Abstract
Cardiac hypertrophy is a major predictor of future morbidity and mortality. Recent investigation has centered around identifying the molecular signaling pathways that regulate cardiac myocyte reactivity with the goal of modulating pathologic hypertrophic programs. One potential regulator of cardiomyocyte hypertrophy is the calcium-sensitive phosphatase calcineurin. We show here that calcineurin enzymatic activity, mRNA, and protein levels are increased in cultured neonatal rat cardiomyocytes by hypertrophic agonists such as angiotensin II, phenylephrine, and 1% fetal bovine serum. This induction of calcineurin activity was associated with an increase in calcineurin Aβ (CnAβ) mRNA and protein, but not in CnAα or CnAγ. Agonist-dependent increases in calcineurin enzymatic activity were specifically inhibited with an adenovirus expressing a noncompetitive peptide inhibitor of calcineurin known as cain [Lai, M. M., Burnett, P. E., Wolosker, H., Blackshaw, S. & Snyder, S. H. (1998) J. Biol. Chem. 273, 18325–18331]. Targeted inhibition of calcineurin with cain or an adenovirus expressing only the calcineurin inhibitory domain of AKAP79 attenuated cardiomyocyte hypertrophy and atrial natriuretic factor expression in response to angiotensin II, phenylephrine, and 1% fetal bovine serum. These data demonstrate that calcineurin is an important regulator of cardiomyocyte hypertrophy in response to certain agonists and suggest that cyclosporin A and FK506 function to attenuate cardiac hypertrophy by specifically inhibiting calcineurin.
Cardiac hypertrophy is an adaptive response to various intrinsic and extrinsic stimuli. This hypertrophy can be broadly defined as increased two-dimensional cell surface area and re-expression of fetal genes. Prolonged hypertrophy is associated with decompensation, dilated cardiomyopathy, arrhythmia, fibrotic disease, sudden death, or heart failure (1). To elucidate the mechanisms underlying hypertrophy, investigators have used cultured rat neonatal ventricular cardiomyocytes because they respond to various stimuli and undergo hypertrophic growth similar to the adult myocardium (2).
Treatment of cultured cardiomyocytes with hypertrophic agonists such as phenylephrine (PE), angiotensin II (AngII), and endothelin-1 (ET-1) results in activation of G protein-coupled receptors (GPCR) and down-stream pathways such as the mitogen-activated protein kinase- (MAPK) signaling cascade (reviewed in ref. 3). Treatment of cultured cardiomyocytes with pharmacologic inhibitors or transient transfection of activated and dominant negative MAPK-signaling factors has implicated an important role for extracellular signal-regulated kinases in cardiomyocyte hypertrophy (reviewed in refs. 4 and 5). However, other studies have disputed the importance of extracellular signal-regulated kinase signaling in cardiomyocyte hypertrophy (6–10) and have instead implicated important roles for p38 MAPK (10–12) or c-Jun N-terminal kinase (9, 13, 14). Despite the differing accounts, it is likely that each of the MAPK-signaling branches plays a role in some aspect of agonist-induced cardiomyocyte hypertrophy (15).
We have recently implicated another intracellular signaling pathway in cardiomyocyte hypertrophy through the calcium-dependent phosphatase calcineurin (16). Cardiac-specific expression of a constitutively active form of calcineurin A in transgenic mice generated hypertrophy that progressed to heart failure (16). The pharmacologic inhibitors of calcineurin, cyclosporin A, and FK506, prevent hypertrophy of cultured neonatal cardiomyocytes induced by AngII or PE (16). Similarly, cyclosporin A and FK506 were shown to inhibit cardiac hypertrophy in transgenic mouse models of hypertrophic cardiomyopathy and in pressure-overloaded rat hearts (17). Although the beneficial effects of these drugs were attributed to calcineurin inhibition, each has multiple biological effects that are independent of calcineurin.
To evaluate the necessity of calcineurin in cardiomyocyte hypertrophy, without the side-effects of cyclosporin A or FK506, we targeted calcineurin by expressing two distinct noncompetitive peptide inhibitors by using adenoviral-mediated gene transfer [Adcain and AdAKAP (A-kinase anchoring protein)]. These constructs attenuated agonist-induced calcineurin activity, cardiomyocyte hypertrophy, and elevated atrial natriuretic factor (ANF) expression.
Materials and Methods
Primary Cardiomyocytes Cultures, Transfections, and Immunocytochemistry.
Cardiomyocyte cultures were isolated by enzymatic disassociation of 1- to 2-day-old neonatal rat hearts as described previously (18). After isolation, cardiomyocytes were cultured overnight in M199 media supplemented with 15% fetal bovine serum (FBS), penicillin/streptomycin (100 units/ml), and l-glutamine (2 mM) and thereafter cultured in serum-free media supplemented with Nutridoma (Boehringer Mannheim). Cardiomyocytes were plated on gelatinized 6- and 10-cm plates at a preadherent density of 0.5–1.0 and 1.5–2.5 million cells, respectively.
Cardiomyocytes were prepared for immunocytochemistry as described previously (16). Anti-α-actinin antibody (Sigma) and anti-ANF polyclonal antiserum (Peninsula Laboratories) were added at dilutions of 1:800 and 1:300, respectively. Alternatively, cells were hybridized with anti-flag monoclonal M2 antibody (Sigma) at a dilution of 1:500. Nuclear staining was performed with 0.5 μg/ml bisbenzimide.
Transfections were performed by the calcium phosphate method with 8 μg of a luciferase reporter containing multimeric nuclear factor of activated T cells (NFAT)-binding sites, 2 μg of a plasmid encoding NFAT3, and 2 μg of the mammalian pECE-flag expression vector.
Measurements of cell surface area was performed on α-actinin-labeled cardiomyocytes by using video edge detection and NIH image analysis software, similar to previous descriptions (10, 13).
Plasmid Construction.
A cDNA fragment corresponding to the 3′ of cain was obtained as a mouse expressed sequence tag. A 582-bp fragment corresponding to amino acids 1989–2182 was generated by PCR and subcloned into the SmaI site of the pECE-flag. To generate recombinant adenovirus, the flag-cain cDNA fragment was subcloned into pACCMVpLpA (19). A cDNA fragment encoding amino acids 60–358 of AKAP79 (lacking the protein kinase C- and protein kinase A-binding sites) was generated by PCR and subcloned into pECE-flag, which was then subcloned into pACCMVpLpA (gift from John D. Scott, Vollum Institute, Portland, OR).
Replication-Deficient Adenovirus Production.
Adβgal is a replication-deficient, E1A-deleted, recombinant adenovirus expressing a nuclear localized β-galactosidase gene (20). To generate recombinant adenovirus, the pACCMVpLpA-cain or AKAP plasmids were cotransfected with pJM17 in HEK293 cells (19). Each adenovirus was plaque purified, expanded, and titered by detection of visible plaques in a HEK293 monolayer by agarose overlays (21). Cardiomyocyte cultures were infected at a multiplicity of infection of 100 plaque-forming units/cell in 2 ml (6-cm dish) or 5 ml (10-cm dish) of DMEM (GIBCO/BRL) supplemented with 2% FBS for 2 hr at 37°C in a humidified, 5% CO2 incubator. Under these conditions, ≈98% of the cells were infected (assessed by flag antibody-directed immunocytochemistry).
Western Blotting.
Protein extracts for Western blotting were generated from two, 10-cm plates of cardiomyocytes per lane in extraction buffer (20 mM sodium phosphate/150 mM NaCl/2 mM MgCl2/0.1% Nonidet P-40/10% glycerol/10 mM NaF/0.1 mM sodium orthovanadate/10 mM sodium pyrophosphate/1 mM DTT, 10 μg/ml leupeptin/10 μg/ml aprotinin/10 μg/ml pepstatin/10 μg/ml N-tosylphenylalanyl chloromethyl ketone/10 μg/ml N-tosyllysyl chloromethyl ketone). Samples were subjected to SDS-PAGE, transferred to poly(vinylidene difluoride) membrane, and immuno-detected with the ECF kit (Amersham). Antibodies used in this study were against calcineurin Aα (CnAα) and CnAβ (Transduction Laboratories, Lexington, KY), or a isoform-specific CnAα, CnAβ, or CnAγ antibody (Santa Cruz Biotechnology), or a glyceraldehyde-3-phosphate dehydrogenase antibody (Research Diagnostics, Flanders, NJ). Blots were quantified for fluorescence with a Storm 860 PhosphorImager (Molecular Dynamics).
Reverse Transcription–PCR (RT-PCR) mRNA Quantitation.
Analysis of mRNA levels for CnAα, CnAβ, CnAγ, ANF, and L7 (ribosomal protein, control) were performed with primers designed to detect either mouse or rat gene products. CnAα used primers 5′-ACTGGCATGCTCCCCAGCGGA and 5′-GTGCCGTTAGTCTCTGAGGCG, resulting in a 244-bp and 214-bp product (two spliced forms). CnAβ used primers 5′-CCACAGGGATGTTGCCTAGTG and 5′-GTCCCGTGGTTCTCAGTGGTA, resulting in a 242-bp and 212-bp product. CnAγ used primers 5′-TCCCACAGGCACACTCCCACT and 5′-CAGGGCTTTCTTTCCATGGTC, generating a 279-bp fragment. ANF detection used primers 5′-GCCCTGAGCGAGCAGACCGA and 5′-CGGAAGCTGTTGCAGCCTA, generating a 202-bp fragment. L7 detection used primers 5′-GAAGCTCATCTATGAGAAGGC and 5′-GAGACGGAGCAGCTGCAGCAC, generating a 240-bp fragment. RT-PCR reactions were performed with 1 μg of total RNA in the presence of [α-32P]dCTP as recommended by the manufacturer (Titan one tube RT- PCR, Boehringer Mannheim), followed by 22 cycles of PCR amplification.
Calcineurin Phosphatase Activity Assay.
Cardiomyocytes were collected in 1.5 ml of trypsin-EDTA (GIBCO/BRL), pelleted, and lysed in 100 μl of calcineurin assay buffer (BioMol, Plymouth Meeting, PA). The Quantizyme Assay System AK-804 was performed by using 3 μg of protein according to the manufacturer's procedure (BioMol). Calcineurin phosphatase activity was measured spectrophotometrically by detecting free-phosphate released from the calcineurin-specific RII phosphopeptide.
Statistical Analysis.
Data are presented as means ± SE. The data were evaluated for significance by using a nonpaired Student's t test or one-way ANOVA followed by Bonferroni multiple comparison test using instat software (Graphpad, San Diego, CA).
Results
Calcineurin Is Activated by Hypertrophic Agonist in Cultured Cardiomyocytes.
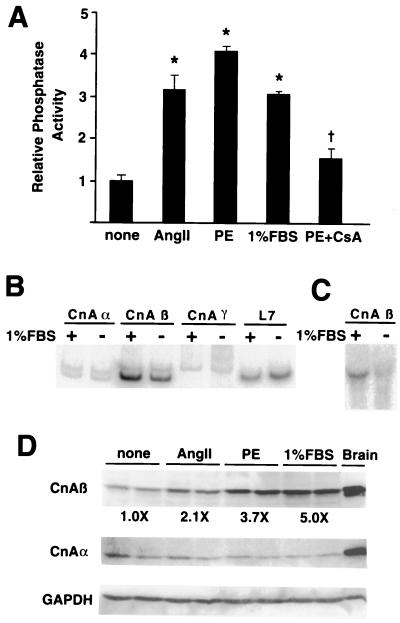
Because cardiomyocyte hypertrophy is associated with enhanced calcium handling, we investigated the enzymatic activity of calcineurin (calcium activated) in cultured cardiomyocytes treated with AngII, PE, or 1% FBS. We determined that calcineurin activity was significantly increased by AngII (3.2 ± 0.3 fold), PE (4.1 ± 0.1 fold), and 1% FBS (3.1 ± 0.1 fold) (P < 0.0001). PE-activated phosphatase activity was significantly attenuated by cotreatment with 500 ng/ml of cyclosporin A (P < 0.001) (Fig. 1A).
Figure 1.
Calcineurin is activated by agonist-stimulated hypertrophy. Cultured cardiomyocytes were stimulated for 48 hr with AngII (100 nM), 1% FBS, or PE (50 μM). (A) Calcineurin phosphatase activity was induced by each agonist whereas cyclosporin A (CsA) significantly attenuated PE-induced calcineurin activity. The data represent two independent experiments, each performed in triplicate. (B) RT-PCR analysis demonstrated a significant induction of CnAβ, but not CnAα, CnAγ, or L7 mRNA in response to 1% FBS in cardiomyocytes. (C) Northern analysis confirms the increase in CnAβ mRNA levels. (D) Western blot analysis demonstrated an increase in CnAβ protein but not CnAα and GAPDH in response to agonist. Rat brain is shown as an enriched source of both CnAα and CnAβ. *, P < 0.0001 vs. control; †, P < 0.001 vs. PE.
There are three known genes that encode catalytic calcineurin A protein, CnAα, CnAβ, and CnAγ (22). Isoform-specific RT-PCR demonstrated a hypertrophy-associated induction in only CnAβ mRNA levels (2.5-fold), whereas no changes were noted in CnAα, CnAγ, or L7 (control) (Fig. 1B). Two splice variants were observed for both CnAβ and CnAα. Interestingly, only the 212-bp splice form of CnAβ was up-regulated by agonist stimulation. The specific increase in CnAβ mRNA levels was further confirmed by Northern blot analysis (3.2-fold) (Fig. 1C).
Consistent with mRNA levels, Western blotting demonstrated an agonist-dependent increase in CnAβ protein level but no change in CnAα or GAPDH (Fig. 1D). It was not possible to resolve protein migration differences due to alternate splicing in CnAβ, nor was CnAγ protein detectable in cardiomyocytes (data not shown). Taken together, these data indicate that hypertrophic agonists induce calcineurin activity in cultured cardiomyocytes, which is associated with a specific increase in CnAβ mRNA and protein.
The Cain Inhibitory Peptide Attenuates Calcineurin Activity and NFAT Transcriptional Responses.
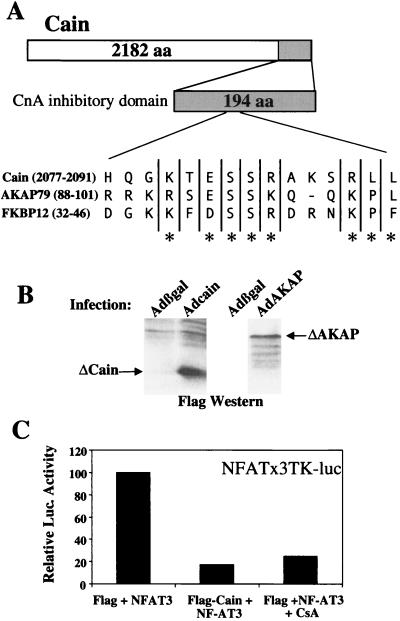
Cyclosporin A and FK506 inhibit calcineurin indirectly through immunophilin proteins (22). Because these drug-immunophilin complexes have multiple effects, we designed a unique strategy to diminish calcineurin activity by expressing an inhibitory protein domain. Using a yeast-two hybrid screen, two groups identified a novel, noncompetitive inhibitor of calcineurin referred to as cain or cabin 1 (23, 24). The calcineurin binding/inhibitory domain was limited to a 38-aa region located in the C terminus of this protein. Interestingly, the calcineurin inhibitory domain of cain contains a conserved motif that is also found in the noncompetitive calcineurin inhibitory domain of AKAP79 and FKBP12 (25, 26) (Fig. 2A).
Figure 2.
Strategy for targeted inhibition of calcineurin. (A) The noncompetitive calcineurin inhibitory domain of cain contains a putative motif that is conserved in AKAP79 and FKBP12. (B) Adenoviral expression of a 194-aa cain peptide (21 kDa) or a 299-aa AKAP79 peptide (33 kDa) in neonatal cardiomyocytes shows stable expression by flag Western blotting. (C) Cotransfection of a NFAT-dependent luciferase reporter plasmid, an expression vector encoding NFAT3, and a flag-tagged cain (194 aa) expression vector or cyclosporin inhibited reporter activation.
An expression vector encoding the inhibitory domain of cain was transfected into cardiomyocytes along with an NFAT-dependent reporter and a NFAT3 expression vector. Cain expression or cyclosporin extinguished NFAT3 transcriptional activity, a measure of calcineurin activity (Fig. 2C). cDNA fragments encoding a 194-aa fragment of cain or a 299-aa fragment of AKAP79 (lacking the protein kinase C- and protein kinase A-binding sites) were fused to a flag epitope and used to generate replication-deficient adenovirus. Western blotting against the flag epitope demonstrated that each adenovirus generated a stable protein product in neonatal cardiomyocytes (Fig. 2B).
Adcain Attenuates Endogenous Calcineurin Activity.
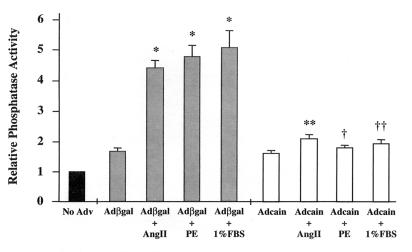
Calcineurin phosphatase activity was measured in cardiomyocytes stimulated with AngII, PE, or 1% FBS and infected with either Adcain or Adβgal (control). Adenoviral infection preceded agonist stimulation by 24 hr to allow adequate expression. Consistent with Fig. 1, calcineurin phosphatase activity was significantly increased by AngII, PE, and 1% FBS in cardiomyocytes infected with Adβgal control (P < 0.001) (Fig. 3). However, Adcain infection significantly reduced the increase in calcineurin activity induced by each agonist (P < 0.0001) (Fig. 3).
Figure 3.
Agonist induced increases in calcineurin activity are inhibited by Adcain. Cultured cardiomyocytes were infected with either Adβgal or Adcain, and 24 hr later, stimulated with AngII (100 nM), PE (50 μM), or 1% FBS for 48 hr. Agonist-stimulated cardiomyocytes infected with Adβgal had increased calcineurin phosphatase activity, whereas Adcain infection prevented this increase. The data represent two independent experiments performed in triplicate. *, P < 0.001 vs. Adβgal control; **, P < 0.0001 vs. Adβgal + AngII; †, P < 0.0001 vs. Adβgal + PE; ††, P < 0.0001 vs. Adβgal + 1% FBS.
Targeted Inhibition of Calcineurin Prevents Cardiomyocyte Hypertrophy.
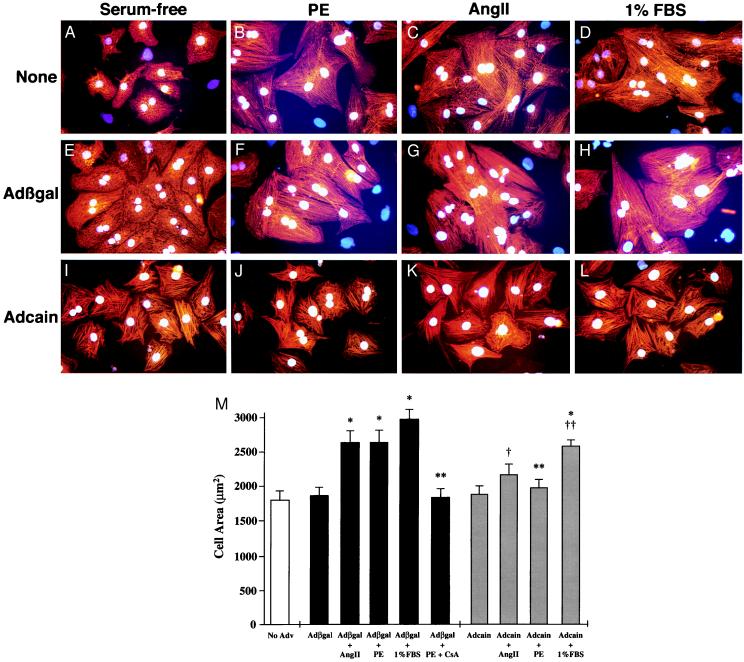
To establish the necessity of calcineurin in cardiomyocyte hypertrophy, we investigated the effects of Adcain infection on the morphological changes induced by AngII, PE, and 1% FBS over 48 hr. Cardiomyocytes were infected with Adcain (Fig. 4 I–L), Adβgal (Fig. 4 E–H), or simply left in serum-free media (Fig. 4 A–D). The data demonstrate that Adcain infection attenuates cardiomyocyte hypertrophy in response to AngII, PE, or 1% FBS (Fig. 4 I–L). Adenoviral infection with either Adβgal (Fig. 4 E–H) or a nonexpressing adenovirus did not inhibit agonist-induced hypertrophy (data not shown). Importantly, Adcain infection did not induce cardiomyocyte apoptosis nor did it affect the morphology (Fig. 4I) and viability of unstimulated cells (data not shown). These data indicate that Adcain is not compromising the “health” of infected cardiomyocytes.
Figure 4.
Adcain infection attenuates agonist induced hypertrophy. Cardiomyocytes were subjected to Adcain infection (I–L), Adβgal infection (E--H), or were left uninfected (A–D); 24 hr later, cells were either untreated (A, E, and I), treated with 50 μM PE (B, F, and J), 100 nM Ang II (C, G, and K), or 1% FBS (D, H, and L) for 48 hr. Cardiomyocytes were identified with α-actinin antibody (red signal) and nuclei were stained with bis-benzamide (blue/white). (M) Adcain infection or cyclosporin (CsA) treatment prevented the increase in cardiomyocyte cell area induced by AngII and PE, and attenuated the 1% FBS-stimulated increase. The data are means ± SEM from 50–75 random cells measured in each group and were confirmed in two additional experiments. *, P < 0.001 vs. Adβgal control; **, P < 0.01 vs. Adβgal + PE; †, P < 0.05 vs. Adβgal + Ang II; ††, P < 0.05 vs. Adβgal + 1% FBS.
Quantitation of hypertrophy was performed by video edge detection on large groups of myocytes (see Materials and Methods). The data demonstrate significant increases in cardiomyocyte surface area with AngII, PE, or 1% serum stimulation in control Adβgal-infected cells (P < 0.001) (Fig. 4M). Adcain infection prevented AngII and PE-stimulated increases in cell surface area, and significantly attenuated the 1% FBS-mediated increase (P < 0.05) (Fig. 4M).
Targeted Inhibition of Calcineurin Inhibits ANF Expression.
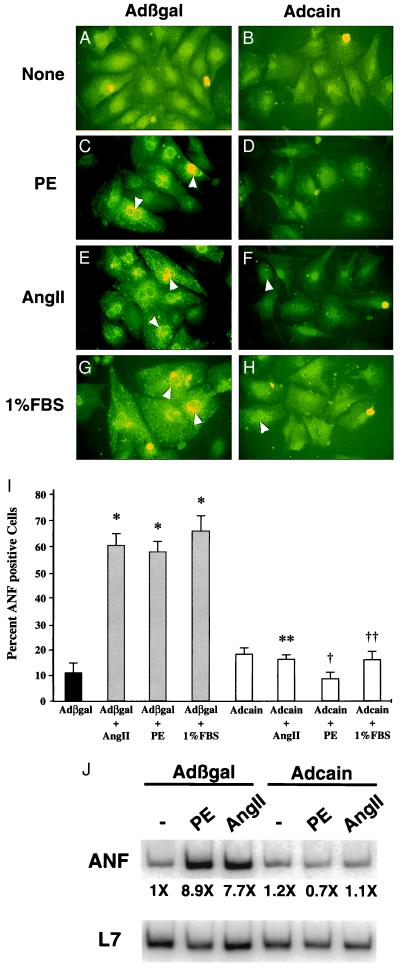
Increased ANF expression is a hallmark of cardiac hypertrophy and is readily detected by immunocytochemistry as perinuclear staining (12, 13). Cardiomyocyte cultures were infected with either Adcain or Adβgal, followed 24 hr later by agonist stimulation for an additional 48 hr. Stimulation with PE, AngII, or 1% FBS induced ANF protein expression in control Adβgal-infected cells (Fig. 5 C, E, and G), whereas Adcain infection blocked ANF expression (Fig. 5 D, F, and H). The ANF panels correspond to the α-actinin-stained panels shown in Fig. 4 E–L. Quantitation of ANF expression demonstrated a significant increase in ANF-positive cells in response to AngII, PE, and 1% serum (P < 0.0001), whereas Adcain abrogated this increase (P < 0.0001) (Fig. 5I). To confirm these results, RT-PCR was performed to quantify ANF mRNA levels (Fig. 5J). The data demonstrate that PE and AngII induced an ≈8-fold increase in ANF mRNA, which was inhibited with Adcain (48 hr).
Figure 5.
Adcain inhibits ANF expression. The same panels of cardiomyocytes shown in Fig. 4 were colabeled with ANF antibody (green). (A, C, E, and G) Cardiomyocytes infected with Adβgal showed increased ANF protein expression after agonist stimulation, whereas Adcain-infected cells (B, D, F, and H) did not respond to agonist. The white arrowheads indicate positive perinuclear ANF staining. The arrowheads in F and H represent Adcain cardiomyocytes that were barely expressing ANF. (I) Quantitation of percent of ANF expressing cells demonstrated significant expression in Adβgal-infected cultures, whereas Adcain blocked agonist-induced expression. (J) RT-PCR quantitation demonstrated that Adcain blocked ANF mRNA expression in agonist stimulated cardiomyocyte cultures. *, P < 0.0001 vs. Adβgal control; **, P < 0.0001 vs. Adβgal + Ang II; †, P < 0.0001 vs. Adβgal + PE; ††, P < 0.0001 vs. Adβgal + 1% serum.
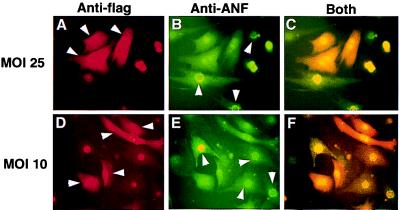
Adcain infection was performed at a multiplicity of infection of 100 plaque-forming units/cell in 2 ml of media (6-cm dish) for 2 hr. To control for detrimental effects associated with adenoviral infection itself or effects due to nonmyocytes in the cultures, we decreased infectivity to 10 and 25 plaque-forming units/cell, resulting in ≈30% and 70%, respectively, of the cardiomyocytes being infected. Co-immunostaining demonstrated (Fig. 6) that PE-induced ANF expression (green) was abated in cells expressing cain (red), but robustly expressed in neighboring cells void of cain. These data demonstrate that cain specifically inhibits the hypertrophic response in a cell autonomous manner.
Figure 6.
Concentration independence of Adcain inhibition. Cardiomyocytes were infected with Adcain at a multiplicity of infection of 25 (A–C) or 10 (D–F) and treated with 50 μM PE for 48 hr. Cardiomyocytes were immunostained with a primary mAb against the flag-epitope (cain) in red and for ANF in green. The data demonstrate that cells expressing cain (arrowheads in A and D) lack ANF expression, and cells expressing ANF (arrowheads in B and E) lack cain expression. (C and F) Overlaid images of both antibodies are shown. The 21-kDa cain-flag peptide fusion was found in both the cytosol and nucleus.
AdAKAP Infection Blocks Cardiomyocyte Hypertrophy.
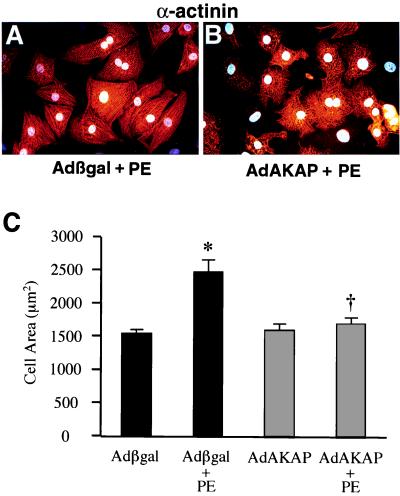
Although the expressed domain of cain was sufficient to inhibit calcineurin in cardiomyocytes, it is formally possible that this region has other biologic effects. A specific domain in the docking protein AKAP79 was previously shown to inhibit calcineurin in a noncompetitive manner (25). We designed an additional adenoviral strategy to inhibit calcineurin and validate the specificity of cain by expressing the calcineurin inhibitory domain of AKAP79 (amino acids 60–358). Interestingly, the calcineurin inhibitory subdomain of AKAP79 is also present in cain and FKBP12 (Fig. 2). Similar to Adcain infection, AdAKAP prevented PE-induced calcineurin activity (data not shown). AdAKAP also blocked agonist-induced cellular hypertrophy and ANF expression (Fig. 7A-C, and data not shown). Collectively, these data indicate that Adcain and AdAKAP attenuate cardiomyocyte hypertrophy by specifically inhibiting calcineurin.
Figure 7.
AdAKAP (60–358 amino acids) infection attenuates cardiomyocyte hypertrophy. (A and B) AdAKAP infection, but not Adβgal, attenuated morphologic cardiomyocyte hypertrophy induced by PE. (C) Quantitation of cell surface area demonstrated a significant increase in Adβgal-infected cells treated with PE, which was prevented by AdAKAP infection. *, P < 0.001 vs. Adβgal; †, P < 0.001 vs. Adβgal + PE.
Discussion
Hypertrophic Agonists Use Calcium and Calcineurin.
It is well established that GPCR ligands such as AngII, PE, and ET-1 are capable of inducing cardiomyocyte hypertrophy through the activation of a wide array of intracellular signaling pathways (3). These agonists have been shown to significantly induce inotropy and calcium transients in cultured cardiomyocytes (27–30), of sufficient magnitude to saturate calmodulin (31). In this report, we establish that calcineurin is also activated by certain GPCR ligands in cultured neonatal cardiomyocytes.
A critical question that remains is the mechanism whereby inotropic increases in calcium might lead to calcineurin activation in cardiomyocytes. Calcineurin activation and subsequent NFAT transcriptional responses have previously been shown to require a sustained elevation in intracellular calcium concentrations (32). Given the oscillatory nature of calcium handling in cardiomyocytes, the aforementioned study might suggest that calcineurin only responds to elevated basal calcium levels and not to increases in transient calcium release. However, a subsequent study revealed that NFAT transcriptional responses can be regulated by cumulative calcium oscillations, demonstrating that calcium transients increase both the efficacy and the information content of calcium signals leading to gene expression and cell differentiation (33). Because AngII, ET-1, and PE are not thought to increase basal calcium levels in cardiomyocytes, it suggests a working model whereby enhanced calcium transients lead to calcineurin activation. Alternatively, subcellular calcium triggers might act as the inducing signal for calcineurin activation. Indeed, targeted disruption of calreticulin, an endoplasmic reticulum calcium handling protein, results in a loss of inducible NFAT3 nuclear translocation in cultured cells (34).
Targeted Inhibition of Calcineurin Attenuates Cardiomyocyte Hypertrophy.
In a previous study, we reported that cyclosporin A and FK506 could prevent cardiomyocyte hypertrophy in response to AngII and PE (16) and could prevent cardiac hypertrophy in various rodent models of heart disease (17). However, it is becoming increasingly clear that cyclosporin and FK506 have multiple biological effects independent of calcineurin. To directly target calcineurin activity, we used a strategy involving the calcineurin inhibitory protein cain/cabin 1 (23, 24) and AKAP79 (25, 26). Adenoviral expression of the calcineurin inhibitory domain from either protein repressed cardiomyocyte hypertrophy in response to agonist but did not detrimentally affect unstimulated cells. Because cyclosporin A and FK506 also attenuate cardiomyocyte hypertrophy, these collective data solidify the notion that calcineurin is an important regulator of cardiac reactive signaling.
Multiple Necessary Factors Regulate the Hypertrophic Program.
The observations presented in this study indicate that calcineurin is required for hypertrophic growth of cardiomyocytes in culture. However, this does not exclude the requirement of other intracellular signaling pathways. A number of studies have implicated a requirement for a wide range of signaling factors in cardiac hypertrophy. Hearts of transgenic mice overexpressing a dominant negative G protein fail to efficiently hypertrophy in response to pressure overload, suggesting that GPCR are necessary in cardiac responsiveness (35). Downstream of GPCR, a number of regulatory factors are implicated in cardiac hypertrophy. Pharmacologic inhibition of MAPK-signaling factors has demonstrated a necessary role of p38 and extracellular signal-regulated kinase signaling in mediating hypertrophic growth of cultured cardiomyocytes (10–12, 15). Using adenoviral gene transfer, dominant negative SEK1 (MKK4), a regulator of c-Jun N-terminal kinase activation, prevented the hypertrophic response of cardiomyocytes to ET-1 (14).
Collectively, these studies underscore the multifactorial nature of hypertrophic reactive signaling pathways in cardiomyocytes, suggesting that many regulatory pathways are coordinately required for full responsiveness, but that inhibition of central pathways can short-circuit the entire response. Additional studies will be required to elucidate the interconnectivity between calcineurin and other intracellular signaling pathways to gain a greater understanding of the coordinate regulation of cardiac hypertrophy.
Acknowledgments
We thank Dr. Bruce C. Trapnell for assistance with adenovirus. This work was supported by National Institutes of Health Grants HL-69562, HL-62927, a Scholar award from the Pew Foundation, and local American Heart Affiliate grant-in-aid to J.D.M. H.W.L was supported by National Institutes of Health Training Grant T32 ES07051.
Abbreviations
- AKAP
A-kinase anchoring protein
- AngII
angiotensin II
- PE
phenylephrine
- ANF
atrial natriuretic factor
- CnAα
β, or γ, calcineurin Aα, β, or γ
- ET-1
endothelin-1
- GPCR
G protein-coupled receptors
- MAPK
mitogen-activated protein kinase
- RT-PCR
reverse transcription-PCR
- NFAT
nuclear factor of activated T cells
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Levy D, Garrison R J, Savage D D, Kannel W B, Castelli W P. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Sadoshima J, Izumo S. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 3.Dorn G W, II, Brown J H. Trends Cardiovasc Med. 1999;9:26–34. doi: 10.1016/s1050-1738(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 4.Sugden P H. Circ Res. 1999;84:633–646. doi: 10.1161/01.res.84.6.633. [DOI] [PubMed] [Google Scholar]
- 5.Olson E N, Molkentin J D. Circ Res. 1999;84:623–632. doi: 10.1161/01.res.84.6.623. [DOI] [PubMed] [Google Scholar]
- 6.Thorburn J, Frost J A, Thorburn A. J Cell Biol. 1994a;126:1565–1572. doi: 10.1083/jcb.126.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorburn J, McMahon M, Thorburn J Biol Chem. 1994;269:30580–30586. [PubMed] [Google Scholar]
- 8.Post G R, Goldstein D, Thuerauf D J, Glembotski C C, Brown J H. J Biol Chem. 1996;271:8452–8457. doi: 10.1074/jbc.271.14.8452. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez M T, Sah V P, Zhao X L, Hunter J J, Chien K R, Brown J H. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 10.Zechner D, Thuerauf D J, Hanford D S, McDonough P M, Glembotski C C. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemoto S, Sheng Z, Lin A. Mol Cell Biol. 1998;18:3518–3526. doi: 10.1128/mcb.18.6.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Huanf S, Sah V P, Ross J, Heller Brown J, Han J, Chien K R. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Su B, Sah V P, Heller Brown J, Han J, Chien K R. J Biol Chem. 1998;272:5423–5426. doi: 10.1074/jbc.273.10.5423. [DOI] [PubMed] [Google Scholar]
- 14.Choukroun G, Hajjar R, Kyriakis J M, Bonventre J V, Rosenzweig A, Force T. J Clin Invest. 1998;102:1311–1320. doi: 10.1172/JCI3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerk A, Michael A, Sugden P H. J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molkentin J D, Lu J R, Antos C L, Markham B, Richardson J, Robbins J, Grant S R, Olson E N. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sussman M A, Lim H W, Gude N, Taigen T, Olson E N, Robbins J, Colbert M C, Gualberto A, Wieczorek D F, Molkentin J D. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 18.De Windt L J, Willemsen P H, Popping S, Van der Vusse G J, Reneman R S, Van Bilsen M. J Mol Cell Cardiol. 1997;8:2095–2106. doi: 10.1006/jmcc.1997.0444. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Foix A M, Coats W S, Baque S, Alam T, Gerard R D, Newgard C B. J Biol Chem. 1992;267:25129–25134. [PubMed] [Google Scholar]
- 20.Sussman M A, Baque S, Uhm C S, Daniels M P, Price R L, Simpson D, Terracio L, Kedes L. Circ Res. 1998;82:94–105. doi: 10.1161/01.res.82.1.94. [DOI] [PubMed] [Google Scholar]
- 21.Mittereder N, March K L, Trapnell B C. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klee C B, Ren H, Wang X. J Biol Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- 23.Sun L, Youn H D, Loh C, Stolow M, He W, Liu J O. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- 24.Lai M M, Burnett P E, Wolosker H, Blackshaw S, Snyder S H. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- 25.Coghlan V M, Perrino B A, Howard M, Langeberg L K, Hicks J B, Gallatin W M, Scott J D. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 26.Kashishian A, Howard M, Loh C, Gallatin W M, Hoekstra M F, Lai Y. J Biol Chem. 1998;273:27412–27419. doi: 10.1074/jbc.273.42.27412. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y J, Gopalakrishnan V. Circ Res. 1991;69:239–245. doi: 10.1161/01.res.69.1.239. [DOI] [PubMed] [Google Scholar]
- 28.Mebazaa A, Mayoux E, Maeda K, Martin L D, Lakatta E G, Robotham J L, Shah A M. Am J Physiol. 1993;265:H1841–H1846. doi: 10.1152/ajpheart.1993.265.5.H1841. [DOI] [PubMed] [Google Scholar]
- 29.Touyz R M, Fareh J, Thibault G, Tolloczko B, Lariviere R, Schiffrin E L. Am J Physiol. 1996;270:H857–H868. doi: 10.1152/ajpheart.1996.270.3.H857. [DOI] [PubMed] [Google Scholar]
- 30.Eble D M, Qi M, Waldschmidt S, Lucchesi P A, Byron K L, Samarel A M. Am J Physiol. 1998;274:C1226–C1237. doi: 10.1152/ajpcell.1998.274.5.C1226. [DOI] [PubMed] [Google Scholar]
- 31.Persechini A, Cronk B. J Biol Chem. 1999;274:6827–6830. doi: 10.1074/jbc.274.11.6827. [DOI] [PubMed] [Google Scholar]
- 32.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Nature (London) 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 33.Dolmetsch R E, Xu K, Lewis R S. Nature (London) 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 34.Mesaeli N, Nakamura K, Zvaritch E, Dickie P, Dziak E, Krause K H, Opas M, MacLennan D H, Michalak M. J Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akhter S A, Luttrell L M, Rockman H A, Iaccarino G, Lefkowitz R J, Koch W J. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]