Abstract
Background
Hemangiomas are the most common type of congenital anomaly in childhood. Although many resolve spontaneously, intervention is required when their growth could damage vital adjacent structures. Various therapeutic approaches to childhood hemangiomas with different types of laser have been described previously. The objective of this study was to determine whether the cooling of the epidermis during irradiation of hemangiomas with a Nd:YAG laser prevents thermal damage and decreases the number of sessions required to treat these lesions.
Methods
Between 1993 and 2001, 110 patients aged 3 months to 4 years, with cutaneous hemangiomas were treated with a Nd:YAG laser. The lesion was cooled with ice prior to, during, and after the irradiation. During each session the laser beam passed through the pieces of ice. The laser power was between 35–45 W with a pulse length of 2–10 seconds.
Results
After 6 months of follow-up, from the first session of laser treatment, total resolution was obtained in 72 (65.5%) patients. A second or third session followed in 30 out of 38 patients in which, the initial results were good, moderate, or poor. The parents of the remaining eight children refused this second session and these patients excluded from the study Complications were seen in nine (8.8%) patients. One patient had postoperative bleeding which stopped spontaneously, while atrophic scars occurred in six (5.8%) patients, and hypertrophic scars in two (1.9%) patients.
Conclusions
Nd:YAG laser irradiation in conjunction with ice protection of the epidermis produces good cosmetic results for the treatment of cutaneous hemangiomas in children, and decreases the number of sessions for treatment of these lesions.
Keywords: Children, Hemangioma, Nd:YAG Laser
Background
A hemangioma is a benign congenital vascular malformation and the most common type of tumor in infancy. It has been reported that 1.1 – 2.6% of newborns suffer from this vascular lesion, and this number increases to 10.1% by 12 months of age [1]. They are usually absent at birth but appear and enlarge during the first 6 months of life, and often continue to grow until 12 months of age. After this proliferative phase, slow involution follows in most cases, with almost complete disappearance by 5–10 years of age [2-4]. However, in 10 – 20% of cases with cutaneous hemangioma, complications appear such as ulceration and bleeding, and the involvement of important functions such as vision, respiration, hearing, or feeding. These complications, along with cosmetic disfigurement, are a clear indication for treatment and should immediately lead to a suitable therapy [4-8]. Although laser irradiation has been used to induce photo-thermal destruction of hemangiomas, thermal damage to the epidermis remains a concern [6,9,10]. The objective of this study was to determine the efficacy of combining Nd:YAG photocoagulation with the protective use of ice for definitive treatment of hemangiomas in the pediatric population.
Methods
From January 1993 to December 2001, 110 patients with cutaneous hemangiomas were treated in our department with a Nd:YAG laser (Neodym:YAG laser, MCW 100, 1989 Germany, Aesculap). Eight-two (74.5%) were female, and 28 (25.5%) were male, and their ages ranged from 3 months to 4 years with a mean age of 9 months. In all cases the diagnosis of hemangiomas was confirmed clinically. The anatomic location of the hemangiomas varied, such as on the cheek, flank, scalp, torso, shoulder, hand, lip, forehead, eyelid, eyebrow, and nose. All the patients in this study did not receive any other treatments such as steroids, interferon, e.c.t. Preoperative evaluation in all 110 patients included medical history, a complete physical examination, and blood count (i.e., hematocrit and, platelet count). The indications for treatment were as follows: in 72 (65.5%) patients the hemangioma had affected a vital function such as, vision, respiration, hearing, or feeding; in 25 (22.7%) patients there was recurrent ulceration and hemorrhage; and in 13 (11.8%) patients significant cosmetic disfigurement and psychological problems related to appearance were present. Each hemangioma was categorized after measuring its length, width, and height. On the basis of area (length × width) they were divided into three categories: (a) 1 to 4 cm2, (b) 4 to 10 cm2, and (c) greater than 10 cm2; on the basis of height, they were divided into three categories: (A) 1 to 2 cm, (B) 2 to 3 cm, and (C) greater than 3 cm. Table 1 shows the patient allocations on these bases. Lesions were measured in cube centimeters and recorded in a database.
Table 1.
| Height | ||||
| Area | A (1–2 cm) | B (2–3 cm) | C (>3 cm) | |
| a (1–4 cm2) | 72 | 8 | - | |
| b (4–10 cm2) | 18 | 2 | 2 | |
| c (> 10 cm2) | - | 4 | 4 | |
All patients were treated under general anesthesia. Our technique consists of the following procedures: The surface of the lesion was cooled by application of broken pieces of ice, for 10–15 minutes prior to irradiation with a Nd:YAG laser. The laser-induced coagulation was performed in a repetitive manner with the hand-piece, of the irradiation delivery system, being held perpendicular to the skin with the lesion at its focal point. The laser beam was guided through a piece of ice that was in direct contact with the skin in order to protect the epidermis from the thermal damage of irradiation. Moreover, compressing the tissue with this piece of ice can increase the depth of penetration of the laser beam. The laser power was between 35–45 W, with a pulse length of 2–10 seconds. Laser energy was delivered to all areas of the vascular lesion. The average time for the laser photocoagulation procedure varied from 15 to 30 minutes depending on the extent of the lesion. The session lasted until edema appeared in the lesion, and its consistency became hard. After radiation the lesion was protected with continuous application of ice for 5–10 minutes. In all cases the patients wore protective eye covering. A topical application of antibiotic ointment was all that was necessary for the treated area. The treatment results were assessed by measuring the change in size of the lesion, and were classified as follows: "Excellent" 90–100 % area reduction, "Good" 50–89% area reduction, "Moderate" 20–49% area reduction, and "Poor" 0–19% area reduction. In cases where the lesion remained, after the first session of therapy, a second or third session of therapy followed. Postoperatively, the patients were seen at 3 weeks, 6 weeks, 3 months, 6 months, 1 year and yearly thereafter.
Results
The postoperative hospital stay for all patients ranged from 1 to 2 days. Three weeks after treatment all lesions appeared with edema and with a slightly hard consistency. The size of the lesions had decreased only slightly. At the 6 week follow-up edema was no longer present, and all lesions had decreased in size so as not to interfere with vital functions. At the 6 month follow-up the results were clinically evident (see Table 2) (Fig. 1). A second session followed in 30 out of 38 patients in which, the initial results were good, moderate, or poor. The parents of the remaining eight children refused this second session either because of social inconvenience (six children), or because they were disappointed with the initial results (two children) and these patients dropped out from the study. Six months after the second session 24 of these patients had excellent results and six patients had moderate results (Fig. 2). A third session followed for these six patients. Six months after the third session the results were good in all patients (Fig. 3). Complications were encountered in nine (8.8%) patients. One patient had postoperative bleeding, which stopped spontaneously within 1 hour. Atrophic scars occurred in six patients (5.8%). In three of them the scars appeared after the second session and were small in size; a surgical excision was performed with good results. In the other three the scars presented after the third session and were large in size; no surgical excision was done. Large hypertrophic scars occurred in two patients (1.9%) after the third session; no surgical excision was accomplished.
Table 2.
| Results | Height | ||||
| A | B | C | |||
| (1–2 cm) | (2–3 cm) | (>3 cm) | |||
| a | (1–4 cm2) | ||||
| Excellent | 72 (100%) | ||||
| Good | 8 (100%) | ||||
| b | (4–10 cm2) | ||||
| Area | Good | 15 (83,33%) | 1 (50%) | ||
| Moderate | 3 (16,67%) | 1 (50%) | 1 (50%) | ||
| Poor | 1 (50%) | ||||
| c | (>10 cm2) | ||||
| Moderate | 2 (50%) | 2 (50%) | |||
| Poor | 2 (50%) | 2 (50%) | |||
Excellent: 90 to 100 percent reduction. Good: 50 to 89 percent reduction. Moderate: 20 to 49 percent reduction. Poor: 0 to 19 percent reduction.
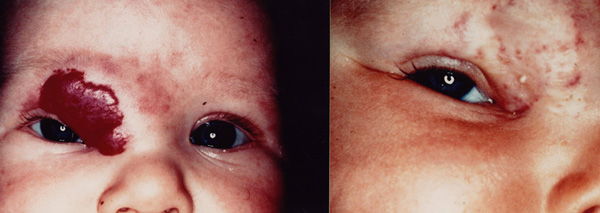
Figure 1.
a) Superficial hemangioma on the right periorbital region in a 5-month-old girl. b) Clinical response after one session with a Nd:YAG photocoagulation.
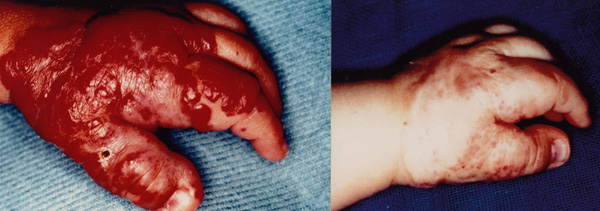
Figure 2.
a) Massine hemangiomas on the left hand in a 6-month-old girl. b) Regression after three Nd:YAG laser treatments.
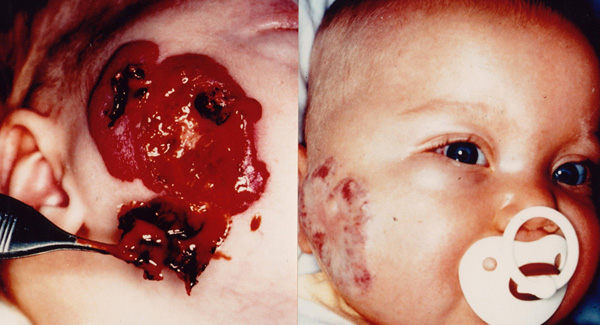
Figure 3.
a) Ulcerated and bleeding hemangioma on the right side of cheek in a 4-month-old boy. b) The same patient after two treatments with a Nd:YAG laser.
Discussion
Most hemangiomas do not require treatment, as most of them resolve spontaneously. Batta et al reported a series of 121 infants with early haemangiomas. They observed that about 40% of these showed complete clearance or minimal residual sign at the age of 1 year without treatment [11]. Nevertheless, some complications are indications for therapy. A number of treatment modalities have been proposed for the treatment of hemangiomas, including: systemic therapy with corticosteroids, interferon-a2a, surgical procedures, compression, sclerosis treatment, embolization, intralesional injection of corticosteroids, and cryotherapy [6,12-16].
One of the most significant advances in the treatment of hemangiomas has been the advent of laser technology, and its clinical application in pediatric surgery at the end of 1960's [17]. Since then, there have been many reports of the treatment of these lesions using different types of laser, including the argon, CO2, the Nd:YAG laser, the flash lamp-pumped pulsed dye laser (FPDL) and more recently the long-pulsed tunable dye laser (LPTDL) [18,19]. The FPDL is recommended as the most effective treatment for port-wine stains and superficial skin teleangiectasia [5,7,20]. Satisfactory results have also been obtained with argon laser [21]. Nevertheless, the restricted effective depth (of only 1–2 mm), and the rather non-specific coagulation of vascular lesion associated, with a risk scaring limits, the use of the argon laser in children. [22].
Although most authors agree that the initial therapeutic modality in the management of hemangiomas should be the administration of corticosteroids (systemic or interstitial) [23,24], some others have questioned their efficacy [25]. We did not use corticosteroid therapy as there are well-documented risks, such as disseminated varicella and herpes infection. The major adverse effects of the corticosteroids (growth retardation, cushingoid characteristics) are also a reasons to limit their use in children.
Landthaler et al. reported a series of hemangiomas treated with different types of lasers. They observed that in the superficial hemangiomas the results with the FPDL were excellent or good, but in the deep part of the lesion was not influenced by the FPDL [7]. They concluded that the Nd:YAG laser is the treatment modality of choice for thick hemangiomas. We have chosen the Nd:YAG laser as a therapeutic modality because its irradiation penetrates approximately 8 mm into soft tissue, and the scattering in the tissue effects deep photocoagulation.
There are several methods of cooling utilized with laser treatment including cryogen spray cooling, contact cooling incorporated into laser hand-pieces and air cooling [19,26,27]. In our special therapeutic technique we used ice to prevent laser-induced thermal injury to the epidermis, but modified the methods of other authors, who cooled the lesions with cryogen spray prior and during actual irradiation [28,29]. Our cooling of the surface of the lesion with application of broken pieces of ice prior to irradiation, provoked vasoconstriction of superficial vessels with consequent diminution of blood in these, which in turn helped to diminish the absorption of laser beam, and minimized adverse thermal effects on the skin. During irradiation we used a piece of ice to protect the epidermis and compress the lesion, which helped the beam to penetrate and induce photocoagulation in the deep layers of the lesion, thus producing even better results. Also, immediately after irradiation we protected the surface of lesion with ice because the increased residual temperature can provoke, thermal damage. Our use of ice produced excellent clinical and cosmetic results in the treatment of hemangiomas with a Nd:YAG laser, in contrast to Landthaler et al. who observed superficial scarring in all patients treated by a Nd : YAG laser. They used an output power of 50 W with exposure time of up to 1 second [7]. They performed coagulation in four infants (aged 4-months to 14-months) and observed regression of the lesions in three of them. The fourth child, an 11-month-old girl with a pulsating extensive hemangioma of the lower tip, treated with two laser sessions with no effect on the size of hemangioma. Lesions in this series were very similar in 10 patients included in our study. Our results were excellent in all of them. Moreover, we treated an hemangioma similar to the above described hemangioma of the 11-month-old girl with excellent results.
Our results clearly demonstrate the ability of the Nd:YAG laser to successfully treat cutaneous hemangiomas of various sizes. In small and shallow (height <2 cm, area <4 cm2) hemangiomas we have obtained excellent results after only one session of laser treatment. Larger and deeper hemangiomas (height > 2 cm, area > 4 cm2) were reduced by approximately 50% during the first session, while for the residual lesion a second or third session was needed to achieve excellent results, contrasting the results of Clymer et al. who reported an average of approximately five treatments to achieve the final result [3]. They used interstitial Nd:YAG coagulation in eight children with hemangiomas aged 2 months to 8 years. The power settings were between 15 and 25 W, with a pulse length of 0.3 to 1.0 seconds. They observed regression in the size of hemangiomas in all of their patients with good cosmetic results. In our series an average of 1.3 treatments per patient were required to obtain optimal results. When the 72 patients with small and shallow hemangiomas (height < 2 cm, area < 4 cm2) were removed from the analysis the remaining 30 patients needed an average of 2.2 treatments to achieve the end results. We believe that the ice cooling of the epidermis with manual compression of ice cube upon the lesion permitted us to increase the power setting, the exposure time, and the depth of penetration of the laser beam.
We conclude that use of the Nd:YAG laser in conjunction with ice protection of the epidermis is a very useful instrument in the treatment of massive and deep hemangiomas (height > 2 cm, area > 4 cm2) in childhood, in terms of minimizing the adverse thermal effects on the skin, and decreasing the number of sessions required to treat these lesions.
Authors' contributions
I Vlachakis designed the study, followed up patients, did patients assessments, and writing of the report.
S Gardikis helped with study design, analyzed the data, and drafted the final report.
E. Michailoudi helped with study design, contributed to the writing of the report.
G. Charissis conceived the study, helped with design of the study, recruited patients, did all laser treatments, contributed to the writing of the report, and acquired funding for the study.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ioannis Vlachakis, Email: johnbl@otenet.gr.
Stefanos Gardikis, Email: sgardik@med.duth.gr.
Eleni Michailoudi, Email: charissi@ics.forth.gr.
Georgios Charissis, Email: charissi@ics.forth.gr.
References
- Amir J, Metzker A, Krinler MB, Reisner SH. Strawberry haemangioma in preterm infants. Pediatr Dermatol. 1986;3:331–332. doi: 10.1111/j.1525-1470.1986.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Berlien HP, Muller G, Wldschmidt J. Lasers in pediatric surgery. Prog Pediatr Surg. 1990;25:5–22. doi: 10.1007/978-3-642-87707-0_2. [DOI] [PubMed] [Google Scholar]
- Clymer M, Fortune S, Reinisch L, Toriumi D, Werkhaven J, Ries R. Interstitial Nd:YAG photocoagulation for vascular malformations and hemangiomas in childhood. Arch Otolaryngol Head Neck Surg. 1998;124:431–436. doi: 10.1001/archotol.124.4.431. [DOI] [PubMed] [Google Scholar]
- Fishman S, Mulliken J. Hemangiomas and vascular malformations in infancy and childhood. Pediatr Clin North Am. 1993;40:1177–1200. doi: 10.1016/s0031-3955(16)38656-4. [DOI] [PubMed] [Google Scholar]
- Garden JM, Bakus AD, Passer AS. Treatment of cutaneous hemangiomas by the flashlamp-pumped dye laser: prospective analysis. J Pediatr. 1992;120:555–560. doi: 10.1016/s0022-3476(05)82481-3. [DOI] [PubMed] [Google Scholar]
- Glassberg E, Lask G, Rabinowitz L, Tunnessen W. Capillary hemangiomas: Case study of a novel laser treatment and a review of therapeutic options. J Dermatol Surg Oncol. 1989;15:1214–1223. doi: 10.1111/j.1524-4725.1989.tb03235.x. [DOI] [PubMed] [Google Scholar]
- Landthaler M, Hohenleuther U, El-Raheem TA. Laser therapy of childhood hemangiomas. Br J Dermatol. 1995;133:275–281. doi: 10.1111/j.1365-2133.1995.tb02629.x. [DOI] [PubMed] [Google Scholar]
- Shapshay S, David L, Zeitels S. Neodymium – YAG laser photocoagulation of hemangiomas of the head and neck. Laryngoscope. 1987;97:323–329. [PubMed] [Google Scholar]
- Achauer B, Vander Kam V. Capillary hemangioma (Strawberry Mark) of infancy: Comparision of Argon and Nd-YAG laser treatment. Plast Reconstr Surg. 1989;84:60–69. [PubMed] [Google Scholar]
- Anvari B, Tanenbaunt BS, Hoffman W, Said S, Milner T, Liaw L, Nelson JS. Nd:YAG laser irradiation in conjunction with cryogen spray cooling deep and spatially selective photocoagulation in animal models. Phys Med Biol. 1997;42:265–282. doi: 10.1088/0031-9155/42/2/001. [DOI] [PubMed] [Google Scholar]
- Batta K, Goodyear H, Moss C, Williams H, Hiller L, Waters R. Randomized controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: Results of 1-year analysis. Lancet. 2002;360:521–527. doi: 10.1016/S0140-6736(02)09741-6. [DOI] [PubMed] [Google Scholar]
- Enjolras O, Riche MC, Merland JJ, Escand JP. Management of alarming hemangiomas in infancy: a review of 25 cases. Pediatrics. 1990;85:491–498. [PubMed] [Google Scholar]
- Ezekowitz RA, Mulliken JB, Folkan J. interferon α-2a therapy for life-threatening hemangiomas in infancy. N Engl J Med. 1992;326:1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- Stringer G. Giant hemangioma: treatment with intermittent pneumatic compression. J Pediatr Surg. 1987;22:7–10. doi: 10.1016/s0022-3468(87)80004-0. [DOI] [PubMed] [Google Scholar]
- Woods JE. Extended use of sodium tetracyl sulfate in treatment of hemangiomas and other related conditions. Plast Reconstr Surg. 1987;79:542–549. doi: 10.1097/00006534-198704000-00005. [DOI] [PubMed] [Google Scholar]
- Sloan GM, Reinisch JF, Nichter LS, Saber WL, Lew K, Morwood DT. Intralesional corticosteroid therapy for infantile hemangioma. Plast Reconstr Surg. 1989;83:459–467. doi: 10.1097/00006534-198903000-00009. [DOI] [PubMed] [Google Scholar]
- Hendersen BM, Goldman L, Martin LW, Rockwell RJ. The laser in pediatric surgery. J Pediatr Surg. 1968;3:263–270. doi: 10.1016/0022-3468(68)90010-9. [DOI] [PubMed] [Google Scholar]
- Garden JM, Bakus AD, Paller AS. Treatment of cutaneous hemangiomas by the flashlamp pulsed dye laser: prospective analysis. J Pediatr. 1992;120:555–560. doi: 10.1016/s0022-3476(05)82481-3. [DOI] [PubMed] [Google Scholar]
- Scherer K, Lorenz S, Wimmershoff M, Landthaler M, Hohenleutner U. Both the flashlamp-pumped dye laser and the long-pulsed tunable dye laser can improve results in port-wine stain therapy. Br J Dermatol. 2001;145:79–84. doi: 10.1046/j.1365-2133.2001.04285.x. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Kelly KM, Van Gemert MJ, Nelson JS. Comparing the effectiveness of 585-nm vs. 595-nm wavelength pulsed dye laser treatment of port wine stains in conjunction with cryogen spray cooling. Lasers Surg Med. 2002;31:352–8. doi: 10.1002/lsm.10102. [DOI] [PubMed] [Google Scholar]
- Dixon JA, Rotering RH, Huether SE. Patients evaluation of argon laser therapy of port wine stain, decorative tattoo and essential telangiectasia. Lasers Surg Med. 1984;4:181–190. doi: 10.1002/lsm.1900040210. [DOI] [PubMed] [Google Scholar]
- Brauner G, Schliftman A, Cosman B. Evaluation of Argon laser surgery in children under 13 years of age. Plast Reconstr Surg. 1991;87:37–43. doi: 10.1097/00006534-199101000-00007. [DOI] [PubMed] [Google Scholar]
- Brown SH Jr, Neerhout RC, Fonkalsrud EW. Prednisone therapy in the management of large hemangiomas in infants and children. Surgery. 1972;71:168–173. [PubMed] [Google Scholar]
- Kushner BJ. The treatment of periorbital infantile hemangioma with intralesional corticosteroid. Plast Reconstr Surg. 1985;76:517–524. doi: 10.1097/00006534-198510000-00005. [DOI] [PubMed] [Google Scholar]
- Bartoshesky LE, Bull M, Feingold M. Corticosteroid treatment of cutaneous hemangiomas: how effective? a report on 24 children. Clin Pediatr. 1978;17:625–638. doi: 10.1177/000992287801700807. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Nanda VS, Nelson JM. treatments of port-wine stain birtmarks using the 1.5-msec Pulsed Dye Laser at high fluences in conjuction with cryogen spray cooling. Dermatl Surg. 2002;28:309–313. doi: 10.1046/j.1524-4725.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- Torres JH, Tunnell JW, Pikkula BM, Anvari B. An analysis of heat removal during cryogen spray cooling and effects of simultaneous airflow application. Lasers Surg Med. 2001;28:477–86. doi: 10.1002/lsm.1077.abs. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Anvari B, Nelson JS. Cryogen spray cooling for spatially selective photocoagulation of hemangiomas: a new methodology with preliminary clinical reports. Plast Reconstr Surg. 1998;102:459–463. doi: 10.1097/00006534-199808000-00029. [DOI] [PubMed] [Google Scholar]
- Nelson JS, Milner TE, Anvari B, Tanenbaum BS, Kimel S, Svaasand LO, Jacques SL. Dynamic epidermal cooling during pulsed laser treatment of port-wine stain. A new methodology with preliminary clinical evaluation. Arch Dermatol. 1995;131:695–700. doi: 10.1001/archderm.131.6.695. [DOI] [PubMed] [Google Scholar]