Abstract
Dendritic spines are a key structure in neuronal plasticity. Enhanced activity is commonly associated with an increase in spine size and density. Purkinje cell dendrites are characterized by a proximal and a distal compartment on which climbing fibers and parallel fibers, respectively, impinge. The proximal region has a very low spine density, whereas the distal region has a high density. Previous experiments showed that after climbing fiber deletion, Purkinje cells become hyperactive, and a large number of spines develop on the proximal dendrites. Here we show that the same hyperspiny transformation occurs in the proximal dendrites of adult Purkinje cells by depressing electrical activity with tetrodotoxin. Thus, spines in different dendritic compartments are created or maintained independently from the level of Purkinje cell-firing rate and when the afferent activity is blocked. This conclusion supports the view that spinogenesis is the expression of an intrinsic program and the two regions of the dendritic tree respond differently to activity block because of differences in the inputs that they receive. On tetrodotoxin treatment, climbing fibers become atrophic and may sprout thin collateral ramifications directed mainly toward the granular layer. All changes are reversible on tetrodotoxin removal. Therefore, Purkinje cells provide a model where spines in different compartments of the same neuron are differently regulated by the activity of their local afferents. In addition, electrical activity is also essential to maintain the full climbing fiber innervation.
Keywords: Purkinje cell, climbing fiber, tetrodotoxin
Dendritic spines are the principal postsynaptic targets of excitatory afferents, and they are considered a main site of brain plasticity. The factors that regulate their growth during development and modulate their shape and function in the mature brain are of basic importance in understanding the mechanisms of neuronal plasticity (1). Many studies have shown that spines are under the control of intrinsic factors and environmental conditions, electrical activity being an important parameter (2–4). The role of electrical activity in the adult brain, however, has been little investigated.
The Purkinje cells (PCs) of the cerebellar cortex provide an excellent model for studying this issue, because two well-defined compartments of their dendritic arbors, characterized by a clear-cut difference in spine density, can be distinguished. The proximal compartment, consisting of the primary, secondary, and tertiary branches on which the climbing fiber (CF) terminal arbor impinges, appears smooth under the light microscope. They were described as free of spines in Golgi preparations by Cajal (5) as well as by all subsequent investigators (see ref. 6) until Larramendi and Victor (7) could demonstrate rare spines contacted by CF boutons by using electron microscopy. The distal compartment is made up of spiny branchlets, which have a fuzzy appearance because of the very high density of long-necked spines contacted by the parallel fibers (5, 7) (Fig. 1, Inset).
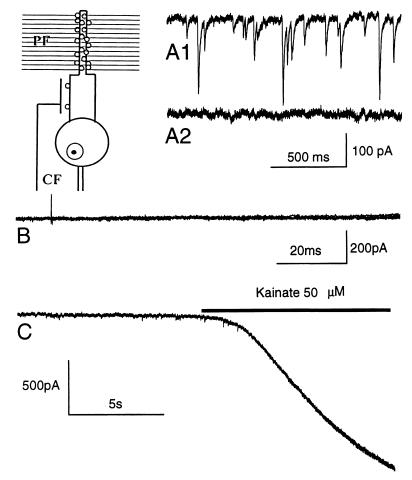
Figure 1.
Patch-clamp recording of PCs. Spontaneous inward currents elicited GABAergic afferents in control conditions (A1). These currents are not present in a cell from a TTX-treated cerebellum (A2). (B) Several superimposed traces from the same cell by applying stimuli of increasing intensity (from 1 V to 100 V); no evoked current is present. (C) In the same cell, the application of 50 μM kainate induces a large inward current, suggesting that glutamate receptors are functional. Holding potential, −70 mV. (Inset) Schematic representation of a PC with a CF impinging on the rare spines of proximal dendrites, and many parallel fibers (PF) ending on spiny branchlets with numerous spines.
A large number of new spines develop on the PC proximal dendrites either when the CF input is deleted (8) or when the axonal flow in olivocerebellar fibers is blocked by colchicine (9). In the former case, they regress on CF reinnervation (10). The hypothesis has been formulated (8, 11) that spine formation is an intrinsic property of PCs, CFs having a local repressive action on their development. However, it is not known whether electrical activity plays a role in these processes.
Recent experiments have shown that PCs in culture develop dendritic branches and protrusions resembling spines only in the presence of granule cells (12), but not when electrical activity is blocked by tetrodotoxin (TTX; ref. 13). This finding suggests that electrical activity of parallel fibers is the main determinant of PC spinogenesis.
To better clarify this issue, we have studied the effect of blocking electrical activity of the adult cerebellum on the number and density of spines in PC dendrites. Because we found a striking change in the proximal dendritic compartment, we also investigated the structural profile of the CF input.
MATERIALS AND METHODS
Toxin Delivery.
We used adult Wistar albino rats (Charles River Breeding Laboratories; body weight 150–250 g). The experimental plan was designed according to the guidelines of Italian law for care and use of experimental animals (DL 116/92) and approved by the Italian Ministry of Health. Long-lasting drug infusion (3–14 days) was performed by means of osmotic minipumps (Alzet 2001 and 2002, Alza) provided with an infusion cannula inserted into the posterior vermal cortex. TTX (Sigma) was dissolved in citrate buffer (pH 4.8). Control animals were infused with vehicle only. All surgical procedures were performed under general anesthesia obtained by intraperitoneal injection of a mixture of ketamine (100 mg/kg; Ketalar, Parke Davis) and xylazine (5 mg/kg; Rompum, Bayer, Wuppertal, Germany).
Immunocytochemistry.
At the end of the survival period, the animals were killed by an overdose of anesthetic and transcardially perfused with 4% paraformaldehyde in 0.12 M phosphate buffer (PB; pH 7.4). Free-floating cryostat cerebellar sections (30 μm thick) were stained by a monoclonal anticalbindin antibody (1/3,000, Swant, Bellinzona, Switzerland) to label PCs, visualized by the avidin-biotin-peroxidase (Vectastain Elite kit, Vector Laboratories) method, by using diaminobenzidine (DAB) as a chromogen. The effect of TTX infusion on cerebellar neuron activity was assessed by cytochrome-oxidase histochemistry (14), by incubating cerebellar sections for 2–4 hr in 0.12 M PB (pH 7.4)/0.05% DAB/0.03% cytochrome C (type III, Sigma) at 37°C.
Lucifer Yellow Staining.
In vibratome parasagittal sections (200 μm thick) from fixed cerebella of both vehicle and TTX-treated rats, individual PCs were impaled by glass microelectrodes (tip diameter 1–2 μm) and filled by iontophoretic injections (2–20 nA of negative current) of biotinylated Lucifer yellow (3% in distilled water, Molecular Probes). The slices were postfixed 30 min in 2.5% glutaraldehyde in PB, incubated with the avidin-biotin-peroxidase complex overnight in PB with 0.1% Triton X-100 at room temperature, and reacted with DAB.
Olivocerebellar Axon Visualization.
In 13 animals, the olivocerebellar system was visualized by means of the anterograde axonal tracer biotinylated dextran amine (Mr 10,000, Molecular Probes) applied according to previously described protocols (15). Briefly, while the animals were under deep anesthesia, four iontophoretic injections were made through a ventral approach into the right inferior olive by means of glass micropipettes (inner tip diameter 20–30 μm) filled with a few microliters of a 10% biotinylated dextran amine solution in 0.12 M PB (pH 7.4). Tracer injections were made 3 days before minipump implantation.
At the end of the survival period, the animals were killed and perfused as described above. The cerebella were cut in 30-μm-thick sagittal sections, collected free-floating in PBS, and incubated for 2–4 hr in the avidin-biotin-peroxidase complex. Finally, they were reacted in a DAB solution with 0.04% nickel ammonium sulfate to yield a black reaction product.
To quantify CF modifications, complete labeled arbors were selected from single sections according to previously established criteria (16). Because climbing fiber arbors have a maximal extension of 25 μm in the frontal plane (16), some of them may be completely included in a single section. These arbors were reproduced by means of the neurolucida software (Microbrightfield, Colchester, VT) based on a Pentium PC connected to an E-800 Nikon microscope with a ×100 oil-immersion lens. Statistical analysis was made by Student’s t test.
Electron Microscopy.
Ultrastructural analysis was performed on two vehicle- and three TTX-infused rats killed after 7 days of treatment and in three rats infused with TTX for 14 days and allowed to survive for 28 days. Under general anesthesia, the animals were transcardially perfused with 1,000 ml of Karnowsky double aldehyde fixative solution (1% paraformaldehyde/1% glutaraldehyde in 0.12 M PB). The brains were left in situ for 30 min at 4°C and then dissected and kept in the same fixative at 4°C overnight. The cerebellar vermis was cut in 1-mm-thick sagittal slices further reduced into small tissue blocks, which were rinsed with 8% dextrose/0.5% CaCl2 in 0.4 M PB (pH 7.4) for 24 h at 4°C. They were postfixed in 2% osmium tetroxide and embedded in Epon/Araldite resin (Fluka). Ultrathin sections were cut from cerebellar cortex by an LKB 8800 Ultratome microtome, collected on filmed grids, and stained by uranyl acetate and lead citrate. The preparations were examined in an EM Philips 410 electron microscope (Philips, Eindhoven, The Netherlands).
Proximal dendrites and branchlets were identified according to specific morphological features (6, 7), and only those having a diameter above and below 2 μm, respectively, were retained for analysis. Number of spines with or without innervation, total sampled membrane length, and spine intervals were blindly evaluated by means of a magnetic graphic tablet coupled to a PC 80486 with the sigmascan Scientific Measurement System (Jandel, San Rafael, CA). Statistical analysis was made by Student’s t test.
Patch-Clamp Recording.
Five rats, treated with TTX for 3 to 6 days, were anesthetized with halothane and decapitated. The cerebellar vermis was quickly cooled by immersion into O2/CO2-bubbled ice-cold extracellular saline solution (in mM: 125 NaCl/2.5 KCl/1.25 NaH2PO4/1 MgCl2/2 CaCl2/26 NaHCO3/20 glucose). Vibratome-cut parasagittal slices (200 μm thick) were transferred to a recording chamber placed in an experimental setup equipped with a Zeiss Axioscop Fs upright microscope. During recording, bicuculline and kainate (Sigma) were applied at 20 μM and 50 μM, respectively. PCs were whole-cell recorded by an EPC7 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany). The composition of the intracellular solution was (in mM): 130 CsCl/20 tetraethylammonium/10 Hepes/4 Na2 ATP/0.1 CaCl2/2 MgCl2/1 EGTA, pH 7.3. The signal was digitized and stored on a Macintosh Quadra 650. Stimulations were applied through a second pipette filled with the extracellular solution. Data analysis was performed with the program igor pro (WaveMetrics, Lake Oswego, OR).
RESULTS
By means of a minipump, we applied TTX to the parenchyma of the cerebellar vermis at concentrations from 30 to 1,000 μM for 14 days. We found that above a value of 100 μM, the survival of the rats was sporadic, whereas with a concentration of 70–80 μM, the majority of rats survived and showed a severe ataxia, which suggested widespread interference with normal activity of the cerebellum. Therefore, in the described experiments we applied the latter concentration for a period of 3 to 14 days and we analyzed only those rats affected by severe ataxia.
Electrophysiological and Histological Control of Activity Block.
To check that electrical activity was effectively blocked, in five rats treated with TTX for 3–6 days, we recorded the electrophysiological activity from PCs in cerebellar slices with the patch-clamp technique. Recording was started as soon as possible after removal of the minipump (about 1 h) to avoid a significant washout of the drug and lasted up to 2–3 h. PCs located in the posterior vermis were selected for our investigations. Spontaneous inhibitory currents caused by ongoing synaptic activity by GABAergic (GABA, γ-aminobutyric acid) afferents were absent (Fig. 1A1–2). In addition, it was not possible to evoke excitatory postsynaptic currents even with stimulation intensities exceeding those routinely used to generate parallel fiber and CF responses (Fig. 1B) (17). By contrast, application of kainate, a glutamate receptor agonist, elicited a large inward current (Fig. 1C), showing that the absence of excitatory postsynaptic currents evoked by presynaptic stimulation was not caused by loss of receptor function. Thus, TTX was effective in blocking both spontaneous and presynaptically evoked activity in the PCs.
To study the spatial distribution of electrical activity depression, we stained cerebella by cytochrome-oxidase histochemistry, which has been widely used as an endogenous metabolic marker for neuronal activity (18). In vehicle-treated cerebella (n = 2), the staining was homogeneously distributed and was most intense in the granule cell layer, as previously described (19). In contrast, in the TTX-treated rats (n = 3) staining was weaker over a large though variable region, with the posterior cortical lobules being usually totally affected. Cytochrome-oxidase activity in the brainstem was unchanged.
Spine Formation.
Fig. 2 a–b shows PCs stained by an anticalbindin antibody after 6 days of treatment with vehicle and TTX, respectively. With vehicle (a), a proximal dendrite, recognizable by the large diameter, is typically smooth and no spines are clearly detectable. In contrast, with TTX (b), the dendrite is covered with numerous spines. The latter feature was present in wide areas often extending to most of the TTX-treated cerebellar cortex and consistently in the entire posterior vermis of nine rats (at least 10 sections in each rat), where all PCs showed hyperspiny dendrites. Similar results were obtained at 14 days of treatment. In contrast, smooth dendrites were evident in the entire extent of the vehicle-treated cerebella, even close to the minipump cannula.
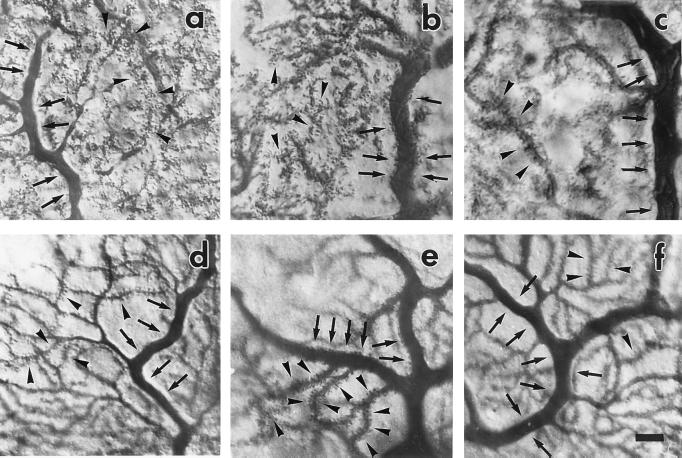
Figure 2.
PC modifications induced by TTX treatment. Calbindin immunostaining (a–c) and single Lucifer yellow-injected cells (d–f); proximal dendrites of PCs from vehicle-treated cerebella (a and d) appear typically smooth (arrows), whereas after 6 days of TTX treatment (b and e), they are covered with spines (arrows). If TTX is discontinued for 28 days after 14 days of infusion (c and f), the proximal dendrites (arrows) appear smooth again, as in a and d. Spiny branchlets (arrowheads) are consistently studded with numerous spines. (Scale bar = 15 μm.)
To better appreciate these changes at the single-cell level, we labeled PCs intracellularly by Lucifer yellow in rats treated with vehicle (five cells, two rats at 6 and 14 days of treatment) and TTX (five cells, three rats at 7 and 14 days). In the vehicle-infused rats, the proximal dendrites were smooth, while long-necked spines were abundantly present in the spiny branchlets (Fig. 2d, 6 days). In contrast, in the TTX-infused rats the proximal dendritic branches, except for a short region close to the soma, appeared to be covered with numerous spines (Fig. 2e, 7 days).
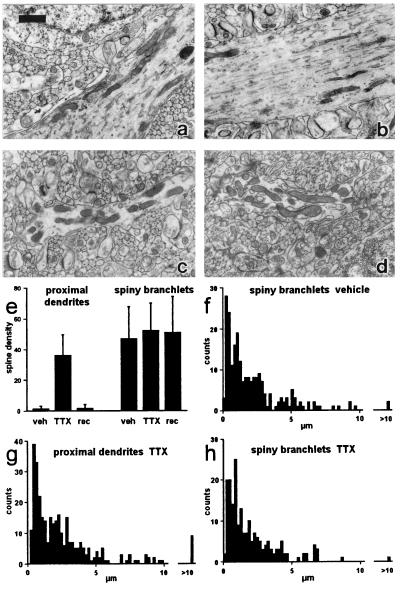
These light-microscope results were confirmed by a quantitative ultrastructural analysis performed on randomly selected electronmicrographs, taken from the posterior lobe of cerebella treated for 7 days. As shown in Table 1, the proximal dendrites of vehicle-perfused cerebella had a very low spine density (Fig. 3 a and e), whereas those of the TTX-treated ones had a high density (Fig. 3 b and e) and the difference was highly significant (P = 6.2 × 10−17). In the branchlets, the density was very high in both conditions (Fig. 3 c–e), and the difference was not significant (P = 0.31). Fig. 3 f–h shows the interspine interval histograms for proximal dendrites in TTX-treated rats (f) and for branchlets in vehicle (g) and TTX (h) conditions. A similar histogram could not be made in the proximal dendrites of vehicle-treated rats, because it was rare to find more than one spine in a single sample (only in 3 of 36 samples was there more than one spine). All histograms showed strikingly similar modal values near 1 μm. It should be noted that spine density values in proximal and distal control dendrites are similar to those reported in mice (7), i.e., 1.5 and 35, respectively, per 100 μm membrane length. They are relative values and do not reflect the actual number of spines per unit area.
Table 1.
Mean spine density (Density) per 100 μm of dendritic membrane length and mean diameter in in proximal dendrites and in spiny branchlets in vehicle and TTX-treated rats, and after 28 days of recovery
| Proximal dendrites
|
Spiny
branchlets
|
|||||
|---|---|---|---|---|---|---|
| Vehicle (n = 36) | TTX (n = 35) | Recovery (n = 36) | Vehicle (n = 32) | TTN (n = 25) | Recovery (n = 33) | |
| Density ± SD | 1.1 ± 1.9 | 36.1 ± 13.6 | 1.6 ± 2.4 | 46.90 ± 20.7 | 52.1 ± 27.1 | 51.0 ± 23.2 |
| Diameter | 3.3 | 3.8 | 4.2 | 1.3 | 1.4 | 1.2 |
| TML | 1282.7 | 1074.8 | 1389.3 | 631.9 | 414.1 | 571.0 |
TML = total sampled membrane length, μm.
Figure 3.
Ultrastructural analysis of spines. (a–d) Samples of dendritic profiles. Proximal dendrites: vehicle (a), TTX (b); spiny branchlets: vehicle (c), TTX (d). (e) Mean density values per 100 μm and standard deviations under the four conditions. (f–h) Spine interval histograms; ordinates: number of intervals; bin width: 0.2 μm. (Scale bar = 1 μm.)
These results show that by decreasing the electrical activity of the adult rat cerebellum, a large number of new spines appear in the proximal dendrites, while in the branchlets there are no significant changes. The similarity of the interspine interval distribution between branchlets and proximal dendrites suggests that in the absence of activity, the spine density tends to reach a default maximal level in the whole dendritic territory.
Climbing Fiber Modifications.
During development, changes in spine density in the visual system have been related to the rearrangement of the presynaptic arbor (20, 21). In addition, in the absence of electrical activity, growing axons may be derailed to improper target cells (22). Therefore, we studied whether the new spinogenesis in the PC proximal dendrites was related to changes in the structural profile of the CF terminal arbor. A typical arbor is made by four branch orders (16, 23). The thick proximal branches give rise to thin terminal tendrils bearing numerous small varicosities (Fig. 4a).
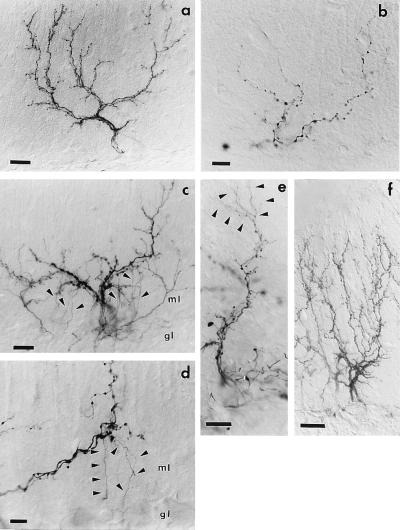
Figure 4.
CF remodeling after TTX treatment. A normal CF (a) is made of several thick ramifications and numerous fine varicose tendrils. After 14 days of TTX infusion (b–d), most tendrils have been lost, whereas the remaining proximal branches bear several enlarged boutons (b). In c and d, the arbors bear numerous sprouts (arrowheads) that originate from the proximal branches and elongate toward the granular layer (gl). At a higher magnification (d), they appear as thin unbranched processes, devoid of varicosities, which terminate in the upper granular layer with small boutons. Fourteen days after minipump removal (e), CF arbors are characterized by several thin varicose branches (arrowheads), which emanate from the thick proximal stems and elongate into the molecular layer. At 14 and 28 days (f), all CFs have reacquired the normal structure (ml = molecular layer). (Scale bars: a, c, e = 25 μm, b, d, f = 15 μm.)
In the vehicle-treated rats, CF morphology and morphometric evaluations (two rats) matched those previously reported for intact arbors (16). By contrast, in TTX-treated cerebella, CF showed severe regressive changes that were already evident at 7 days. Morphometric analysis carried out after 14 days of TTX treatment (two rats) revealed a significant decrease in the total arbor length (P = 0.02), number (P = 0.001), and density (P = 0.006) of varicosities (Table 2). A sample of a highly atrophic arbor is shown in Fig. 4b. The arbor is characterized by the virtual absence of the most distal branch orders and by a highly reduced number and density of varicosities whose size is larger.
Table 2.
Quantitative morphometric analysis of CF arbors in vehicle and TTX-treated cerebella, and after 28 days of recovery
| Vehicle (n = 5) | TTX (n = 7) | Recovery (n = 5) | |
|---|---|---|---|
| Length ± SD | 1787.9 ± 384.8 | 1111.1 ± 438.8 | 1495.0 ± 155.2 |
| Varicosities ± SD | 355.0 ± 84.5 | 137.9 ± 71.1 | 297.6 ± 31.9 |
| Density ± SD | 20.2 ± 4.8 | 11.7 ± 3.8 | 19.9 ± 0.4 |
Mean total length, number of varicosities and their density per 100 μm are reported in the three experimental conditions.
Moreover, in 12 of 36 arbors from four rats perfused with TTX for 14 days (none in 36 arbors from four vehicle-treated rats), we could observe thin branches almost devoid of varicosities arising from the thick CF trunks in the molecular layer. Most of these processes elongated unbranched toward the upper granular layer, where they ended with tiny terminal clubs (Fig. 4 c and d). They were clearly different from the very few descending collaterals reported in the normal cerebellum (6, 15, 24), which typically originate at the level of the PC perikaryon. The presence of such new ramifications indicates that the absence of electrical activity does not prevent outgrowth processes, and that the regressive phenomena are caused by a lack of receptivity by the PCs. The limited number of growing terminal arbors that are associated with a mild atrophy suggests that the outgrowth is a transient event.
Recovery from TTX-Induced Alterations.
To see whether the change in spine density was a reversible phenomenon, in eight rats TTX administration was discontinued at 14 days, and the cerebella were examined after a recovery period of 2–4 weeks. In these animals, proximal PC dendrites appeared smooth (Fig. 2 c and f), as in the vehicle-treated rats. As shown in Table 1, at 4 weeks the average spine density in the proximal dendrites had significantly recovered (P = 7.7 × 10−17), whereas no significant difference existed between rats infused with vehicle and those after recovery (P = 0.31). Also, in the branchlets the density value after recovery was not significantly different either from vehicle- (P = 0.46) or TTX- (P = 0.83) treated rats.
Concerning CF phenotype, 2–4 weeks after TTX was discontinued, arbors reacquired their normal morphological features. Fig. 4f shows one such arbor, whose pattern is within the normal range. A quantitative analysis was performed after a 28-day recovery period (two rats). As shown in Table 2, there was a significant difference in the recovery values for number (P = 0.0006) and density of varicosities (P = 0.001) relative to TTX-treated rats and no difference relative to vehicle-treated rats (P = 0.21 and P = 0.92, respectively). Concerning total length, the recovery values were not significantly different from vehicle-treated cerebella (P = 0.17), whereas they just failed to be significantly different from those of TTX-treated cerebella (P = 0.065).
DISCUSSION
Spine Formation Is an Intrinsic Property of PCs.
Spinogenesis in neurons is controlled by intrinsic and environmental factors (1–4, 25). Among extrinsic influences, neural afferent activity plays an important role both during development (21) and in the mature brain. In the intact adult brain, an increase in spine density has been reported following enhanced neuronal activity (26), exposure to an enriched environment (27), a single-experience learning event (28), and in relation to long-term potentiation (see ref. 29). In mice submitted to exercise during late development, PCs show a greater number of spines compared to inactive animals (30). In cultured hippocampal neurons, an increased spine density has been reported after enhanced spontaneous activity, whereas blockade of electrical activity by TTX leads to a reduced density at 3 days (31, 32). The main view on this issue is that neuronal activation favors spine formation, and this fact has been related to learning (27).
In our work, we investigated the role played by neural activity by taking as a model the adult PC with its two main inputs, the parallel and CFs, which impinge on separate dendritic domains. When applying TTX to the adult cerebellum, a large number of new spines appeared on the proximal PC dendrites. TTX is known to block Na+ spikes in both presynaptic and postsynaptic elements. The effectiveness of the TTX treatment was shown by the following observations: (i) the animals were ataxic; (ii) cytochrome oxydase activity was decreased in most of the cerebellum, including the posterior vermis, which was the focus of our analysis; (iii) no synaptic responses could be recorded in PCs after stimulation of afferent pathways, although postsynaptic activity could be evoked by glutamate receptor activation. The specificity of the TTX action is demonstrated by lack of spinogenesis even close to the minipump cannula when vehicle was applied. Thus, formation of a high number of spines on the proximal dendritic compartment was allowed by the block of climbing fiber input activity. Our findings are congruent with the view that spinogenesis is an intrinsic property of the PCs (8, 11), although the importance of extrinsic activity-independent mechanisms cannot be excluded.
Such a view is in contrast with the notion that spines in PC dendrites are grown under the influence of parallel fibers. Recent in vitro studies showed that dendritic development and spine formation depend on the presence (12) and on the electrical activity of granule cells (13). The discrepancy might be caused by the different experimental conditions. In our in vivo experiments, spinogenesis might have been controlled by nonneuronal elements such as astrocytes, which may induce the proliferation of PC spines through diffusible substances (33, 34). In addition, astrocytes and astrocytic factors are known to increase PC survival in culture (35). Similar diffusible substances may also be released by inactive parallel fibers. It is thus possible that in vivo environmental factors exert an activity-independent trophic influence on PCs, which allow or induce the expression of their phenotype. Alternatively, it is possible that dendritic branching and spine formation are separate and independent processes, and only the former is regulated by granule cell influences. Thus, spine formation could be started only after the PC dendritic tree has developed under parallel fiber control. In culture conditions, compartments in PC dendrites have not been distinguished. However, in our TTX experiments the typical high spine density in the spiny branchlets was maintained. Therefore, in the adult cerebellum, parallel fiber activity is not necessary to maintain these dendritic spines. This fact provides further support for the view that the activity-independent property for spinogenesis is not limited to the proximal dendritic compartment, but applies to the whole arbor.
Formation of a large number of new spines on the proximal compartment of PC dendrites also occurs after inferior olive lesion (8). In addition to a phasic excitatory action, CFs exert a tonic depression of the firing rate on PCs, as shown by the long-lasting PC hyperactivity after inferior olive inactivation or lesion (36–38). Thus, spinogenesis occurs not only when the PC electrical activity has been depressed by TTX, but also when releasing the tonic inhibitory action of the CFs enhances its firing rate. This consideration leads us to conclude that this process is set up by this neuron independently from its own electrical activity.
Climbing Fiber–PC Interactions.
CF deletion by lesions of the inferior olive induces a hyperspiny transformation of the proximal dendritic compartment of the PCs, leading to the conclusion that CFs exert a local repressive action on spinogenesis (8, 11). Our experiments show that this local repression of spinogenesis is removed by depressing electrical activity. We propose that a diffusible substance, whose release is activity dependent, mediates the local inhibitory action. This view is supported by the demonstration that block of axonal flow in the olivocerebellar fibers by colchicine also induces spinogenesis (9). TTX treatment also induces a remarkable atrophy of CF arbors, sometimes accompanied by the development of thin processes extending toward the granular layer. During development, electrical activity plays a basic role in the formation of connections with the proper target (22) and for the refinement of a highly specific pattern of connections typical of the adult brain (39). Our results show the importance of electrical activity in maintaining such a highly ordered connectivity in the adult.
There are two possible mechanisms that might explain the terminal withdrawal. First, electrical silence in the CF might induce a direct atrophy with a consequent PC deafferentation and with elimination of the substance with spine-repressing action on the proximal dendrites, as occurs following an inferior olive lesion. This possibility is unlikely because the same arbor may develop new branches, and therefore it is likely that CF atrophy is the consequence of a lack of receptivity by the PCs. Second, absence of electrical activity may prevent the release of the trophic substance from the normal terminal arbor. The ensuing changes in the postsynaptic membrane, including spinogenesis, lead to the loss of those postsynaptic cues, which are necessary to maintain the CF contacts with its target. This hypothesis is supported by the fact that atrophic changes in CF arbor similar to those here described after TTX treatment are present after PC loss (16, 40, 41). According to this view, the CF atrophy would be the direct consequence not of the absence of electrical activity but of the loss of target support (40). Interestingly, sprouting in motoneuron terminals occurs in response to the motor nerve block (42) and also after application to the neuromuscular junction of synaptic blockers, which enhance acetylcholine receptor density (43). Therefore, this observation supports the concept that changes in postsynaptic membrane characteristics induce a remodeling of the presynaptic fibers.
Concerning outgrowth phenomena, it is interesting to note that the developing processes are directed toward the upper granule cell layer, which is normally the target of a few descending collaterals arising at the PC level (6, 15, 24).
Concluding Remarks.
Spinogenesis in PCs appears to be the expression of a program that does not depend on their electrical activity and does not require electrical activity of the afferents. Such a program may be intrinsically expressed (8, 11) or may be fully developed in the entire dendritic arbor when these neurons are in their proper in vivo environment. In the spiny branchlets, parallel fiber activity is not necessary to maintain the normal set of spines, whereas, in the proximal compartment, activity of the CF induces a repression. Electrical activity is also essential to maintain the typical highly ordered pattern of connections in the adult.
Acknowledgments
We thank Prof. D. Cantino for helpful advice on electron microscopy, Prof. F. Tempia for assistance in patch-clamp recording, and Prof. R. J. Harvey for critically reading the manuscript. This work was supported by grants from the European Community (ERBBIO4-CT96-0774), Ministero dell’Università e della Ricerca Scientifica e Tecnologica (MURST), and the Consiglio Nazionale delle Ricerche (CNR). M.B. and L.M. were recipients of a fellowship from the Ottolenghi Foundation.
ABBREVIATIONS
- CF
climbing fiber
- PC
Purkinje cell
- TTX
tetrodotoxin
- DAB
diaminobenzidine
- PB
phosphate buffer
References
- 1.Shepherd G M. J Neurophysiol. 1996;75:2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- 2.Horner C H. Prog Neurobiol. 1993;41:281–321. doi: 10.1016/0301-0082(93)90002-a. [DOI] [PubMed] [Google Scholar]
- 3.Harris K M, Kater S B. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 4.Kossel A H, Williams C V, Schweizer M, Kater S B. J Neurosci. 1997;17:6314–6324. doi: 10.1523/JNEUROSCI.17-16-06314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramón y Cajal S. Histologie du système nerveux de l’homme et des vertébrés. Paris: Maloine; 1911. [Google Scholar]
- 6.Palay S L, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Berlin: Springer; 1974. [Google Scholar]
- 7.Larramendi L M H, Victor T. Brain Res. 1967;5:15–30. doi: 10.1016/0006-8993(67)90216-8. [DOI] [PubMed] [Google Scholar]
- 8.Sotelo C, Hillman D E, Zamora A J, Llinás R. Brain Res. 1975;98:574–581. doi: 10.1016/0006-8993(75)90374-1. [DOI] [PubMed] [Google Scholar]
- 9.Baetens D, Tribollet E, Garcia-Segura L M. Neurosci Lett. 1983;38:239–244. doi: 10.1016/0304-3940(83)90375-0. [DOI] [PubMed] [Google Scholar]
- 10.Rossi F, van der Want J J L, Wiklund L, Strata P. J Comp Neurol. 1991;308:536–554. doi: 10.1002/cne.903080404. [DOI] [PubMed] [Google Scholar]
- 11.Sotelo C. Prog Brain Res. 1978;48:149–170. doi: 10.1016/S0079-6123(08)61021-3. [DOI] [PubMed] [Google Scholar]
- 12.Baptista C A, Hatten M E, Blazeski R, Mason C A. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 13.Schilling K, Dickinson M H, Connor J A, Morgan J I. Neuron. 1991;7:891–902. doi: 10.1016/0896-6273(91)90335-w. [DOI] [PubMed] [Google Scholar]
- 14.Wong-Riley M T T. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- 15.Rossi F, Wiklund L, van der Want J J L, Strata P. J Comp Neurol. 1991;308:513–535. doi: 10.1002/cne.903080403. [DOI] [PubMed] [Google Scholar]
- 16.Rossi F, Borsello T, Vaudano E, Strata P. Neuroscience. 1993;53:759–778. doi: 10.1016/0306-4522(93)90622-m. [DOI] [PubMed] [Google Scholar]
- 17.Konnerth A, Llano I, Armstrong C M. Proc Natl Acad Sci USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong-Riley M T T. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- 19.Hevner R F, Liu S, Wong-Riley M T T. Neuroscience. 1995;65:313–342. doi: 10.1016/0306-4522(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 20.Sretavan D W, Shatz C J, Stryker M P. Nature (London) 1988;336:468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- 21.Dalva M B, Ghosh A, Shatz C J. J Neurosci. 1994;14:3588–3602. doi: 10.1523/JNEUROSCI.14-06-03588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catalano S M, Shatz C. Science. 1998;281:559–562. doi: 10.1126/science.281.5376.559. [DOI] [PubMed] [Google Scholar]
- 23.Strata P, Rossi F. Trends Neurosci. 1998;21:407–413. doi: 10.1016/s0166-2236(98)01305-8. [DOI] [PubMed] [Google Scholar]
- 24.Scheibel M E, Scheibel A B. J Comp Neurol. 1954;101:733–763. doi: 10.1002/cne.901010305. [DOI] [PubMed] [Google Scholar]
- 25.Luo L, Hensech T K, Ackerman L, Barbel S, Jan L Y, Jan Y N. Nature (London) 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 26.Bundman M C, Gall C M. Hippocampus. 1994;4:611–622. doi: 10.1002/hipo.450040511. [DOI] [PubMed] [Google Scholar]
- 27.Moser M B, Trommald M, Andersen P. Proc Natl Acad Sci USA. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowndes M, Stewart M G. Brain Res. 1994;654:129–136. doi: 10.1016/0006-8993(94)91578-4. [DOI] [PubMed] [Google Scholar]
- 29.Hosokawa T, Rusakov D A, Bliss T V P, Fine A. J Neurosci. 1995;15:5560–5573. doi: 10.1523/JNEUROSCI.15-08-05560.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pysh J J, Weiss G M. Science. 1979;206:230–232. doi: 10.1126/science.482938. [DOI] [PubMed] [Google Scholar]
- 31.Papa M, Segal M. Neuroscience. 1996;71:1005–1011. doi: 10.1016/0306-4522(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 32.Segal M. Trends Neurosci. 1995;18:468–471. doi: 10.1016/0166-2236(95)92765-i. [DOI] [PubMed] [Google Scholar]
- 33.Seil F J, Eckenstein F P, Reier P J. Exp Neurol. 1992;117:85–89. doi: 10.1016/0014-4886(92)90114-6. [DOI] [PubMed] [Google Scholar]
- 34.Seil F J. Neuroscience. 1997;77:695–711. doi: 10.1016/s0306-4522(96)00546-5. [DOI] [PubMed] [Google Scholar]
- 35.Jeffrey P L, Meaney J, Tolhurst O, Weinberger R P. J Neurosci Methods. 1996;67:167–175. [PubMed] [Google Scholar]
- 36.Montarolo P G, Palestini M, Strata P. J Physiol (London) 1982;332:187–302. doi: 10.1113/jphysiol.1982.sp014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benedetti F, Montarolo P G, Rabacchi S. Exp Brain Res. 1984;55:368–371. doi: 10.1007/BF00237287. [DOI] [PubMed] [Google Scholar]
- 38.Savio T, Tempia F. Exp Brain Res. 1985;57:456–463. doi: 10.1007/BF00237832. [DOI] [PubMed] [Google Scholar]
- 39.Goodman C S, Shatz C J. Cell. 1993;72:77–98. doi: 10.1016/s0092-8674(05)80030-3. [DOI] [PubMed] [Google Scholar]
- 40.Rossi F, Strata P. Prog Neurobiol. 1995;47:341–369. [PubMed] [Google Scholar]
- 41.Rossi F, Jankovski A, Sotelo C. J Neurosci. 1995;15:2040–2056. doi: 10.1523/JNEUROSCI.15-03-02040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown M C, Ironton R. Nature (London) 1977;265:459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- 43.Holland R L, Brown M C. Science. 1980;207:649–651. doi: 10.1126/science.6243417. [DOI] [PubMed] [Google Scholar]