Abstract
Embryo implantation, endometrial stromal cell decidualization and formation of a functional placenta are critical processes in the establishment and maintenance of pregnancy. Interleukin (IL)-11 signalling is essential for adequate decidualization in the mouse uterus and IL-11 promotes decidualization in the human. IL-11 action is mediated via binding to the specific IL-11 receptor α (IL-11Rα). The present study examined immunoreactive IL-11 and IL-11Rα in cycling rhesus monkey endometrium, at implantation sites in cynomolgus and rhesus monkeys and in human first trimester decidua and defined distinct spatial and temporal patterns. In cycling rhesus monkey endometrium, IL-11 and IL-11Rα increased in both basalis and functionalis regions during the secretory compared with the proliferative phase, with changing cellular locations in luminal and glandular epithelium and stroma. The patterns were similar overall to those previously described in human endometrium. Differences were seen in immunostaining during implantation in cynomologus and rhesus monkey. In the cynomolgus, very little staining for IL-11 or IL-11Rα was seen in syncytio- and cyto-trophoblast cells in the villi between days 12 and 150 of pregnancy although there was moderate staining in cytotrophoblast in the shell between days 12 and 17 and in subpopulations of cytotrophoblast cells invading the arteries at day 17. By contrast in the rhesus monkey between days 24 and 35 of pregnancy and in human first trimester placenta, cyto- and syncytio-trophoblast in the villi but not cytotrophoblast in the shell were positively stained. The most intense staining for both IL-11 and IL-11Rα was present within the decidua in the maternal component of implantation sites in all three primates but moderate staining was also present in maternal vascular smooth muscle and glands perivascular cells and epithelial plaques. These results are consistent with a role for IL-11 both during decidualization and placentation in primates.
Introduction
Embryo implantation, endometrial stromal cell decidualization and formation of a functional placenta are critical processes in the establishment and maintenance of pregnancy. The molecular events of early implantation in humans are difficult to study due to the lack of tissues available. However, gene targeting studies have identified a small number of cytokines that are unequivocally required for implantation in mice, including interleukin 11 (IL-11) and leukemia inhibitory factor (LIF) [1,2].
IL-11 was initially described as a growth factor synergising with other factors in the regulation of hematopoiesis. More recently it has been demonstrated to have pleiotropic actions, including an anti-inflammatory role, in various cell types [3,4]. IL-11 belongs to a family of cytokines that includes oncostatin M, IL-6, LIF, cardiotropin 1 and ciliary neurotropic growth factor. Activities of this family of cytokines are mediated via high affinity receptor complexes composed of a ligand-specific alpha (α) subunit and a common signalling subunit gp130. IL-11 binds to a complex of IL-11 receptor (R) α and gp130 [5]. Female mice with a null mutation of the gene encoding IL-11Rα are infertile due to a defective uterine decidualization response to the implanting blastocyst [1,6]. In both rats and mice, IL-11 mRNA is expressed in the primary decidual zone while mRNA for both receptor components, IL-11Rα and gp130, are expressed throughout the peri-implantation period [1,7]. Furthermore, comparison of the predicted IL-11 amino acid sequences of mouse, rat, non-human primate and human reveals that IL-11 is highly conserved among species [7].
Although there are many similarities in the processes of implantation and placentation between the rodent and human, important differences exist. In the human, implantation is initiated in the secretory phase of the menstrual cycle during which time the endometrium is extensively remodelled in preparation for the implanting blastocyst. In particular, the terminal differentiation or decidualization of endometrial stromal cells occurs spontaneously at this time (even in the absence of a blastocyst) and continues throughout pregnancy [8-11]. However, in the mouse and rat, decidualization occurs post-implantation in response to the blastocyst and continues throughout pregnancy [12].
IL-11 and IL-11Rα mRNA are expressed in human endometrium throughout the menstrual cycle [13-15]. Immunoreactive IL-11 increases in luminal epithelium and glands in the mid-late secretory phase but is most intense in the decidualized stromal cells late in the cycle [13,16]. IL-11Rα protein has also been detected in the endometrium throughout the menstrual cycle [16]. During the first trimester of pregnancy, IL-11Rα mRNA and IL-11 protein are present in decidua and in chorionic villi [15] but reduced in anembryonic compared to normal pregnancies, identifying a possible critical role for IL-11 signalling in pregnancy [15]. Functions of IL-11 in the human endometrium have been examined using in vitro cell culture models; IL-11 progresses endometrial stromal cell decidualization and also increases decidualized and non-decidualized stromal cell survival [17,18].
In the human, early stages of implantation are difficult to study due to the lack of availability of tissue. As a prelude to examining the function of IL-11 in human implantation and placentation, the aim of this study was to determine the localisation of IL-11 and IL-11Rα at implantation sites of rhesus and cynomolgus macaque and in first trimester human decidua and placenta.
Materials and Methods
Tissue collection
Adult cynomolgus monkeys (Macaca fascicularis) were housed at the California Regional Primate Centre. All animal procedures conformed to the requirements of the Animal Welfare Act, and the protocols were approved by the Institutional Animal Use and Care Administrative Committee at the University of California, Davis, USA. The timing of pregnancies has been described previously [19]. Briefly, animals were bred twice during the ovulation stage of the cycle and the second mating was designated day 0 of pregnancy. Pregnancy was verified by ultrasound at day 12 of pregnancy. Intact uterus was removed at days 12 (n = 2), 14 (n = 1), 17 (n = 1), 27 (n = 1), 31 (n = 1), 49 (n = 1), 74 (n = 1), 150 (n = 1) of pregnancy. Tissues were fixed with 4% paraformaldehyde in phosphate buffer for 4 hrs and processed to paraffin wax blocks.
Rhesus monkeys (Macaca mulatta) were maintained in the Fu-Zhou Primate Research Centre, China. All experimental work was approved by the Animal Ethics Committee at the Institute of Zoology, Chinese Academy of Sciences, Beijing. Estrous cycles of female monkeys were monitored for two to three cycles before sampling. Uterine tissue was collected 1 day before ovulation (ov-1, n = 3) and at 5 (ov+5, n = 3), 10 (ov+10, n = 3) and 15 (ov+15, n = 2) days after ovulation. For the tissue from pregnant monkeys, animals were permitted to mate over a period of 3 days at the anticipated time of ovulation. The second day of mating was designated day 0 of pregnancy. The presence of a conceptus was confirmed by ultrasound diagnosis and rectal examination. Samples of uterus were collected from pregnant monkey implantation sites between day 24 and day 35 (n = 8). At the appropriate time of either the estrous cycle or pregnancy, the monkeys were sacrificed, the uterus was removed (with or without placenta) and appropriately selected wedges of full thickness uteri (myometrium, endometrium, placenta of < 0.5 mm in diameter) were fixed in buffered formalin overnight at 4°C, washed in Tris-buffered saline (pH 7.4) and processed to paraffin wax blocks.
Human decidua and placenta were collected from healthy women undergoing elective termination. All were singleton pregnancies at 8–9 weeks of gestation without any known fetal abnormality. Tissue from term pregnancy was collected after spontaneous vaginal delivery. All tissue collection followed informed consent and was under approval from Monash Medical Centre Human Research and Ethics Committee, Clayton, Vic., Australia. All tissues were fixed in 10% neutral buffered formalin for 12–16 hrs at 4°C and processed to paraffin wax blocks.
Immunohistochemistry
Immunohistochemistry for IL-11 was conducted using a monoclonal anti-huIL-11 antibody (5E3) produced in our laboratory raised against recombinant human IL-11 (a kind gift of Genetics Institute). A second monoclonal antibody (MAB618; R&D Systems Inc., Minneapolis, USA) was also used to verify the specificity of immunostaining in the macaque tissues as previously described [13]. Immunostaining for IL-11Rα was performed using a monoclonal anti-huIL-11Rα (4D12) as previously described [18]. A commercially available antibody (sc-993, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) confirmed specificity of the IL-11Rα antibody in macaque uterus.
Paraffin sections (5 μm) of endometrium from all stages of the rhesus monkey menstrual cycle and implantation sites, cynomolgus monkey implantation sites, human placenta and decidua were dewaxed in histosol and rehydrated through descending grades of ethanol. Endogenous peroxidase activity was quenched by immersion in 3% H2O2 in methanol for 10 min. Sections were then incubated with blocking solution containing 10% horse serum (H0146; Sigma Aldrich Inc. Missouri, USA), 5% human serum and TBS (pH 7.4) for 30 min. Primary antibodies were applied diluted to 5 μg/ml (IL-11) and 2.5 μg/ml (IL-11Rα) in blocking solution for 18 hrs at 4°C. Antibody localisation was detected by sequential application of biotinylated horse anti-mouse IgG (Vector Laboratories, Burlingame, CA, USA) in blocking solution, and an avidin-biotin-complex conjugated to HRP (Vectastain ABC Elite kit; Vector Laboratories). The substrate used was diaminobenzidine (DAB) (Zymed, San Francisco, USA), which forms an insoluble brown precipitate, and nuclei were counterstained blue with Harris hematoxylin (Sigma Diagnostics, St. Louis, USA). Human term placenta was used as a positive control and a section from a single block was included in each staining run for quality control. An isotype matched negative control at the same concentration as the primary antibody was included for each tissue.
Immunohistochemistry for cytokeratin in rhesus monkey implantation sites was conducted using anti-human cytokeratin (CAM 5.2; Becton Dickinson Immunocytochemistry Systems, San Jose, California, USA) using a technique similar to that above except that the primary antibody, pre-diluted by the manufacturer, was applied for 18 hrs at 4°C. Immunohistochemistry for cytokeratin in cynomolgus monkey tissues was performed similarly but tissue sections were digested with 0.1% pepsin (Sigma Aldrich, St Louis, USA) prior to incubation with antibody.
Assessment of immunostaining
Immunostaining in individual cellular compartments within each section was scored blind by two independent observers from 0 (negative) to +++ (maximal staining intensity) relative to the positive and negative controls.
Results
Immunohistochemistry for cytokeratin
For all three sets of tissues, immunostaining for cytokeratin was used to identify both trophoblast and epithelial cells in adjacent sections to those examined for IL-11 and IL-11Rα. Immunostaining on cynomolgus monkey day 12, 14 and 17 pregnant tissues clearly identified the cytotrophoblast shell, villous syncytio- and cyto-trophoblast cells, epithelial plaque cells (Figure 1A), and cytotrophoblast cells that invaded arteries (Figure 1G). In the rhesus monkey, trophoblast cells, glandular epithelial cells, and the placental-endometrial interface within the trophoblast shell were clearly identified (Figure 2O). When primary antibody was replaced with mouse IgG (negative control) the lack of staining verified the specificity of the antibody (Figure 2O insert). In human placenta and decidua, immunostaining identified trophoblast and epithelial cells (results not shown).
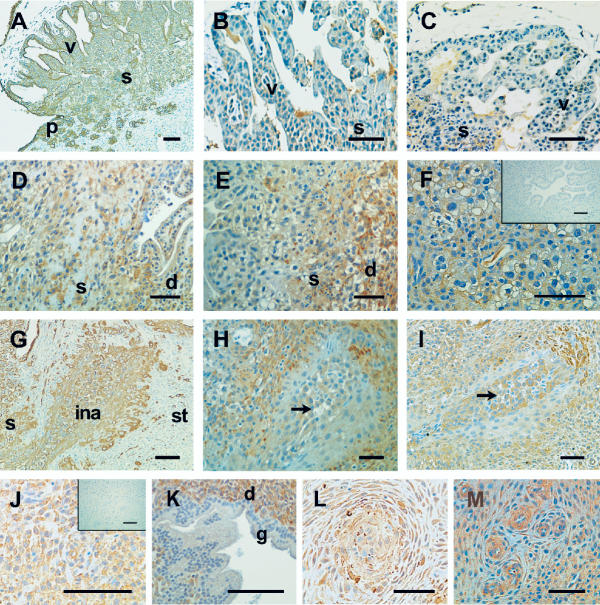
Figure 1.
Photomicrographs representing examples of immunostaining for cytokeratin, IL-11 and IL-11Rα in cynomolgus monkey implantation sites at days 12, 14 and 17 of pregnancy. Positive cells are shown as brown pigment with hematoxylin counterstain. (A) Cytokeratin staining at day 14 gestation showing chorionic villi (v), cytotrophoblast shell (s,) and epithelial plaque cells (p); (B and C) Day 12 gestation showing villi and shell. (B) IL-11 staining; (C) IL-11Rα staining. (D and E) Day 17 gestation showing shell and decidua; (D) IL-11 staining, decidua (d); (E) IL-11Rα staining. (F) IL-11Rα staining at day 14 gestation showing epithelial plaque cells. Insert represents negative control. (G-M) Day 17 gestation: (G) cytokeratin staining identifying trophoblast cells in: the shell, invaded artery (ina), and stroma (st); (H) IL-11 staining; arrow indicates positively stained trophoblast cells of invaded artery; (I) IL-11Rα staining; arrow indicates positively stained trophoblast cells of invaded artery; (J) IL-11 staining showing decidua, insert represents negative control; (K) IL-11Rα staining showing gland (g) and decidua; (L) IL-11 staining showing spiral artery at centre; (M) IL-11Rα staining showing spiral arteries. Scale bar represents 50 μm.
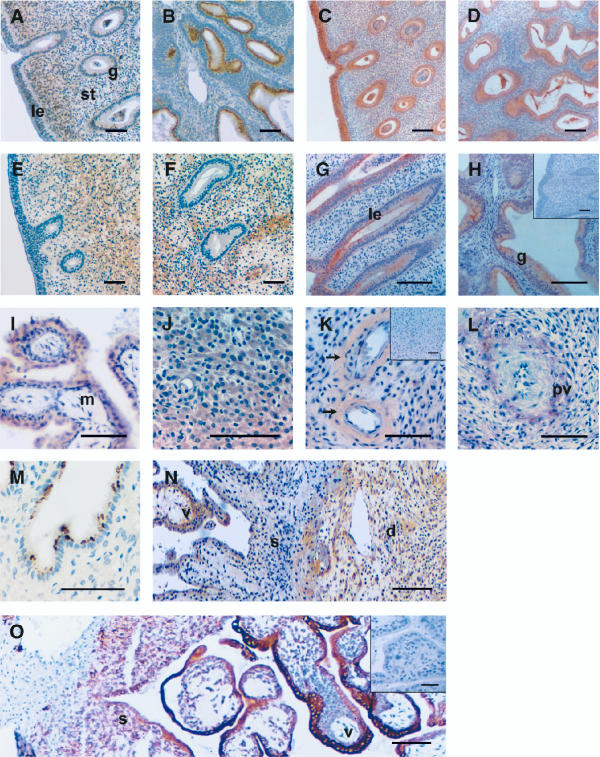
Figure 2.
Photomicrographs representing immunostaining for IL-11, IL-11Rα and cytokeratin in cycling endometrium and implantation sites of the rhesus monkey. Positive cells are shown as brown pigment with hematoxylin counterstain. (A-H) Cycling endometrium. (A and B) IL-11 staining of endometrium at ov+10: (A) luminal epithelium (le), stroma (st) and glands (g) of functionalis; (B) glands and stroma of basalis. (C and D) IL-11Rα staining at ov+10: (C) luminal epithelium, stroma and glands of functionalis; (D) stroma and glands of basalis. (E and F) IL-11 staining at ov+15: (E) luminal epithelium, stroma and glands of functionalis; (F) stroma and glands of basalis. (G and H) IL-11Rα staining at ov+15: (G) luminal epithelium and stroma of functionalis; (H) stroma and glands of basalis. Insert represents the negative control. (I-N) Implantation sites at day 24 of pregnancy. (I-K and M) IL-11 staining: (I) villi, mesenchyme (m); (J) decidua; (K) spiral arterioles (arrowed), insert represents negative control (M) gland showing punctate staining. (L and N) IL-11Rα staining: (L) spiral arteriole and perivascular cells (pv); (N) Low power view of the implantation site showing decidua (d), villi (v), and trophoblast shell (s). (O) Low power view of cytokeratin staining identifying shell and villi. Insert represents negative control. Scale bar represents 50 μm.
Immunohistochemistry for IL-11 and IL-11Rα
Controls
Positive controls for IL-11 and IL-11Rα were term placenta and decidualized stromal cells which showed positive staining in trophoblast and decidual cells respectively (results not shown) [13,17]. To verify the specificity of the antibodies for IL-11 and IL-11Rα, tissues were also stained with commercial antibodies, with similar results (results not shown). When primary antibodies were replaced with mouse IgG (negative control) the lack of staining verified the specificity of the antibodies. (Figure 1F and 1J, 2H, 2K and 2O inserts; Figure 3F and 3G).
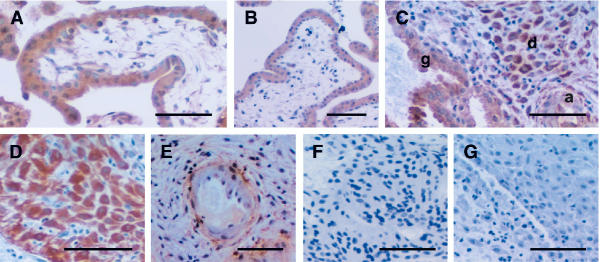
Figure 3.
Photomicrographs representing immunostaining for IL-11 and IL-11Rα in first trimester human placenta and decidua. Positive cells are shown as brown pigment with hematoxylin counterstain. (A) IL-11 staining showing villi; (B) IL-11Rα staining showing villi; (C) IL-11 staining showing glands (g), decidua (d) and spiral artery (a); (D) IL-11Rα staining showing decidua; (E) IL-11Rα staining showing spiral artery; (F) IL-11 negative control; (G) IL-11Rα negative control. Scale bar represents 50 μm.
Cynomolgus monkey implantation sites from day 12 to day 150 of pregnancy
Implantation occurs in the cynomolgus monkey at day 9 of pregnancy. The tissues studied included: day 12 (early lacunar stage; trophoblast penetrates superficial endometrial vessels), day 14 (late lacunar stage; early placental formation), day 17 (prior to blood circulation in the placenta), and day 27–150. The results are shown in Tables 1 and 2, and Figure 1. Almost no immunoreactive IL-11 or IL-11Rα was seen in syncytio- and cyto-trophoblast of the villous in all the tissues examined (Table 1 and 2, Figure 1B and 1C respectively). Likewise, low staining for IL-11 and IL-11Rα was found in stromal mesenchyme of the villous at 12–17 days of pregnancy (Table 1 and 2), but there was increased staining for both receptor and ligand in the stromal mesenchyme by days 27–49 (Table 1 and 2). Interestingly, moderate IL-11 and low IL-11Rα staining was found in the cytotrophoblast shell at days 12–17 of pregnancy (Table 1 and 2; Day 17 shown in Figure 1D and 1E) followed by decrease in staining at later time-points. Very little immunostaining for IL-11 and IL-11Rα was found in cytotrophoblast cells invading arteries during at days 12–14 but staining increased to moderate at day 17 (Table 1 and 2, Figure 1H and 1I). Interestingly, a sub-population of cytotrophoblast cells that invaded arteries showed very low staining for IL-11 while other cytotrophoblast cells showed moderate to high staining at day 17 (arrowed in Figure 1H). A serial section of the same day 17 tissue stained for IL-11Rα showed low to moderate staining of a sub-population of cytotrophoblast cells located in the invaded artery (arrowed in Figure 1I). Immunostaining remained moderate for IL-11 in the cytotrophoblast that invaded arteries until day 27–49, but that for IL-11Rα decreased from day 27–29 (Table 1 and 2 respectively).
Table 1.
IL-11 immunostaining in cynomolgus and rhesus monkey implantation sites, and human first trimester decidua and placenta.
| Implantation sites | ||||||
| Cynomolgus | Rhesus | Human | ||||
| Day of gestation | 12–14 | 17 | 27–49 | 74–150 | 24–35 | 8–9 weeks |
| Trophoblast | ||||||
| Villi – cyto | -/+ | -/+ | -/+ | - | +++ | +++ |
| - syncytio | -/+ | -/+ | -/+ | - | +++ | +++ |
| - mesenchyme | -/+ | - | ++/+ | ++ | + | + |
| Shell | +/++ | ++ | + | + | - | N/A |
| Cyto- invading artery | + | +/++ | ++ | - | +/- | N/A |
| Mother | ||||||
| Decidua | ++ | +++ | +++ | +++ | +++ | +++ |
| VSM | ++ | +++ | +++ | -/+ | + | ++ |
| Endothelial | + | +++ | ++ | -/+ | + | -/+ |
| Glands | + | -/+ | +/++ | + | +++ | ++/+ |
| Perivascular | - | ++/+ | ++ | - | + | +++ |
| Epithelial plaques | ++ | ++/+ | N/A | N/A | N/A | N/A |
Relative staining intensities are represented as: – (no staining), + (low), ++ (moderate) and +++ (high). Trophoblast cellular compartments include: Chorionic villi (villi), villous cytotrophoblast (cyto), villous syncytiotrophoblast (syncytio), villous stromal mesenchyme (mesenchyme), cytotrophoblast shell (shell), cytotrophoblast invading spiral artery (cyto-invading artery). Maternal cellular compartments include: decidua, vascular smooth muscle cells (VSM), endothelial cells, glands, perivascular cells and epithelial plaque.
Table 2.
IL-11Rα immunostaining in cynomolgus and rhesus monkey implantation sites, and human first trimester decidua and placenta.
| Implantation sites | ||||||
| Cynomolgus | Rhesus | Human | ||||
| Day of gestation | 12–14 | 17 | 27–49 | 74–150 | 24–35 | 8–9 weeks |
| Trophoblast | ||||||
| Villi – cyto | - | -/+ | - | - | ++/+ | +++ |
| - syncytio | - | -/+ | - | - | ++/+ | +++ |
| - mesenchyme | -/+ | - | ++/+ | + | + | +++ |
| Shell | + | + | -/+ | + | - | N/A |
| Cyto- invading artery | + | +/++ | -/+ | - | - | N/A |
| Mother | ||||||
| Decidua | ++ | +++ | +++ | +++ | +++ | +++ |
| VSM | -/+ | +++ | +++ | + | + | ++ |
| Endothelial | -/+ | +++ | +/++ | + | - | -/+ |
| Glands | - | -/+ | -/+ | - | -/+ | + |
| Perivascular | - | ++/+ | ++/+ | + | +++ | ++ |
| Epithelial plaques | +/++ | + | N/A | N/A | N/A | N/A |
Relative staining intensities are represented as: – (no staining), + (low), ++ (moderate) and +++ (high). Trophoblast cellular compartments include: Chorionic villi (villi), villous cytotrophoblast (cyto), villous syncytiotrophoblast (syncytio), villous stromal mesenchyme (mesenchyme), cytotrophoblast shell (shell), cytotrophoblast invading spiral artery (cyto-invading artery). Maternal cellular compartments include: decidua, vascular smooth muscle cells (VSM), endothelial cells, glands, perivascular cells and epithelial plaque.
Staining for IL-11 and IL-11Rα in decidua was moderate during days 12–14 and was intense at all later time points (Table 1 and 2; Day 17, Figure 1J and 1K). Vascular smooth muscle and endothelial cell scores were from arteries that were not invaded by trophoblast cells. Staining for IL-11 was moderate and for IL-11Rα was low in vascular smooth muscle cells at days 12–14 but both increased at days 17–49 (Table 1 and 2; Day 17, Figure 1L and 1M respectively). Staining for both IL-11 and IL-11Rα in endothelial cells was maximal at day 17 (Figure 1L and 1M respectively) but decreased by day 27 and thereafter (Table 1 and 2 respectively). Interestingly, IL-11 immunostaining in glandular epithelial cells was low until day 27, but IL-11Rα was almost undetectable in glands in all samples (Table 1 and 2 respectively; Day 17, Figure 1K). Epithelial plaque cells stained moderately for IL-11 and minimally for IL-11Rα at days 12–17 (Table 1 and 2; Day 17, Figure 1F).
Rhesus monkey uterus throughout the menstrual cycle
Full thickness endometrial sections were available for immunostaining. Overall the results show that IL-11 and IL-11Rα immunoreactivity was maximal in the mid-late secretory phase of the cycle compared to the proliferative phase (Table 3). Very little staining for IL-11 and IL-11Rα was found at ov-1 and ov+5. Luminal epithelial cell staining was very low at ov+10 for IL-11 and maximal for IL-11Rα (Table 3 and 4, Figure 3A and 3C respectively). Glandular epithelium showed differences in staining between functionalis and basalis endometrial layers and so were scored separately. The glandular epithelial cells close to the myometrium (basalis) were the most intensely stained cells for IL-11, staining maximally at ov+10 (Table 3, Figure 2B). Similarly, intense staining was found for IL-11Rα in the glands of the functionalis and basalis layers of the endometrium at ov+10 (Table 3, Figure 2C and 2D respectively). In contrast, at ov+10, staining in the stroma was low for both IL-11 and IL-11Rα (Table 3, Figure 2A,2B,2C,2D). Interestingly, by ov+15, IL-11 staining increased in stroma in the functionalis and basalis but IL-11Rα staining remained low (Table 3, Figure 2E,2F,2G,2Hrespectively). In contrast, at ov+15, staining for IL-11 was low, but remained moderate for IL-11Rα, in glandular and luminal epithelium (Table 3, Figure 2E,2F,2G,2H respectively).
Table 3.
IL-11 and IL-11Rα relative immunostaining in cycling rhesus monkey endometrium.
| ov-1 | ov+5 | ov+10 | ov+15 | |||||
| IL-11 | IL-11Rα | IL-11 | IL-11Rα | IL-11 | IL-11Rα | IL-11 | IL-11Rα | |
| Luminal epithelium | - | - | - | - | -/+ | +++ | -/+ | +/++ |
| Glands (Basalis) | - | - | -/+ | - | +++ | ++/+ | -/+ | ++ |
| Glands (Functionalis) | - | - | - | - | -/+ | ++/+ | -/+ | ++ |
| Stroma | - | - | - | -/+ | + | + | +/++ | -/+ |
Relative staining intensities are represented as: - (no staining), + (low), ++ (moderate) and +++ (high). Cellular compartments include: luminal epithelium, glands in the functionalis, glands in the basalis and stroma.
Early rhesus monkey implantation sites
Implantation is initiated in the rhesus monkey on day 9 of pregnancy. The implantation sites examined ranged between day 24 and day 35 of pregnancy. The results are shown in Table 1 and 2, and Figure 2I,2J,2K,2L,2M,2N (day 24 of pregnancy). Very little penetration of individual cytotrophoblast cells into the endometrial stroma was apparent. Overall, the cell types that stained for IL-11 and IL-11Rα did not differ between tissues. In contrast to the cynomolgus monkey, strong staining for IL-11 and IL-11Rα was observed in syncytio- and cyto-trophoblast cells of the chorionic villi (Tables 1 and 2, Figures 2I and 2N). The stromal mesenchyme of the villous showed low staining for both IL-11 and IL-11Rα (Tables 1 and 2, Figures 2I and 2N respectively). Also, in contrast to the cynomolgus monkey, immunoreactive IL-11 (Table 1) and IL-11Rα (Table 2, Figure 2N) was absent from the trophoblast shell.
Within the uterus, intense staining for IL-11 and IL-11Rα was seen in the stroma particularly in the decidual cells (Figures 2J and 2N respectively). Vascular smooth muscle and endothelial cells also stained weakly positive for IL-11 (Table 1, Figure 2K), while staining for IL-11Rα was seen in the peri-vascular cells (Table 2, Figure 2L). In glandular epithelial cells, IL-11 staining was predominant, and, interestingly, in some tissues a punctate pattern was observed (Table 1, Figure 2M), but very little staining was seen for IL-11Rα (Table 2).
Human first trimester placenta and decidua
As for the pregnant rhesus monkey uterus, intense staining for both IL-11 and IL-11Rα was observed in syncytio- and cyto-trophoblast cells of the chorionic villi (Tables 1 and 2, Figure 3A and 3B). Similarly, stromal mesenchyme of the villi stained positively for IL-11 (Figure 3A). In the uterus, decidual cells exhibited very strong staining for ligand and receptor (Tables 1 and 2, Figures 3C and 3D). Indeed, IL-11α was of greatest intensity in decidua (Figure 3D). Interestingly, IL-11 stained strongly in the glandular epithelium (Figure 3C), while very little staining for IL-11Rα was apparent in glands (Table 2). This was similar to the pregnant monkey uterus. Staining for IL-11 and IL-11Rα was high in perivascular cells but low in endothelial cells (Tables 1 and 2, Figures 3C and 3E respectively). Staining was moderate for both IL-11 and IL-11Rα in smooth muscle cells (Tables 1 and 2, Figures 3C and 3E).
Discussion
IL-11 signalling has been identified as critical for female fertility in the mouse [1,6]. This is the first study to establish that both IL-11 and IL-11Rα protein are present in the cycling rhesus monkey endometrium and in early implantation sites of cynomolgus and rhesus monkeys. Both proteins were also detected in human first trimester placenta and decidua.
In the cycling rhesus monkey endometrium, staining for IL-11 and IL-11Rα increased during the secretory phase of the oestrous cycle compared to the proliferative phase. Similarly, studies in the human identified IL-11 immunostaining to be maximal in the late secretory phase of the menstrual cycle [13,14,20]. However, a third study reported very little staining for IL-11 in cycling human endometrium, probably reflecting differences in antibody or immunohistochemical staining techniques, although a significant increase in IL-11 gene expression was found in secretory phase endometrium compared to proliferative phase endometrium [15]. Maximal IL-11 and IL-11Rα immunostaining in rhesus monkey endometrium was found during the mid-late secretory phase. Maximal IL-11Rα staining was found in the mid-secretory phase, the time when implantation is most likely to occur. Furthermore, all the major cellular compartments stained positively for IL-11 although the glandular epithelial cells in the basalis were the most highly stained cell type. Staining for IL-11Rα was maximal in the luminal epithelium and glands. This is in accordance with previous findings that showed strong staining for IL-11Rα in glandular and luminal epithelium in cycling human endometrium, although with little cyclical variation [16,20]. In the human, in the late secretory phase, strongest IL-11 staining is seen in the decidualizing stromal cells, but this process does not occur until later in the rhesus monkey.
There are very few functional studies in the human identifying a role for IL-11 in the endometrium. IL-11 has been shown to be involved in stromal cell decidualization and decidualized stromal cell survival in vitro [17,18]., but whether such actions in vivo result from IL-11 of epithelial or other cellular origin is not yet known. In addition, the cyclical variation in staining for IL-11 and IL-11Rα indicates a role for IL-11 signalling in uterine receptivity. It is likely that epithelial IL-11 has additional, as yet unidentified, functions perhaps on the implanting blastocyst.
As it is not possible to examine early implantation sites in the human, samples were collected from pregnant cynomolgus and rhesus monkeys. During both the early and late lacunar stage of implantation in the cynomolgus monkey, very little staining was seen for IL-11, and this was in the epithelial plaques, cytotrophoblast in the trophoblast shell, glands, decidual and vascular smooth muscle cells. Staining in the trophoblast shell was variable likely reflecting the different populations of trophoblast cells present. In contrast, virtually no staining for IL-11Rα was present either in the trophoblast or the glands, and only pale staining was present in the stroma, endothelial and smooth muscle cells. It therefore seems that IL-11 has a minimal, if any, role in early migration of trophoblast within the arterioles, since very little IL-11 is detectable during the lacunar stage.
In contrast there was a dramatic increase in staining for both IL-11 and IL-11Rα at day 17 of pregnancy compared to the earlier time points. At this time, large numbers of cytotrophoblast cells invade the arterial wall most likely from the lumen [21]. Sub-populations of trophoblast cells that invaded arteries, along with smooth muscle and endothelial cells, and many cells in the trophoblast shell showed staining for IL-11 and IL-11Rα. This suggests IL-11 may facilitate trophoblast invasion into the spiral arteries, a critical step in placentation.
Later in gestation, as seen in both the cynomolgus and rhesus monkey implantation sites, the most intriguing finding was the absence, or very little staining for both IL-11 and IL-11Rα from the trophoblast shell. There appears to be a switching off of IL-11 production in the trophoblast shell and a switching on in the villi by day 24 of pregnancy in the rhesus monkey. By the third week of pregnancy, the cytotrophoblast cells within the anchoring villi and trophoblast shell are heterogeneous and respond to adjacent constituents [22]. Heterogeneity also exists in cytotrophoblast cells in proximal, mid and distal regions (adjacent to the trophoblast shell) of the cell columns [22]. Thus, the observation that sub-populations of trophoblast cells stained for IL-11 and IL-11Rα likely reflects differences in IL-11 function during trophoblast migration.
The most consistent finding was the high IL-11 and IL-11Rα in the decidua in all three primate species. This likely reflects an involvement in the continuing process of decidualization of endometrial stromal cells, as demonstrated in vitro for the human [17]. The intense staining for IL-11 and IL-11Rα in human first trimester decidua was not surprising since stromal differentiation continues to provide decidual cells well into pregnancy. This confirms previous data showing immunoreactive IL-11 and IL-11Rα mRNA in such cells [15]. Both IL-11 and IL-11 Rα protein were also present in syncytio- and cyto-trophoblast of the chorionic villi in first trimester pregnancy, in accord with the documented IL-11Rα mRNA expression in these cells [15]. Thus, very similar expression patterns are present in the rhesus monkey and human implantation sites.
The most striking difference in IL-11 and IL-11Rα staining between species was the lack of staining in villous trophoblast in the cynomolgus monkey compared to the rhesus monkey and the human. Inconsistencies may be related to differences in tissue fixation but this is unlikely since staining was similar in the decidual cells, and trophoblast cells that invaded arteries in all three species. The results indicate that IL-11 has multiple roles in implantation and early placentation. The potential importance of IL-11 signalling in early placentation is emphasised by the defective production of IL-11 and IL-11Rα in decidua and placenta of women with anembryonic pregnancies [15].
The data presented in this paper thus indicate a complex and critical role for IL-11 signalling in preparing the endometrium for implantation, early trophoblast invasion and stromal cell decidualization in the primate. IL-11 and IL-11Rα production by cycling endometrium is upregulated at the time an embryo is likely to implant. In addition, the precise location of IL-11 and IL-11Rα during early implantation and placentation suggests that aberrant IL-11 signalling or blocking IL-11 action may lead to reduced fertility. Furthermore, reduced IL-11 signalling in primate endometrium or early pregnant decidua and placenta may be a therapeutic target for treatment of infertility.
Authors' contributions
ED conceived, designed and coordinated the study, carried out and scored the immunohistochemistry studies, and drafted the manuscript. LR prepared antigens, raised and tested the antibodies used for immunohistochemistry. YXL obtained ethics approval for rhesus monkey studies, obtained and prepared rhesus monkey tissues for immunohistochemistry. ACE obtained ethics approval for cynomolgus monkey studies, obtained and prepared cynomolgus monkey tissues for immunohistochemistry, conducted the cynomolgus monkey cytokeratin staining. CS participated in performing the immunohistochemistry studies. EW obtained ethics approval for collection of human placenta and decidua, and prepared tissues for the immunohistochemistry studies. LAS conceived the study and participated in drafting the manuscript.
Acknowledgments
Acknowledgements
We thank Wendy Carter, together with the WEHI monoclonal laboratory for development of the mouse anti-huIL-11 antibody. We are grateful to Samantha Park and Sue Panckridge for assistance in preparing the manuscript and the figures. Support for this Subproject (CIG-99-27) was provided by the CICCR Program of the Contraceptive Research and Development (CONRAD) Program, Eastern Virginia Medical School. Rhesus monkey tissue collection was supported by The Rockefeller Foundation/World Health Organisation Initiative on Implantation, and the CAS Chuang-Xin programme (KSCX-2-SW-201) and NSFC (30270196). Collection of cynomolgus macaque tissues was supported by grant HD10342. The views expressed by the authors do not necessarily reflect the views of CONRAD or CICCR. E.D. and C.S. are supported by CONRAD; L.R. by The Sylvia and Charles Viertel Charitable Foundation, L.A.S. and L.R. by the National Health and Medical Research Council of Australia.
Contributor Information
E Dimitriadis, Email: evdokia.dimitriadis@med.monash.edu.au.
L Robb, Email: robb@wehi.edu.au.
Y-X Liu, Email: liuyx@panda.ioz.ac.cn.
AC Enders, Email: acenders@ucdavis.edu.
H Martin, Email: martin@wehi.edu.au.
C Stoikos, Email: chelsea.stoikos@med.monash.edu.au.
E Wallace, Email: euan.wallace@med.monash.edu.au.
LA Salamonsen, Email: lois.salamonsen@med.monash.edu.au.
References
- Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nature Medicine. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- Du XX, Williams DA. Interleukin-11: Review of molecular, cell biology and clinical use. Blood. 1997;89:3897–3908. [PubMed] [Google Scholar]
- Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia. 1999;13:1307–1315. doi: 10.1038/sj/leu/2401514. [DOI] [PubMed] [Google Scholar]
- Hilton DJ, Hilton AA, Raicevic A, Rakar S, Harrison-Smith M, Gough NM, Begley CG, Metcalf D, Nicola NA, Willson TA. Cloning of a murine IL 11 receptor alpha chain: requirement for gp130 for high affinity binding and signal transduction. EMBO J. 1994;13:4765–4775. doi: 10.1002/j.1460-2075.1994.tb06802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski P, Roopenian D, Gossler A. Maternal IL-11Rα function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243. doi: 10.1101/gad.12.14.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Hartley L, Robb L. Cloning of rat interleukin 11 and interleukin 11 receptor alpha chain and analysis of their expression in rat uterus in the peri-implantation period. Reproduction. 2001;122:593–600. doi: 10.1530/rep.0.1220593. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- Maslar IA, Riddick DH. Prolactin production by human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;135:751–754. doi: 10.1016/0002-9378(79)90386-7. [DOI] [PubMed] [Google Scholar]
- Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein 1 expression. Biol Reprod. 1995;52:609–615. doi: 10.1095/biolreprod52.3.609. [DOI] [PubMed] [Google Scholar]
- Wu W-X, Brooks J, Glasier AF, McNeilly AS. The relationship between decidualization and prolactin mRNA and production at different stages of human pregnancy. J Mol Endocrinol. 1995;14:255–261. doi: 10.1677/jme.0.0140255. [DOI] [PubMed] [Google Scholar]
- Parr MB, Parr EL. The implantation reaction. In: Wynn RM, Jollie WP, editor. Biology of the Uterus. Plenum Press, New York and London; 1989. pp. 233–277. [Google Scholar]
- Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6:907–914. doi: 10.1093/molehr/6.10.907. [DOI] [PubMed] [Google Scholar]
- Cork BA, Li TC, Warren MA, Laird SM. Interleukin-11 (IL-11) in human endometrium: expression throughout the menstrual cycle and the effects of cytokines on endometrial IL-11 production in vitro. J Reprod Immunol. 2001;50:3–17. doi: 10.1016/S0165-0378(00)00089-9. [DOI] [PubMed] [Google Scholar]
- Chen HF, Lin CY, Chao KH, Wu MY, Yang YS, Ho HN. Defective production of interleukin-11 by decidua and chorionic villi in human anembryonic pregnancy. J Clin Endocrinol Metab. 2002;87:2320–2328. doi: 10.1210/jcem.87.5.8478. [DOI] [PubMed] [Google Scholar]
- Enders AC, Lantz KC, Schlafke S. Preference of invasive cytotrophoblast for maternal vessels in early implantation in the macaque. Acta Anat. 1996;155:145–162. doi: 10.1159/000147800. [DOI] [PubMed] [Google Scholar]
- Cork BA, Tuckerman EM, Li TC, Laird SM. Expression of interleukin (IL)-11 receptor by the human endometrium in vivo and effects of IL-11, IL-6 and LIF on the production of MMP and cytokines by human endometrial cells in vitro. Mol Hum Reprod. 2002;8:841–848. doi: 10.1093/molehr/8.9.841. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Robb L, Salamonsen LA. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol Hum Reprod. 2002;8:636–643. doi: 10.1093/molehr/8.7.636. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Sakamoto T, Miyama M, Ogita S, Umesaki N. Interleukin-11 enhances cell survival of decidualized normal human endometrial stromal cells. Gynecol Endocrinol. 2001;15:272–278. [PubMed] [Google Scholar]
- Karpovich N, Chobotova K, Carver J, Heath JK, Barlow DH, Mardon HJ. Expression and function of interleukin-11 and its receptor alpha in the human endometrium. Mol Hum Reprod. 2003;9:75–80. doi: 10.1093/molehr/gag012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC, Blankenship TN. Modification of endometrial arteries during invasion by cytotrophoblast cells in the pregnant macaque. Acta Anat (Basel) 1997;159:169–193. doi: 10.1159/000147983. [DOI] [PubMed] [Google Scholar]
- Enders AC. Cytodifferentiation of trophoblast in the anchoring villi and trophoblastic shell in the first half of gestation in the macaque. Microsc Res Tech. 1997;38:3–20. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<3::AID-JEMT3>3.3.CO;2-B. [DOI] [PubMed] [Google Scholar]