Abstract
Background
The effect of prolonged strenuous exercise (PSE) on left ventricular (LV) systolic function has not been well studied in younger female triathletes. This study examined LV systolic function prior to, during and immediately following PSE (i.e., 40 km bicycle time trial followed by a 10 km run) in 13 younger (29 ± 6 years) female triathletes.
Methods
Two-dimensional echocardiographic images were obtained prior to, at 30-minute intervals during and immediately following PSE. Heart rate, systolic blood pressure, end-diastolic and end-systolic cavity areas were measured at each time point. Echocardiographic and hemodynamic measures were also combined to obtain LV end-systolic wall stress and myocardial contractility (i.e., systolic blood pressure – end-systolic cavity area relation).
Results
Subjects exercised at an intensity equivalent to 90 ± 3% of maximal heart rate. Heart rate, systolic blood pressure, systolic blood pressure – end-systolic cavity area relation and fractional area change increased while end-diastolic and end-systolic cavity areas decreased during exertion.
Conclusions
PSE is associated with enhanced LV systolic function secondary to an increase in myocardial contractility in younger female triathletes.
Keywords: Echocardiography, myocardial contractility, preload, afterload, long-term exercise; heart rate; cardiac fatigue
Background
Dynamic steady state aerobic exercise lasting between 5 and 30 minutes is associated with an increase in cardiac output secondary to an increase in heart rate and stroke volume [1]. The heightened stroke volume is due, in part, to a Starling-mediated increase in end-diastolic volume (i.e. preload reserve) and to enhanced systolic emptying secondary to an increase in myocardial contractility (i.e. contractile reserve) [2]. During dynamic steady state exercise lasting >30 minutes, stroke volume has been shown to gradually decrease while cardiac output remains unaltered due to a compensatory elevation in heart rate [3]. A widely held belief in exercise physiology is that the prolonged strenuous exercise (PSE) mediated decline in stroke volume may be due, in part, to a reduction in preload reserve secondary to the diminished plasma volume associated with dehydration and thermoregulatory peripheral vasodilatation [4]. Although alterations in preload reserve may contribute to the decreased stroke volume, several investigators have found that the decline in left ventricular (LV) systolic performance associated with PSE was independent of changes in LV preload [5] or afterload [6,7]. These findings suggest that the alteration in LV systolic function after PSE may result in "cardiac fatigue" due, in part, to an impairment in myocardial contractility (reviewed in [8]). A limitation of a majority of investigations that have examined the effect of PSE on LV systolic performance was that echocardiographic assessment of LV function was obtained prior to and approximately 20 to 45 minutes after completing prolonged exercise. However, a previous investigation has found that echocardiographic images obtained after cessation of exercise are not representative of the acute LV changes that occur during exertion [9]. Therefore, the time-course of alteration in LV systolic performance during prolonged intense exercise (similar to that observed during a competitive endurance event) is not well known. Finally, there is a paucity of investigations that have examined the effect of PSE on LV systolic function in female athletes. The purpose of this investigation was to assess LV systolic function prior to, during and immediately after performing PSE in female triathletes. We hypothesized that PSE would result in a decline in LV systolic function secondary to a decline in myocardial contractility.
Methods
Study Population
The participants for this investigation consisted of 13 healthy trained female triathletes with normal LV systolic function (Table 1). The subjects were in the early phase of their annual training regimen and had not participated in a competitive endurance event for at least three months prior to testing. Written informed consent was obtained from each subject in accordance with guidelines established by the Health Research Ethics Board at our institution.
Table 1.
Subject Characteristics.
| Variable | Mean ± SD |
| Age (yrs) | 29 ± 6 |
| Weight (kg) | 60 ± 3 |
| VO2max (L·min-1) | 3.2 ± 0.3 |
| VO2max (mL·kg-1·min-1) | 53 ± 4 |
| HRmax (beats·min-1) | 187 ± 8 |
(HRmax = Maximal heart rate)
Baseline Testing
The subjects performed an incremental exercise treadmill test to exhaustion during which time oxygen uptake, carbon dioxide production, minute ventilation and heart rate were continuously measured. Expired gases were collected, sampled and averaged every 15 seconds using a ParvoMedics Metabolic Cart (ParvoMedics, Salt Lake City, UT) and heart rate data was measured using a Polar Vantage XL heart rate monitor (Polar Electro Oy, Finland). The graded exercise test began at a running velocity of 161 m·min-1 and increased by 13.4 m·min-1 every two minutes until the breakaway point in the ventilatory equivalent for carbon dioxide, after which stages were reduced to one minute.
Race Simulation
In an attempt to simulate the bicycle and running portions of an Olympic-distance triathlon event, participants performed a 40 kilometre simulated cycling time trial using self-selected gears on their own bicycles mounted to a Computrainer (Racermate, Seattle WA), followed by a 10 kilometre run on a treadmill (Star Trac, Irvine CA). Incorporation of the two modes of exercise reduced the boredom associated with stationary bicycle exercise and enabled participants to compare the effort with past racing experiences. At 30-minute intervals, the subjects stopped exercising, dismounted from the apparatus and were placed in the left lateral supine position during which time echocardiographic images, heart rate and blood pressure were obtained. The total time to dismount the exercise apparatus, acquire a satisfactory echocardiographic image and resume exercising was 2.8 ± 0.2 minutes. The subjects were weighed prior to and following the race and fluid consumption was recorded for each participant. Body temperature was not monitored, however participants were cooled with fans throughout the exercise session.
Left ventricular imaging
LV imaging was performed with a commercially available ultrasound instrument (Hewlett Packard, Sonos 5500) with a 3.5 MHz transducer. Two-dimensional transthoracic images were obtained from the parasternal short-axis view at the level of the mid-papillary muscles according to American Society of Echocardiography guidelines [10]. LV images were obtained at rest, at 30-minute intervals during exercise and immediately (<45 seconds) after cessation of PSE. The images were analysed offline at a later date and the following measures were obtained and averaged over four cardiac cycles: end-diastolic cavity area (largest endocardial area) and end-systolic cavity area (smallest endocardial cavity area). LV fractional area change and LV end-systolic meridional wall stress were calculated by standard methods [11]. In addition, the systolic blood pressure – end-systolic cavity area relation was used as an estimate of myocardial contractility [5].
Statistical Analysis
Statistical analysis was performed with a one-way repeated measures analysis of variance. If a significant time effect was found then a post-hoc Neuman Keuls test was performed. The α level was set a priori at p < 0.05. Values are expressed as means ± standard deviations (mean ± SD).
Results
Race simulation
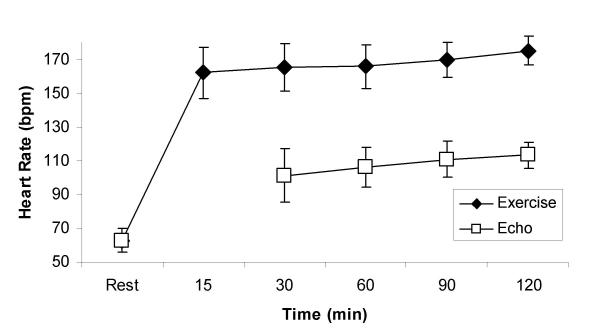
Race simulation data is provided in Table 2. Total exercise time was 121 ± 8 minutes. Subjects exercised at an average intensity equivalent to 90 ± 3% of their maximal heart rate. Heart rate gradually increased from an average of 162 ± 15 beats/min for the initial 15 minutes of exercise to 175 ± 8 beats/min during the final 15 minutes of exercise (Figure 1). Average weight loss following the exercise bout was 0.8 ± 0.6 kg despite an average fluid intake of 1090 ± 455 mL.
Table 2.
Race Simulation Data.
| Variable | Mean ± SD |
| Cycle Time (min) | 72 ± 4 |
| Run Time (min) | 49 ± 5 |
| Total Time (min) | 121 ± 8 |
| Average Time for Image (min) | 2.8 ± 0.2 |
| Weight Loss (kg) | 0.8 ± 0.6 |
| Fluid Consumption (mL) | 1090 ± 455 |
Figure 1.
Heart rate response to prolonged strenuous exercise. Exercise refers to heart rates obtained during exercise. Echo refers to heart rates obtained during left ventricular imaging.
Echocardiographic and hemodynamic measurements
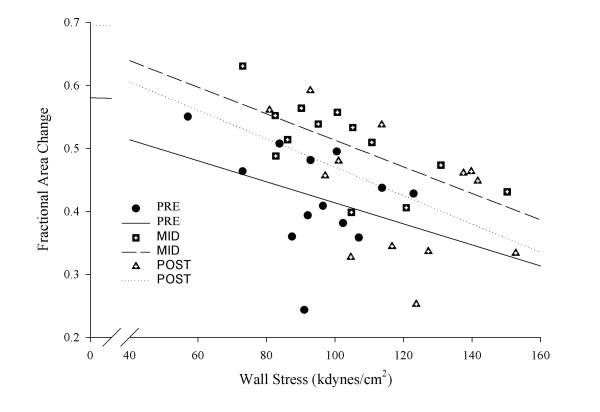
Heart rate, systolic blood pressure and the systolic blood pressure – end-systolic cavity area relation were significantly higher at 30, 60 and 90 minutes of exertion compared to pre-exercise baseline values (Table 3). The systolic blood pressure – end-systolic cavity area relation was significantly lower at the completion of PSE compared to the 60-minute time-period. The end-systolic cavity area was significantly lower than resting values after 30, 60 and 90 minutes of exercise. A similar trend was found for end-diastolic cavity area with the 90-minute measure being significantly lower than rest. Fractional area change was significantly higher after one-hour of exercise and returned to resting values upon cessation of exercise. Left ventricular end-systolic wall stress was significantly higher at 90 minutes and immediately after completing PSE compared to resting values (Table 3). The fractional area change – end systolic wall stress relationship increased with exercise and remained above resting values upon completion of PSE (Figure 2).
Table 3.
Clinical and echocardiographic data at rest and during prolonged strenuous exercise.
| Variable | Pre | 30 min | 60 min | 90 min | Post |
| HR (beats·min-1) | 63 ± 7 | 101 ± 15* | 106 ± 12* | 111 ± 11* | 113 ± 11* |
| SBP (mmHg) | 110 ± 9 | 142 ± 16* | 140 ± 13* | 142 ± 15* | 138 ± 17* |
| EDCA (cm2) | 24 ± 4 | 22 ± 5 | 22 ± 5 | 21 ± 4* | 23 ± 4 |
| ESCA (cm2) | 14 ± 3 | 11 ± 3* | 11 ± 3* | 11 ± 2* | 13 ± 2 |
| FAC (%) | 42 ± 8 | 49 ± 9 | 51 ± 7* | 47 ± 11 | 43 ± 10 |
| SBP – ESCA relation | 8 ± 2 | 13 ± 4* | 13 ± 3* | 13 ± 3* | 11 ± 2*∂ |
| ESWS (Kdynes·cm-2) | 125 ± 23 | 141 ± 37 | 136 ± 29 | 150 ± 34* | 157 ± 29* |
(* = p < 0.05 vs pre; ∂ = p < 0.05 vs 60 minutes; EDCA, End-diastolic cavity area; ESCA, End-systolic cavity area; ESWS, End-systolic wall stress; FAC, Fractional area change; HR, Heart rate; SBP, Systolic blood pressure; SBP-ESCA relation, surrogate for myocardial contractility)
Figure 2.
Left ventricular systolic performance in relation to wall stress prior to, one hour into and immediately following prolonged strenuous exercise.
Discussion
The major new finding of this investigation was that PSE exercise was associated with an enhanced LV systolic function secondary to the heightened myocardial contractility. This finding is contrary to our "a priori" hypothesis and discordant with previous investigations that found a decline in LV systolic function after performing PSE [6,7,12,13]. However, our findings support the findings of others [9,14] who demonstrated that LV systolic function remains above resting levels throughout prolonged exercise.
Previous investigators have found that PSE of varying exercise durations (i.e., 1.6 to 24 hours) was associated with a decline in LV fractional shortening [6,7,12,13] that returned towards pre-exercise baseline values one to two days after cessation of exercise [6]. The mechanism(s) responsible for the reduced LV systolic performance appears to be due, in part, to a decline in end-diastolic volume and subsequent attenuated use of the Starling mechanism and to a reduction in myocardial contractility [6,7,12,13]. A limitation of a majority of previous investigations was that LV systolic function was examined after the cessation of PSE, therefore, the time-course of the decline in LV systolic function and the underlying mechanisms responsible for this change during prolonged exertion are not well known.
In the present investigation, fractional area change increased during exercise and did not decline below pre-exercise baseline values. The heightened systolic performance appears to be secondary to an increase in myocardial contractility (i.e., increased systolic blood pressure – end-systolic cavity area relation) as end-diastolic cavity area (i.e., preload) decreased while LV wall stress (i.e., afterload) increased during exertion. In addition, the fractional area change-end systolic wall stress relationship increased with exercise and remained elevated throughout the 2 hours of PSE (Figure 2). This finding is in contrast to other investigations which demonstrated that the fractional area change-end systolic wall stress relationship declined following PSE lasting three [13] and greater than 12 hours [6]. Although these authors report a decline in LV systolic function following prolonged exercise, they did not measure LV function during exercise. To date only two other investigations have examined LV systolic function throughout (i.e., 1 to 2.5 hours) PSE [9,14] and in both cases exercise was performed by younger healthy male athletes. Consistent with our results, both investigations found that PSE was not associated with LV systolic dysfunction as ejection fraction was always greater than pre-exercise baseline values. Moreover, Palatini and colleagues [9] found that the increased LV ejection fraction was due, in part, to increased myocardial contractility. The mechanism responsible for the heightened myocardial contractility during PSE has not been well studied, however, it may be related to the increased beta-adrenergic stimulation of the myocardium and/or to the force-frequency relation [15]. Regardless of the underlying mechanisms, our findings confirm and extend previous exercise echocardiographic or radionuclide angiographic investigations by revealing that PSE does not appear to negatively alter LV systolic function in younger female triathletes.
Our finding that LV end-diastolic cavity area (i.e., preload) decreased during PSE despite our athletes consuming >250 ml of fluid every 30 minutes during exertion is similar to the findings of Goodman and associates [14]. The attenuated preload reserve has been linked to the increased heart rate associated with performing prolonged exercise [16]. However, in the current investigation, the reduced preload reserve does not appear to be related to an exercise mediated rise in heart rate since the 13% decline in end-diastolic cavity area that occurred during the first 90 minutes of exercise and its subsequent return towards baseline values at the cessation of PSE occurred at similar heart rates. Furthermore, an exercise-mediated increase in sympathetic stimulation and tachycardia have been shown to decrease the time constant of LV pressure fall during isovolumic relaxation and minimal LV pressure while increasing the peak mitral valve pressure gradient, early diastolic filling rate and end-diastolic volume, despite a marked reduction in the duration of diastole [17,18].
It is possible that our attenuated preload reserve may be secondary to ventricular interaction or to a pericardial constraint to LV filling. Douglas and colleagues [19] found disparate left and right ventricular end-diastolic cardiac area responses after performing PSE. More specifically, upon cessation of PSE the right ventricular end-diastolic cavity area increased compared to pre-exercise baseline values while the LV end-diastolic cavity area was smaller than basal values. Therefore, it is possible that sudden postural changes (i.e., a change form the upright exercising posture to the supine left lateral position) that occurred in current investigation may have resulted in increased right ventricular end-diastolic cavity area that via diastolic ventricular interaction (with or without pericardial constraint) may have reduced LV compliance and filling. This hypothesis does not seem likely, however, as the LV end-diastolic cavity area decreased during the first 90 minutes of exercise and returned to baseline values at the cessation of exercise despite similar postural perturbations during all 2-D image acquisitions. Finally, it is possible that heightened LV wall stress associated with PSE may have resulted in reduced LV relaxation and subsequent decrease in LV preload during exertion.
Currently, there has only been one laboratory investigation that has examined the stroke volume response during PSE in younger (31 years) female triathletes [20]. In that investigation, the athletes performed five hours of cycling exercise followed by three hours of treadmill running during which time cardiac output was measured (CO2-rebreathing method). The main finding of this investigation was that stroke volume decreased during the first 30 minutes of cycling exercise and remained lower than pre-exercise values during most of the 8-hour exercise session. The heart rate gradually increased during the prolonged exercise session, however, it did not fully compensate for the reduced stroke volume and as a consequence cardiac output decreased during exertion. A limitation of this examination was that the underlying mechanisms (i.e., loading conditions and myocardial contractility) responsible for the reduced stroke volume were not measured. However, our finding that the PSE mediated decline in preload reserve was offset by an increase in contractile reserve that resulted in no alteration in stroke area during exercise is divergent from the above findings. The disparity of findings between these investigations is not related to the participant's age, mode of exercise, or fluid replenishment, which were similar in both examinations. The only major difference between these investigations was that the subjects in our investigation exercised at a substantially higher exercise intensity and approximately one-quarter of the duration compared to the subjects in the above investigation.
Limitations
A series of limitations that may have affected the results of this investigation must be addressed. First, in order to compare our findings with previous investigations that performed supine echocardiograms post PSE, we elected to have our subjects momentarily stop exercising and quickly lie in the left lateral supine position while the echocardiographic images were acquired. A limitation with our method is that it does not fully represent the loading conditions and heart rate that occur during exercise. In addition, the supine posture should enhance venous return and as a consequence may result in divergent right and left ventricular interactions that may not occur to the same extent during upright exercise. In spite of the above limitations, our results are clearly consistent with recent exercise echocardiographic or radionuclide angiographic examinations that found that PSE did not result in a decline in LV systolic dysfunction [9,14]. Although our images were obtained in the supine position we did observe heightened contractile function at all time points throughout the exercise session. Heart rate values did decline significantly during imaging (Figure 1), however the phenomenon of "cardiac fatigue" was initially described in a resting state in similar supine positions following PSE [6,7,12,13]. If LV contractile dysfunction was evident at rest in the supine position with heart < 100 bpm following PSE in these previous investigations, one would expect to observe changes in contractile function not only during exercise but at heart rates between exercise and resting values, similar to those we observed in our investigation (100–113 bpm). Moreover, our findings are identical to those of Palatini and associates [9] who revealed that the heightened ejection fraction during exercise was due, in part, to an increase in myocardial contractility. Although Palatini's group measured cardiac output during exercise [9], the LV contractile response was identical to what we observed at lower heart rates. These data also parallel the findings of Goodman and colleagues [14], despite the fact that our athletes exercised at significantly higher exercise intensities. Therefore, it is likely that the imaging methods we used in this investigation reflect the contractile status of the LV during exercise despite the alteration in posture and the lower heart rate values during image acquisition.
A second limitation of this investigation is that the systolic blood pressure – end-systolic cavity relation is an indirect measure of end-systolic elastance. However, it is not feasible to perform invasive LV pressure-volume assessments in healthy younger athletes while they perform two hours of PSE. Despite this limitation, our finding that fractional area change increased despite a decline in end-diastolic cavity area (i.e., preload) with a concomitant increase in LV wall stress (i.e., afterload) at a constant exercise heart rate suggests that the heightened systolic function was related to an increase in myocardial contractility.
As previously mentioned, there has been a paucity of investigations that have investigated the effects of PSE in female athletes. Therefore, our purpose was to investigate the effects of PSE on LV systolic function in female triathletes while a secondary purpose was to determine the mechanism(s) responsible for the changes in LV systolic performance. As a result, we were not interested in examining the gender effects of PSE on LV systolic function and as such we did not have a comparison group of male triathletes. However, our results confirm and extend previous findings (in younger male athletes) by revealing that PSE does not appear to result in LV systolic dysfunction in female endurance athletes.
Finally, we chose two divergent modes of exercise (i.e., cycling and treadmill running) since these types of exercise were previously shown to result in a decline in stroke volume, cardiac output and LV systolic function in younger athletic females. Therefore, it is possible that we may have observed a decline in LV systolic function if the subjects performed the same mode of exercise over our allotted time period. A significant number of the investigations that have reported altered LV systolic performance after PSE have obtained LV images immediately following multisport events such as the Hawaii Ironman World Championships (reviewed in [8]). Athletes in these events exercise in three different body positions and echocardiographic data obtained from these studies consistently report altered LV systolic performance following PSE. We therefore chose to mimic such an environment within a laboratory setting to quantify LV systolic performance throughout exercise, rather than following exercise. In addition, although postural changes may alter loading conditions of the LV during exercise, our images were consistent as they were all obtained with the athletes lying supine. Therefore it is our contention that LV function during image acquisition was not affected by changes in postural position during PSE. Alternatively, it may have been possible that a decline in LV systolic function could have occurred if our subjects had exercised for a longer time period (i.e., >2 hours). However, this does not seem realistic based on the findings of O'Toole and associates [20] who revealed that a decline in stroke volume and cardiac output occurred within the first 30–60 minutes of low-intensity cycle ergometer exercise in younger female triathletes.
Conclusions
In summary, PSE as performed by female triathletes was associated with an increase in LV systolic function. The heightened fractional area change during PSE appears to be secondary to the increase in myocardial contractility as this form of exercise was associated with a decline in LV preload and a concomitant increase in LV afterload. These findings confirm a series of recent investigations, in younger males, that found that PSE did not result in LV systolic dysfunction. Moreover, they extend these findings and show that PSE does not appear to result in LV systolic dysfunction in younger female triathletes.
Author's Contributions
JM conceived the study, and participated in its design, coordination and data collection. JM also performed statistical analysis and prepared the manuscript.
MH participated in study design, data collection and manuscript preparation.
DW participated in data collection.
HAQ edited manuscript participated in design of the study.
DT participated in echocardiographic analysis.
RW participated in study design, data collection and echocardiographic analysis.
Acknowledgments
Acknowledgements
We thank Dave Buchaski and the Hewlett-Packard Corporation (Canada) for providing the ultrasound machine for this investigation.
Contributor Information
Jonathan McGavock, Email: mcgavock@ualberta.ca.
Mark Haykowsky, Email: mark.haykowsky@ualberta.ca.
Darren Warburton, Email: darrenwb@interchange.ubc.ca.
Dylan Taylor, Email: dtaylor@cha.ab.ca.
Arthur Quinney, Email: art.quinney@ualberta.ca.
Robert Welsh, Email: rwelsh@cha.ab.ca.
References
- Mahler DA, Matthay RA, Snyder PE, Pytlik L, Loke L. Volumetric responses of right and left ventricles during upright exercise in normal subjects. J Appl Physiol. 1985;58:1818–22. doi: 10.1152/jappl.1985.58.6.1818. [DOI] [PubMed] [Google Scholar]
- Plotnick GD, Becker LC, Fisher ML, Gerstenblith G, Renlund DG, Fleg JL, Weisfeldt ML, Lakatta EG. Use of the Frank-Starling mechanism during submaximal versus maximal upright exercise. Am J Physiol. 1986;251:H1101–5. doi: 10.1152/ajpheart.1986.251.6.H1101. [DOI] [PubMed] [Google Scholar]
- Ekelund LG. Circulatory and respiratory adaptations during prolonged exercise. Acta Physiol Scand. 1967;70:5–38. doi: 10.1111/j.1748-1716.1967.tb03529.x. [DOI] [PubMed] [Google Scholar]
- Smith EE, Guyton AC, Manning RD, White RJ. Integrated mechanisms of cardiovascular response and control during exercise in the normal human. Prog Cardiovasc Dis. 1976;18:421–44. doi: 10.1016/0033-0620(76)90010-4. [DOI] [PubMed] [Google Scholar]
- Haykowsky M, Welsh R, Humen D, Warburton D, Taylor D. Impaired left ventricular systolic function after a half-ironman race. Can J Cardiol. 2001;17:687–90. [PubMed] [Google Scholar]
- Douglas PS, O'Toole ML, Hiller WD, Hackney K, Reichek N. Cardiac fatigue after prolonged exercise. Circulation. 1987;76:1206–13. doi: 10.1161/01.cir.76.6.1206. [DOI] [PubMed] [Google Scholar]
- Vanoverschelde JL, Younis LT, Melin JA, Vanbutsele R, Leclercq B, Robert A, Cosyns J, Detry J. Prolonged exercise induces left ventricular dysfunction in healthy subjects. J Appl Physiol. 1991;70:1356–63. doi: 10.1152/jappl.1991.70.3.1356. [DOI] [PubMed] [Google Scholar]
- McGavock JM, Warburton DE, Taylor D, Welsh RC, Quinney HA, Haykowsky MJ. The effects of prolonged strenuous exercise on left ventricular function: a brief review. Heart Lung. 2002;31:279–92. doi: 10.1067/mhl.2002.126106. [DOI] [PubMed] [Google Scholar]
- Palatini P, Bongiovi S, Macor F, Michieletto M, Mario L, Schiraldi C, Pessina A. Left ventricular performance during prolonged exercise and early recovery in healthy subjects. Eur J Appl Physiol Occup Physiol. 1994;69:396–401. doi: 10.1007/BF00865402. [DOI] [PubMed] [Google Scholar]
- Sahn DJ, Demaria A, Kisslo J, Weyman A. The committee on M-mode. standardization of the American Society of echocardiography: Recommendations regarding the quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- Reichek N, Wison J, St John Sutton M, Plappert TA, Goldberg S, Hirshfeld JW. Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation. 1982;65:99–108. doi: 10.1161/01.cir.65.1.99. [DOI] [PubMed] [Google Scholar]
- Niemela KO, Palatsi IJ, Ikaheimo MJ, Takkunen JT, Vuori JJ. Evidence of impaired left ventricular performance after an uninterrupted competitive 24 hour run. Circulation. 1984;70:350–614. doi: 10.1161/01.cir.70.3.350. [DOI] [PubMed] [Google Scholar]
- Seals DR, Rogers MA, Hagberg JM, Yamamoto C, Cryer P, Ehsani AA. Left ventricular dysfunction after prolonged strenuous exercise in healthy subjects. Am J Cardiol. 1988;61:875–79. doi: 10.1016/0002-9149(88)90362-1. [DOI] [PubMed] [Google Scholar]
- Goodman JM, McLaughlin PR, Liu PP. Left ventricular performance during prolonged exercise: absence of systolic dysfunction. Clin Sci. 2001;100:529–3711. doi: 10.1042/CS20000161. [DOI] [PubMed] [Google Scholar]
- Ross J, Jr, Miura T, Kambayashi M, Eising GP, Ryu RH. Adrenergic control of the force-frequency relation. Circulation. 1995;92:2327–3215. doi: 10.1161/01.cir.92.8.2327. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Gonzalez-Alonso J. Cardiovascular drift during prolonged exercise: new perspectives. Exerc Sport Sci Rev. 2001;29:88–92. doi: 10.1097/00003677-200104000-00009. [DOI] [PubMed] [Google Scholar]
- S Miyazaki, BD Guth, T Miura, C Indolfi, R Schulz, Ross J., Jr Changes of left ventricular diastolic function in exercising dogs without and with ischemia. Circulation. 1990;81:1058–70. doi: 10.1161/01.cir.81.3.1058. [DOI] [PubMed] [Google Scholar]
- CP Cheng, Y Igarashi, WC Little. Mechanism of augmented rate of left ventricular filling during exercise. Circ Res. 1992;70:9–19. doi: 10.1161/01.res.70.1.9. [DOI] [PubMed] [Google Scholar]
- PS Douglas, O'Toole ML, WD Hiller, N Reichek. Different effects of prolonged exercise on the right and left ventricles. J Am Coll Cardiol. 1990;15:64–9. doi: 10.1016/0735-1097(90)90176-p. [DOI] [PubMed] [Google Scholar]
- O'Toole ML, DB Hiller, PS Douglas, JB Pisarello, JL Mullen. Cardiovascular responses to prolonged cycling and running. Annals of Sports Medicine. 1987;3:124–129. [Google Scholar]