Abstract
The larger isoform of the enzyme glutamate decarboxylase, GAD67, synthesizes >90% of basal levels of γ-aminobutyric acid (GABA) in the brain. In contrast, the smaller isoform, GAD65, has been implicated in the fine-tuning of inhibitory neurotransmission. Mice deficient in GAD65 exhibit increased anxiety-like responses in both the open field and elevated zero maze assays. Additionally, GAD65-deficient mice have a diminished response to the anxiolytics diazepam and pentobarbital, both of which interact with GABA-A receptors in a GABA-dependent fashion to facilitate GABAergic neurotransmission. Loss of GAD65-generated GABA does not appear to result in compensatory postsynaptic GABA-A receptor changes based on radioligand receptor binding studies, which revealed no change in the postsynaptic GABA-A receptor density. Furthermore, mutant and wild-type animals do not differ in their behavioral response to muscimol, which acts independently of the presence of GABA. We propose that stress-induced GABA release is impaired in GAD65-deficient mice, resulting in increased anxiety-like responses and a diminished response to the acute effects of drugs that facilitate the actions of released GABA.
γ-Aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in the mammalian nervous system. It is synthesized by two isoforms of glutamic acid decarboxylase, GAD65 and GAD67 (1). The two isoforms, which are coexpressed in GABAergic neurons, differ with respect to cofactor association and subcellular distribution (2, 3). GAD67 is found mainly in the cell body, tightly associated with the cofactor pyridoxal 5′-phosphate, whereas GAD65 is associated mainly with nerve termini, reversibly anchored to the membranes of synaptic vesicles (4–6). Additionally, GAD65 is found at high levels in an apo-enzyme form, which can be activated on cofactor addition (7, 8). Thus, it has been suggested that GAD67 is responsible for the maintenance of basal GABA levels, whereas GAD65 can be rapidly activated in times of high GABA demand (8).
Distinct roles for the two isoforms are further suggested by the phenotypes of GAD65-deficient and GAD67-deficient mice. We and others have shown previously that lack of GAD65 results in a viable phenotype (9, 10). Brains of GAD65−/− mice exhibit a 50% decrease in cofactor-inducible GAD enzymatic activity (9). Yet, young GAD65-deficient mice on the C57BL/6 background exhibit only a slight decrease in brain GABA levels, and adult GAD65-deficient mice exhibit normal GABA content in cortex, cerebellum, and hippocampus (9, 11), indicating that the only remaining isoform of GAD (GAD67) can maintain wild-type GABA levels. Our study revealed that GAD65-deficient mice on the C57BL/6 background are susceptible to infrequent spontaneous and stress-induced seizures. This phenotype is in sharp contrast to GAD67-deficient mice, which exhibit a perinatal lethal phenotype (12, 13). Brains of newborn GAD67−/− mice have only 7% of wild-type levels of GABA at birth, consistent with an inability of the GAD65 protein to produce sufficient GABA for survival.
GABA-A receptors are the predominant receptors for GABAergic neurotransmission (see ref. 14). GABA binds to GABA-A receptors at the GABA binding site and increases the neuronal membrane conductance for Cl− ions, which results in membrane hyperpolarization (15, 16). This hyperpolarization, in turn, leads to reduced neuronal excitability. GABA agonists, such as muscimol, interact directly with the GABA binding site to activate Cl− conductance, and thus function independently of GABA (17). Pentobarbital and other barbiturates allosterically enhance GABAergic neurotransmission by increasing the mean channel open time in a GABA-dependent fashion (18). Diazepam and other benzodiazepines enhance the actions of GABA at the GABA-A receptor by increasing the probability of Cl− channel opening (16, 19) and Cl− conductance (20).
Agents that facilitate GABAergic neurotransmission are typically anxiolytic, whereas agents that inhibit GABAergic neurotransmission are anxiogenic (for reviews see refs. 21 and 22 and references therein). For example, the positive modulators of GABAergic neurotransmission [benzodiazepines (23–25), and barbiturates (26)], have been shown to produce anxiolytic-like effects in rodent ethological models of anxiety. In contrast, the GABA-A receptor antagonists bicuculline (25) and picrotoxin (27) exhibit anxiogenic-like effects.
Here, we have assessed anxiety-related behaviors and responses to GABA-A receptor modulators in GAD65-deficient mice. We show that GAD65-deficient mice exhibit increased anxiety-like behaviors in the open field and elevated zero maze tests. Additionally, these mice fail to respond to the acute effects of benzodiazepines and have a diminished response to barbiturates, in the absence of postsynaptic compensation in GABA-A receptor function.
MATERIALS AND METHODS
Mice.
The disruption of the mGAD65 locus by insertion of a phosphoglycerol kinase-driven neomycin-resistance cassette into the first exon, downstream of the transcription and translation start sites, has been described (9). For these studies, mice were backcrossed for 4–7 generations onto the C57BL/6 background. These mice exhibit infrequent stress-induced seizures (9). Any mouse that had a seizure during or immediately before a test session was eliminated from the analysis for that session. Heterozygotes were intercrossed to generate GAD65+/+ and GAD65−/− littermates. Adult male mice over 3 months of age were group-housed in a pathogen-free transgenic facility. Mice were maintained on a 12-hr light/dark cycle with lights on from 0600 to 1800, with free access to water and rodent chow. Mice were tested during the light cycle, with the experimenter blind to genotype.
Drugs.
Diazepam (5 mg/ml; Schein Pharmaceutical, Florham Park, NJ) was diluted with 0.9% saline/Tween 80 (Sigma; 2 drops of Tween 80 per 10 ml of saline) immediately before administration. Muscimol (Sigma) and pentobarbital (Abbott) were dissolved in saline. Drugs were administered i.p. at a volume of 0.01 ml/g of body weight.
Behavioral Studies.
Open field test. The responses of GAD65+/+ and GAD65−/− mice (n = 15 of each genotype) to open field exposure were analyzed. Mice were placed in the center of a brightly lit open field (50 cm × 50 cm × 50 cm), and their movements were tracked for the ensuing 30 min by using a Poly-track video tracking system (San Diego Instruments, San Diego, CA). Movements were analyzed for total distance traveled during the test, as well as a regional breakdown of central vs. peripheral movements. Dwell times for each region also were determined.
Elevated zero maze.
GAD65+/+ and GAD65−/− mice (n = 10 of each genotype) were analyzed for anxiety levels by using an elevated zero maze. This test is a pharmacologically validated assay of anxiety in animal models that is based on the natural aversion of mice to elevated open spaces (25, 28–30). It is composed of a 6-cm wide ring with an outer diameter of 45 cm containing four equal quadrants of alternating walled (closed) or unwalled (open) sections. The entire ring is elevated to a height of 40 cm. Mice were placed in the walled region at the start of the 5-min test, and movements were analyzed by using a Poly-track video tracking system. Mice were considered to be in the open region when all four paws were entirely on the open portion of the maze.
Behavioral Pharmacology.
Mice were pre-exposed to activity chambers for three (diazepam study) or five (muscimol study) sessions before the drug administration. The chambers consisted of an empty cage (45 cm × 24 cm × 18 cm) crossed with an array of photobeams to monitor horizontal movement (San Diego Instruments). A second rack of photobeams was used to monitor rearing activity. Diazepam (0.5, 1.5, or 4 mg/kg), muscimol (0.5, 1.5, or 4 mg/kg), or vehicle were administered i.p. after a 2-hr habituation period, and animals were monitored for an additional hour. Distance traveled in centimeters, active time (during which the subject is in motion), and rears were determined in 5-min time bins for the entire 3-hr period. Mice were acclimated in the activity boxes for up to 3 hr before the experiment to normalize baseline activity. Variability was minimized by testing only males at the same time of day with the same experimental protocol. Equal numbers of wild-type and GAD65-deficient mice were tested every 3 days with one drug dose in a within-subjects design, such that each mouse received each dose, with no other interruptions throughout the study. Doses were counterbalanced across test sessions. Testing occurred between 0700 and 1100.
Loss of Righting Reflex.
GAD65+/+ (n = 8) and GAD65−/− (n = 7) mice one or two generations backcrossed onto a C57BL/6 background received an i.p. injection of pentobarbital at a dose of 62 mg/kg. Mice were placed in a V-shaped chamber, and the duration of the loss of righting reflex was assessed. Mice were considered to have regained the reflex when they could right themselves three times in a 30-sec time period.
Receptor Binding Studies.
Mice were sacrificed, and their forebrains were quickly dissected out and homogenized in 50 vol of ice-cold 0.05 M Tris-citrate, pH 7.1. Nuclei and unbroken tissues were removed with a low-speed spin (1,000 × g, 10 min, 4°C). Membranes were pelleted at 100,000 × g for 1 hr at 4°C and washed three times with ice-cold 0.05 M Tris-citrate, pH 7.1 by resuspension and centrifugation at these conditions. Membranes were resuspended at 2 mg/ml in the same buffer, and protein concentration was determined (BCA+ method, Pierce). Membranes were stored at −70°C until use. In total, four membrane preparations, representing 6–8 mice of each genotype, were analyzed.
Receptor binding reactions were carried out with 500 mg of brain membranes/ml in a final volume of 0.2 ml containing 0–400 nM 3H-muscimol (8.1 Ci/mmol, Amersham Pharmacia). Nonspecific binding was determined in the presence of 100 mM GABA. After incubation at 0–4°C for 1 hr, the reaction was filtered over a glass filter (GF/B, Whatman), and unbound ligand was removed after vacuum filtration and three rapid washes with 5 ml of ice-cold 0.05 M Tris-citrate, pH 7.1. Filters were soaked in scintillation fluid counted on a standard scintillation counter. Data were combined from four binding experiments, and samples were tested in duplicate. Specific binding was determined and converted to a standard Scatchard plot to determine Kd and Bmax.
RESULTS
GAD65−/− Mice Spend Less Time in the Central Region of the Open Field.
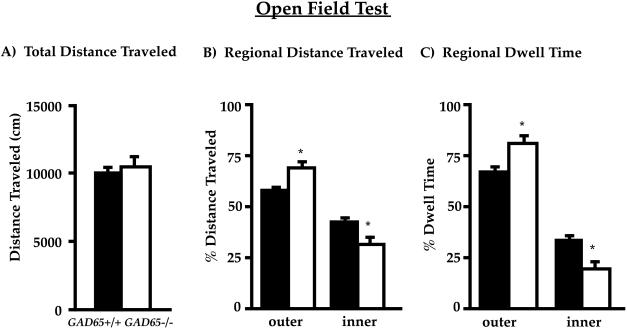
Open field testing revealed no differences in total activity between wild-type and GAD65-deficient mice (see Fig. 1A). However, there were significant differences when regional distributions of activity were analyzed. ANOVA (genotype x region) showed that the percentage of distance traveled in the inner vs. outer region of the open field differed significantly between genotype [F(1,30) = 30, P < 0.001] and region [F(1,30) = 42, P < 0.001]. There was also a significant genotype by region interaction [F(1,30) = 7.5, P < 0.01]. Post hoc multiple comparisons revealed that the differences were caused by the GAD65−/− mice traveling significantly more in the periphery of the open field, and correspondingly less in the central region compared with wild-type mice (GAD65−/− 31 ± 4% vs. GAD65+/+ 42 ± 2%, P < 0.05, Tukey test). There was also a significant difference between GAD65−/− and GAD65+/+ mice in the percentage of time spent in the two regions of the open field [F(1,30) = 30, P < 0.001]. Although both genotypes spent significantly more time in the outer region [F(1,30) = 106, P < 0.001], there was an interaction between genotype and region [F(1,30) = 9.2, P < 0.01]. The interaction reflected the GAD65−/− mice spending significantly less time in the inner, and more time in the outer, region of the open field (Fig. 1C).
Figure 1.
GAD65−/− mice spend more time in the periphery of the open field. Mice were analyzed for locomotion in the open field for 30 min (n = 15 for each genotype). (A) Total distance traveled is unchanged between genotypes. (B) Distance traveled in centimeters in the central region is reduced in GAD65−/− mice. (C) GAD65−/− mice spend less time in the central region of the open field (19 ± 4% vs. 33 ± 3% for GAD65+/+, P < 0.05, Tukey test). Values represent the mean ± SEM. Filled columns, GAD65+/+; open columns, GAD65−/−.
GAD65−/− Mice Spend Less Time on the Open Quadrant of the Elevated Zero Maze.
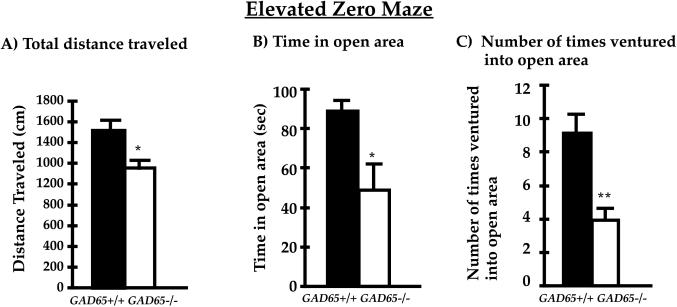
The elevated zero maze is based on the natural aversion of mice to open spaces and is sensitive to the effects of both anxiolytic and anxiogenic compounds (25, 28, 30). It is a circular ring composed of four quadrants of alternating open and closed (walled) regions. As shown in Fig. 2A, there was a small decrease in total distance traveled on the maze for GAD65−/− mice (23% decrease, P < 0.05 between genotypes, Student’s t test). A regional breakdown revealed a significant decrease in time spent on the anxiogenic open arm of the maze, such that GAD65−/− mice spent 50% less time on the open arm (Fig. 2B; GAD65−/− 48 ± 13 sec vs. GAD65+/+ 89 ± 7 sec; P < 0.05 between genotypes, Student’s t test). Additionally, there was a 57% reduction in the number of times GAD65−/− mice ventured onto the open arm, as shown in Fig. 2C. Thus, for all variables examined here, GAD65−/− mice avoid the open arm of the elevated zero maze and thus exhibit increased anxiety.
Figure 2.
Anxiety-related behavior in the elevated zero maze. (A) Total distance traveled is slightly reduced for GAD65−/− mice (n = 10 for each genotype). (B) GAD65−/− mice spend almost 50% less time in the open area of the elevated zero maze. (C) GAD65-deficient mice venture fewer times onto the open arm. Values represent the mean ± SEM. Filled columns, GAD65+/+; open columns, GAD65−/−. ∗, P < 0.05 between genotypes (Student’s t test); ∗∗, P < 0.01 between genotypes (Student’s t test).
The Response to the Anxiolytic Drug Diazepam Is Diminished in GAD65−/− Mice.
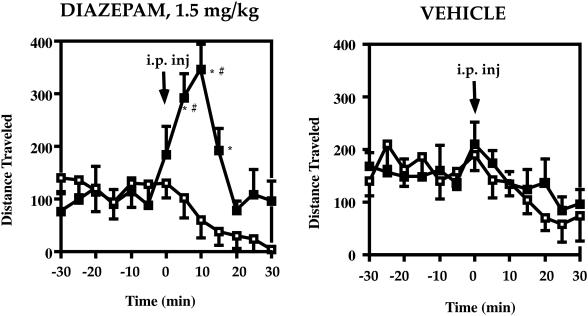
GAD65−/− mice were tested for their responses to the benzodiazepine diazepam, a compound that interacts with the GABA-A receptor and requires GABA for its effect (16, 19, 20). Typically, wild-type mice exhibit increased locomotor activity in response to low doses of diazepam (1.5 mg/kg), which often is interpreted as increased exploration caused by decreased anxiety (27, 30, 31). Wild-type and GAD65-deficient mice were pre-exposed to the behavioral chambers three times before the experiment and for 2 hr in each session before i.p. drug administration. Although there was no difference in baseline activity levels, three-way ANOVA showed a statistically significant genotype by dose interaction [F(3,36) = 5.2, P < 0.01] and a significant time by genotype interaction [F(4,48) = 7.1, P < 0.01], which indicate that the dose-related temporal effects of diazepam depended on genotype. Follow-up comparisons indicated that GAD65-deficient mice showed a dramatically diminished response to diazepam (0.5 and 1.5 mg/kg) as compared with wild-type mice (P < 0.05, Tukey test). Fig. 3 shows that after administration of diazepam (1.5 mg/kg), wild-type mice exhibited a 2.5-fold increase in total distance traveled compared with vehicle at 15-min postinjection, and a 6-fold increase compared with GAD65−/− mice (P < 0.05, Tukey test). This analysis revealed statistically significant differences between GAD65−/− and GAD65+/+ mice at 10-, 15-, and 20-min postinjection (P < 0.05, Tukey test). Fig. 3 also shows that locomotor activity of wild-type mice, after the 1.5 mg/kg dose of diazepam, differed significantly from vehicle at 10- and 15-min postinjection (P < 0.05, Tukey test). Locomotor activity of GAD65−/− mice did not differ from vehicle at any time after administration of diazepam, indicating GAD65−/− mice exhibit a diminished response to diazepam.
Figure 3.
Diminished response to the benzodiazepine receptor agonist diazepam. Distance traveled in centimeters per 5-min time bin is indicated (mean ± SEM) for 30 min before and after i.p. drug administration (indicated with an arrow at time zero). (Left) Diazepam (1.5 mg/kg body weight). (Right) Vehicle. ■, GAD65+/+; □, GAD65−/−. ∗, P < 0.05 between genotypes (Tukey test); #, P < 0.05 drug compared with vehicle (Tukey test).
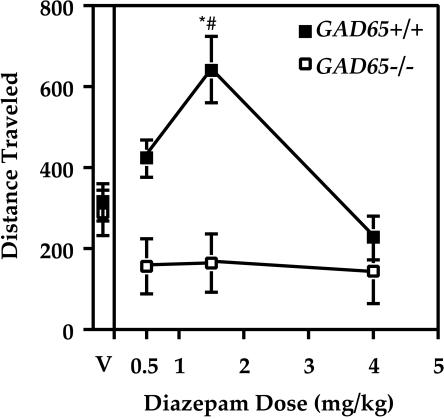
We previously have shown that there is no change in steady-state GABA content in adult C57BL/6-backcrossed GAD65-deficient mice, even though there is a 50% decrease in cofactor-inducible GAD activity in brain extracts (9). However, GAD65−/− mice exhibit a 2.5-fold decrease in K+-stimulated GABA release in vivo (11). Thus, we reasoned that the dose-response curve to diazepam may be shifted in the GAD65−/− mice to reflect a decrease in rapid GABA release. Different doses of diazepam were tested in wild-type and knockout mice, and the distance traveled in the first 10 min after drug administration is plotted as a function of dose in Fig. 4. Two-way repeated measures ANOVA indicated a significant difference between genotype [F(1,12) = 10.6, P < 0.01], as well as a significant effect for dose [F(3,36) = 10.9, P < 0.001]. Wild-type mice showed a typical dose-response curve, with an increase in activity for the 0.5 mg/kg dose and the maximum locomotor activation at 1.5 mg/kg as compared with vehicle (P < 0.05, Tukey test). However, locomotor activity of GAD65−/− mice did not differ from vehicle control at any of the doses of diazepam tested (Fig. 4). Genotypic differences in response to diazepam were significant at both the 0.5 and 1.5 mg/kg dose of diazepam (P < 0.05, Tukey test, Fig. 4). Thus, instead of producing a shift in the dose-response curve, GAD65−/− mice are insensitive to the locomotor activating effect of low doses of diazepam.
Figure 4.
Dose-response curve for diazepam. Distance traveled in centimeters for the first 10 min after drug administration is indicated as a function of diazepam dose. Data are shown as mean ± SEM. Wild-type mice (■) show a typical locomotor activating effect at low doses, which is absent in the GAD65−/− mice (□). V, vehicle. ∗, P < 0.05 between genotypes (Tukey test); #, P < 0.05 compared with vehicle (Tukey test).
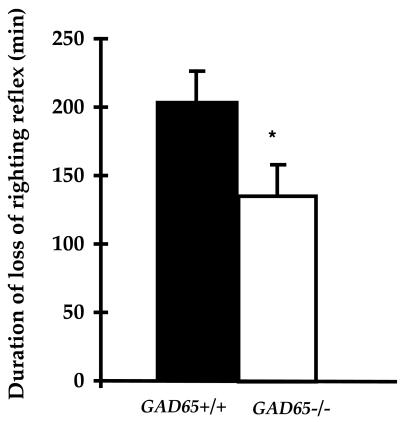
GAD65−/− Mice Exhibit a Diminished Response to Pentobarbital, Another Positive Modulator of GABAergic Neurotransmission.
GAD65−/− mice were tested for their response to a second modulator of GABAergic neurotransmission, the barbiturate pentobarbital, which enhances GABA-induced Cl− influx by GABA-A receptors (32). Mice were given an i.p. injection of pentobarbital (62 mg/kg) and assessed for the duration of the loss of righting reflex (LORR). A mouse was judged to have regained this reflex when able to right itself three times in a 30-sec time period. As shown in Fig. 5, GAD65−/− mice showed a significant reduction in the duration of pentobarbital-induced LORR as compared with GAD65+/+ mice [30% reduction; Kruskal-Wallis ANOVA on Ranks, H(1) = 6.5, P < 0.001]. Thus, as for benzodiazepines, GAD65-deficient mice exhibit an altered response to barbiturates.
Figure 5.
Loss of righting reflex for pentobarbital. Duration of loss of righting reflex after an i.p. injection of 62 mg/g is indicated for GAD65+/+ (filled bar; n = 8) and GAD65−/− (empty bar; n = 7) mice. ∗, Kruskal-Wallis ANOVA on Ranks, H(1) = 6.5, P < 0.001.
GABA-A Receptor Density Is Unchanged in GAD65-Deficient Mice.
We assessed whether the loss of GAD65-generated GABA results in alterations in GABA-A receptor density or affinity to compensate for a possible defect in rapid GABA release. Receptor binding studies were performed by using brain membrane preparations from wild-type and knockout mice. Scatchard analysis revealed that the binding constants for 3H-muscimol (a GABA-A receptor agonist that binds near the GABA site) are unchanged between genotypes (Kd; mean ± SEM; GAD65+/+ 73 ± 7 nM, GAD65−/− 79 ± 13 nM). There is also no difference in total number of binding sites (Bmax; mean ± SEM; GAD65+/+, 2.24 ± 0.14; GAD65−/−, 2.25 ± 0.33). Thus, there is no significant change in postsynaptic GABA-A receptor density in GAD65-deficient mice.
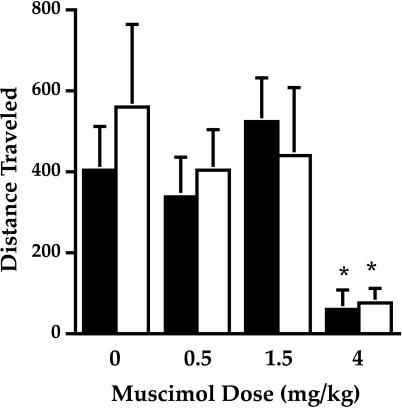
GAD65−/− Mice Exhibit Typical Behavioral Responses to the GABA-A Agonist Muscimol.
If there is no postsynaptic GABA-A receptor alteration in GAD65-deficient mice, then there should be no change in behavioral response of GAD65-deficient mice to muscimol (which interacts with GABA-A receptors and exerts its effect in the absence of GABA). Wild-type mice exhibit a sedative effect in response to muscimol. Mice were exposed to the behavioral chambers for 3 hr before i.p. drug administration. Fig. 6 shows the distance traveled during a 30-min time period starting 15 min after drug administration for several doses of muscimol. Two-way repeated measures ANOVA, with a between subject factor for genotype, showed no significant difference in locomotor behavior between GAD65−/− and GAD65+/+ mice in response to muscimol (0, 0.5, 1.5, and 4.0 mg/kg). There was a significant effect of muscimol dosage [F(3,36) = 7.8, P < 0.001] that was attributable to the effects of the 4.0 mg/kg dose (Fig. 6). Thus, we observed no locomotor activating effect of muscimol and no significant difference between genotypes. However, both genotypes show the sedative effect of the drug at the 4 mg/kg dose (P < 0.05, Tukey test). Thus, as predicted, GAD65−/− mice do not respond differently to the GABA-A receptor agonist muscimol, an agent, which, in contrast to diazepam, does not rely on the presence of GABA for its effect.
Figure 6.
Dose-response curve for muscimol. Distance traveled in centimeters for a 30-min time bin, beginning 15 min after drug administration is indicated as a function of muscimol dose. Data are shown as mean ± SEM. There is no difference between wild-type (filled columns) and GAD65-deficient (open columns) mice. Both genotypes exhibit the sedative effects of muscimol at the highest dose (P < 0.05 compared with vehicle, Tukey test).
DISCUSSION
A distinction between the roles of each of the highly homologous isoforms of the GABA-synthesizing enzyme GAD in inhibitory neurotransmission is starting to emerge. Thus, GAD67 is required for synthesizing sufficient levels of GABA to maintain essential functions that sustain life (12, 13), whereas GAD65-generated GABA appears to be essential for regulating responses to environmental signals. We previously have shown that GAD65-deficient mice exhibit spontaneous seizures, which can be induced by mild stress (9). Furthermore, GABA generated by GAD65 is critical for the loss of visual cortical responses that occur in an eye briefly deprived of vision (11). Here we show that GAD65-deficient mice exhibit increased anxiety-like responses in two behavioral paradigms: the open field test and the elevated zero maze. The wild-type and knockout mice have been backcrossed onto the C57BL/6 background for 4–7 generations and thus still contain some remaining genetic heterogeneity. This heterogeneity should be equally present in both the wild-type and knockout animals, thus highlighting the penetrance of the phenotype. The elevated zero maze, similar to the elevated plus maze, is used to screen compounds for anxiolytic or anxiogenic effects (25, 28–30). Anxiolysis by diazepam typically results in hyperlocomotion (27, 30, 31), which is considered an increase in exploratory activity on reduced anxiety. Additionally, GAD65−/− mice show a loss of response to the locomotor activating effect of the benzodiazepine diazepam, which augments GABA function at GABA-A receptors. GAD65-deficient mice also exhibit a diminished response to the effects of barbiturates. Additionally, we show that there is no postsynaptic change in GABA-A receptor density, based on (i) the behavioral response to muscimol, an agent that acts at GABA-A receptors in the absence of endogenous GABA and (ii) radioligand receptor binding studies. Given that the receptor binding studies were done on crude brain homogenates, alterations in regional GABA-A receptor levels or subunit usage have not been excluded. Nevertheless, the increased anxiety and diminished responses to low doses of diazepam and the sedative dose of pentobarbital in GAD65−/− mice are likely to be a direct effect of a lack of GAD65-generated GABA without a modulation by postsynaptic events. Based on these results, we propose that GAD65-generated GABA is involved in modulating responses to anxiogenic stimuli.
Alterations in GABAergic neurotransmission during times of stress have been proposed to modulate the stress response (33–36), which underlies the therapeutic activity of benzodiazepines in anxiety and stress management. The anxiety-like behavioral effects observed in the GAD65-deficient mice are consistent with the suggested role of GABAergic neurotransmission in animal models of anxiety. For instance, elevation of GABA levels, through inhibition of GABA transaminase (GABA-T), produces anxiolytic-like effects in numerous tests in animal models including conflict (37), open field (38), social interaction (24), light/dark exploration (39), and elevated plus-maze (24) procedures. GABA reuptake inhibitors also have anxiolytic-like effects in punished drinking tests (e.g., refs. 40 and 41). Direct GABA-A agonists (e.g., muscimol) produce anxiolytic profiles in ethological models, such as the plus-maze (e.g., refs. 24, 42, and 43). Accordingly, the prototypical anxiolytic compound diazepam reduces anxiety in the elevated plus-maze (25). Conversely, the noncompetitive GABA-A antagonist picrotoxin produces anxiety-like behavior in conditioned suppression (44–48), social interaction (49), light/dark exploration (27), and elevated plus-maze (50) tests in animal models. The competitive GABA-A antagonist bicuculline also has been shown to increase anxiety in the elevated plus-maze (25).
Based on subcellular localization, membrane association, and cofactor association, GAD65 has been suggested to be involved in generating GABA for rapid release (2, 5, 51). In fact, in vivo microdialysis experiments revealed a defect in GABA release on maximum K+ stimulation in GAD65−/− mice (11). We propose that elevated anxiety in GAD65−/− animals represents a decrement in the ability of GAD65-deficient mice to rapidly release GABA during stressors, rather than changes in sensitivity of postsynaptic GABA-A receptors. In accord with this hypothesis, we find the activity of drugs that depend on endogenous GABA release are blunted in GAD65−/− mice, whereas the effects of drugs that directly activate postsynaptic receptors appear unaffected in mutant animals. Thus, these animals provide a useful model for examining the role of GABAergic neurotransmission in responses to stress and anxiety.
Acknowledgments
We thank Dr. Lora Heisler for thoughtful comments and Raquel Nagy and Anne Neill for technical assistance. This work was supported by grants from the National Institutes of Health (S.B. and L.H.T.) and the March of Dimes (L.H.T.) and by a fellowship from the Juvenile Diabetes Foundation International (S.F.K.).
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Erlander M G, Tillakaratne N J, Feldblum S, Patel N, Tobin A J. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman D L, Houser C R, Tobin A J. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldblum S, Erlander M G, Tobin A J. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- 4.Christgau S, Schierbeck H, Aanstoot H J, Aagaard L, Begley K, Kofod H, Hejnaes K, Baekkeskov S. J Biol Chem. 1991;266:21257–21264. [PubMed] [Google Scholar]
- 5.Christgau S, Aanstoot H J, Schierbeck H, Begley K, Tullin S, Hejnaes K, Baekkeskov S. J Cell Biol. 1992;118:309–320. doi: 10.1083/jcb.118.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reetz A, Solimena M, Matteoli M, Folli F, Takei K, De Camilli P. EMBO J. 1991;10:1275–1284. doi: 10.1002/j.1460-2075.1991.tb08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh M, Uchimura H. Neurochem Res. 1981;6:1283–1289. doi: 10.1007/BF00964349. [DOI] [PubMed] [Google Scholar]
- 8.Martin D L, Martin S B, Wu S J, Espina N. J Neurosci. 1991;11:2725–2731. doi: 10.1523/JNEUROSCI.11-09-02725.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kash S F, Johnson R S, Tecott L H, Noebels J L, Mayfield R D, Hanahan D, Baekkeskov S. Proc Natl Acad Sci USA. 1997;94:14060–14065. doi: 10.1073/pnas.94.25.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji F Y, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 11.Hensch T K, Fasiolini M, Matasa N, Stryker M P, Baekkeskov S, Kash S. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condie B G, Bain G, Gottlieb D I, Capecchi M R. Proc Natl Acad Sci USA. 1997;94:11451–11455. doi: 10.1073/pnas.94.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R G, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Proc Natl Acad Sci USA. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieghart W. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 15.Bormann J. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 16.Study R E, Barker J L. Proc Natl Acad Sci USA. 1981;78:7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen R W. Annu Rev Pharmacol Toxicol. 1982;22:245–277. doi: 10.1146/annurev.pa.22.040182.001333. [DOI] [PubMed] [Google Scholar]
- 18.Guidotti A, Corda M G, Wise B C, Vaccarino F, Costa E. Neuropharmacology. 1983;22:1471–1479. doi: 10.1016/0028-3908(83)90115-6. [DOI] [PubMed] [Google Scholar]
- 19.Rogers C J, Twyman R E, Macdonald R L. J Physiol. 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eghbali M, Curmi J P, Birnir B, Gage P W. Nature (London) 1997;388:71–75. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- 21.Biggio G, Concas A, Costa E, editors. GABAergic Synaptic Transmission: Molecular, Pharmacological, and Clinical Aspects. Vol. 47. New York: Raven; 1992. [Google Scholar]
- 22.Mohler H, Benke D, Benson J, Luscher R, Fritschy J. In: The GABA Receptors. Enna S, Bowery N, editors. Totowa, NJ: Humana; 1997. pp. 11–36. [Google Scholar]
- 23.Pesold C, Treit D. Brain Res. 1994;638:295–301. doi: 10.1016/0006-8993(94)90662-9. [DOI] [PubMed] [Google Scholar]
- 24.Corbett R, Fielding S, Cornfeldt M, Dunn R W. Psychopharmacology. 1991;104:312–316. doi: 10.1007/BF02246029. [DOI] [PubMed] [Google Scholar]
- 25.Dalvi A, Rodgers R J. Psychopharmacology. 1996;128:380–397. doi: 10.1007/s002130050148. [DOI] [PubMed] [Google Scholar]
- 26.Lister R G. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 27.Shimada T, Matsumoto K, Osanai M, Matsuda H, Terasawa K, Watanabe H. Gen Pharmacol. 1995;26:205–210. doi: 10.1016/0306-3623(94)00148-g. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd J K, Grewal S S, Fletcher A, Bill D J, Dourish C T. Psychopharmacology. 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 29.Pellow S, File S E. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 30.Griebel G, Sanger D J, Perrault G. Psychopharmacology. 1996;124:245–254. doi: 10.1007/BF02246664. [DOI] [PubMed] [Google Scholar]
- 31.Seale T W, Niekrasz I, Garrett K M. NeuroReport. 1996;7:1803–1808. doi: 10.1097/00001756-199607290-00023. [DOI] [PubMed] [Google Scholar]
- 32.Peters J A, Kirkness E F, Callachan H, Lambert J J, Turner A J. Br J Pharmacol. 1988;94:1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drugan R C, Basile A S, Ha J H, Ferland R J. Brain Res. 1994;661:127–136. doi: 10.1016/0006-8993(94)91189-4. [DOI] [PubMed] [Google Scholar]
- 34.Fluck E, Hogg S, Jones R B, Bourne R, File S E. Pharmacol Biochem Behav. 1997;58:269–274. doi: 10.1016/s0091-3057(96)00554-0. [DOI] [PubMed] [Google Scholar]
- 35.Wilson M A, Biscardi R. Exp Brain Res. 1994;101:297–306. doi: 10.1007/BF00228750. [DOI] [PubMed] [Google Scholar]
- 36.Bowers G, Cullinan W E, Herman J P. J Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanger D. Life Sci. 1985;36:1503–1513. doi: 10.1016/0024-3205(85)90374-1. [DOI] [PubMed] [Google Scholar]
- 38.Barros H, Tannhauser S, Tannhauser M, Tannhauser M. Brazil J Med Biol Res. 1992;25:281–287. [PubMed] [Google Scholar]
- 39.de Angelis L. Drug Dev Res. 1992;25:331–338. [Google Scholar]
- 40.Giusti A, Guidotti A, Danysz W, Auta J, Costa E. Drug Dev Res. 1990;21:217–225. [Google Scholar]
- 41.Agmo A, Pruneda R, Guzman M, Gutierrez M. Arch Pharmacol. 1991;344:314–322. doi: 10.1007/BF00183006. [DOI] [PubMed] [Google Scholar]
- 42.Nastiti K, Benton D, Brain P. Behav Pharmacol. 1991;2:121–128. [PubMed] [Google Scholar]
- 43.Rodgers R, Dalvi A. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 44.Soubrie P, Thiebot M-H, Simon P. Pharmacol Biochem Behav. 1979;10:463–469. doi: 10.1016/0091-3057(79)90218-1. [DOI] [PubMed] [Google Scholar]
- 45.Prado de Carvalho L, Venault P, Rossier J, Chapouthier G. Soc Neurosci Abstr. 1983;9:128. [Google Scholar]
- 46.Quintero S, Henney H, Lawson P, Mellanby J, Gray J. Psychopharmacology. 1985;85:244–251. doi: 10.1007/BF00428424. [DOI] [PubMed] [Google Scholar]
- 47.Corda M, Biggio G. Neuropharmacology. 1986;25:541–544. doi: 10.1016/0028-3908(86)90181-4. [DOI] [PubMed] [Google Scholar]
- 48.Stutzmann J, Böhme G, Cochon M, Roux M, Blanchard J. Psychopharmacology. 1987;91:74–79. doi: 10.1007/BF00690930. [DOI] [PubMed] [Google Scholar]
- 49.File S, Lister R. Neuropharmacology. 1984;23:793–796. doi: 10.1016/0028-3908(84)90113-8. [DOI] [PubMed] [Google Scholar]
- 50.Rodgers R, Cole J. In: Ethology and Psychopharmacology. Cooper S, Hendrie C, editors. Chichester: Wiley; 1994. pp. 9–44. [Google Scholar]
- 51.Martin D L, Martin S B, Wu S J, Espina N. Neurochem Res. 1991;16:243–249. doi: 10.1007/BF00966087. [DOI] [PubMed] [Google Scholar]