Google Scholar]
- Mood K., Saucier C., Ishimura A., Bong Y. S., Lee H. S., Park M., Daar I. O. Oncogenic met receptor induces cell-cycle progression in Xenopus oocytes independent of direct Grb2 and Shc binding or Mos synthesis, but requires phosphatidylinositol 3-kinase and raf signaling. J. Cell Physiol. 2006;207:271–285. doi: 10.1002/jcp.20564. [DOI] [PubMed] [Google Scholar]
- Nebreda A. R., Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr. Opin. Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Holgado-Madruga M., Maroun C., Fixman E. D., Kamikura D., Fournier T., Charest A., Tremblay M. L., Wong A. J., Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J. Biol. Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- Ong S. H., Dilworth S., Hauck-Schmalenberger I., Pawson T., Kiefer F. ShcA and Grb2 mediate polyoma middle T antigen-induced endothelial transformation and Gab1 tyrosine phosphorylation. EMBO J. 2001;20:6327–6336. doi: 10.1093/emboj/20.22.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C. S., Schmidt M., O’Brien S. J., Blair D. G., Vande Woude G. F. Mechanism of met oncogene activation. Cell. 1986;45:895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Pickham K. M., Meyer A. N., Li J., Donoghue D. J. Requirement of mosXe protein kinase for meiotic maturation of Xenopus oocytes induced by a cdc2 mutant lacking regulatory phosphorylation sites. Mol. Cell. Biol. 1992;12:3192–3203. doi: 10.1128/mcb.12.7.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A. L., Runyan R. B., Mostov K. E. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 1998;204:64–79. doi: 10.1006/dbio.1998.9091. [DOI] [PubMed] [Google Scholar]
- Ponzetto C., Zhen Z., Audero E., Maina F., Bardelli A., Basile M. L., Giordano S., Narsimhan R., Comoglio P. Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility. J. Biol. Chem. 1996;271:14119–14123. doi: 10.1074/jbc.271.24.14119. [DOI] [PubMed] [Google Scholar]
- Rodrigues G. A., Naujokas M. A., Park M. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol. Cell. Biol. 1991;11:2962–2970. doi: 10.1128/mcb.11.6.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. A., Park M. Dimerization mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol. Cell. Biol. 1993;13:6711–6722. doi: 10.1128/mcb.13.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues G. A., Park M., Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;16:2634–2645. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues S. P., Fathers K. E., Chan G., Zuo D., Halwani F., Meterissian S., Park M. CrkI and CrkII function as key signaling integrators for migration and invasion of cancer cells. Mol. Cancer Res. 2005;3:183–194. doi: 10.1158/1541-7786.MCR-04-0211. [DOI] [PubMed] [Google Scholar]
- Sattler M., et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1:479–492. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- Saucier C., et al. The Shc adaptor protein is critical for VEGF induction by Met and ErbB2 receptors and for early onset of tumor angiogenesis. Proc. Natl. Acad. Sci. USA. 2004;101:2345–2350. doi: 10.1073/pnas.0308065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier C., Papavasiliou V., Palazzo A., Naujokas M. A., Kremer R., Park M. Use of signal specific receptor tyrosine kinase oncoproteins reveals that pathways downstream from Grb2 or Shc are sufficient for cell transformation and metastasis. Oncogene. 2002;21:1800–1811. doi: 10.1038/sj.onc.1205261. [DOI] [PubMed] [Google Scholar]
- Schmidt C., Bladt F., Goedecke S., Brinkmann V., Zschiesche W., Sharpe M., Gherardi E., Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Toyoda M., Takayama H., Horiguchi N., Otsuka T., Fukusato T., Merlino G., Takagi H., Mori M. Overexpression of hepatocyte growth factor/scatter factor promotes vascularization and granulation tissue formation in vivo. FEBS Lett. 2001;509:95–100. doi: 10.1016/s0014-5793(01)03126-x. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Minowa O., Mori C., Shiota K., Kuno J., Noda T., Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Weidner K. M., Di Cesare S., Sachs M., Brinkmann V., Behrens J., Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- Yu W., O’Brien L. E., Wang F., Bourne H., Mostov K. E., Zegers M. M. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol. Biol. Cell. 2003;14:748–763. doi: 10.1091/mbc.E02-06-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. W., Vande Woude G. F. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J. Cell. Biochem. 2003;88:408–417. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]