Abstract
Previous studies on the effect of repeated electro-acupuncture (EA) treatments in rats with steriod-induced polycystic ovaries (PCO), EA has been shown to modulate nerve growth factor (NGF) concentration in the ovaries as well as corticotropin releasing factor (CRF) in the median eminence (ME). In the present study we tested the hypothesis that repeated EA treatments modulates sympathetic nerve activity in rats with PCO. This was done by analysing endothelin-1 (ET-1), a potent vasoconstrictor involved in ovarian functions, as well as NGF and NGF mRNA expression involved in the pathophysiological process underlying steroid-induced PCO.
The main result in the present study was that concentrations of ET-1 in the ovaries were significantly lower in the PCO group receiving EA compared with the healthy control group (p < 0.05). In the hypothalamus, however, ET-1 concentrations were found to be significantly higher in the PCO group receiving EA than in the healthy control group (p < 0.05). Concentrations of ovarian NGF protein were significantly higher in the PCO control group compared with the healthy control group (p < 0.001), and these concentrations decreased significantly after repeated EA treatments compared with those in the PCO control group (p < 0.05) and were found to be the same as those in the healthy control group. In conclusion, these results indicate that EA modulates the neuroendocrinological state of the ovaries, most likely by modulating the sympathetic nerve activity in the ovaries, which may be a factor in the maintenance of steroid-induced PCO.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of reproductive ages [1]. The aetiology of PCOS is unclear, but there are indications that the syndrome is associated with increased sympathetic nerve activity [2-4]. A single intramuscular injection of estradiol valerate (EV) in cycling rats results in loss of oestrus cyclicity, anovulation, and the formation of follicular cysts [4-6]. These changes exhibited by the steroid-induced polycystic ovary (PCO) share many of the endocrinological and morphological abnormalities seen in human PCOS, and an association with increased sympathetic nerve activity has been suggested [4,7]. We recently showed that a treatment known to influence the activity of the sympathetic nervous system – repeated treatments of electro-acupuncture (EA) – improved hormonal and metabolic profiles and induced ovulation in women with PCOS and anovulation [8]. Even if human PCOS cannot be reproduced exactly in a rat model, the steroid-induced PCO model may be a great help in studying the physiological mechanisms of reported effects of EA.
Endothelin-1 (ET-1) is a 21-amino acid peptide, considered to be a potent and long-lasting vasoconstrictor [9]. ET-1 exerts a wide spectrum of biological activities in addition to vasoconstriction and is reported to be involved in ovarian functions in the human [10]. A recent study found that women with PCOS had elevated concentrations of plasma ET-1 compared with a healthy control group [11]. This finding supports the theories of a link between ET-1 and increased sympathetic nerve activity associated with PCOS in women. Nerve growth factor (NGF) is synthesized by and released from ovarian cells and the presence of NGF receptors on nerve fibres innervating the ovary suggests that NGF is one of the target-derived neurotrophins involved in the development and maintenance of ovarian innervation [12]. Recent data indicate that abnormally elevated concentrations of ovarian NGF after a single dose of EV may contribute to ovarian dysfunction since an activation of ovarian NGF synthesis precedes the formation of follicular cysts [4]. This increased synthesis was correlated with exaggerated steroid secretory activity in the ovaries [7]. Recently we found that repeated EA treatments in the steroid-induced rat PCO model used in this study significantly reduced increased concentrations of ovarian NGF and of median eminence (ME) corticotropin-releasing factor (CRF) [13,14], which indicates a functional interaction between activity in the nervous and endocrine systems.
In the present study we tested the hypothesis that repeated EA treatments modulates sympathetic nerve activity in rats with steroid-induced PCO. This was done by analysing ET-1 concentrations in the CNS and in endocrine target organs such as the adrenal gland and the ovary. In addition, the concentration of NGF protein and the expression of NGF mRNA in the ovary, the adrenal gland, and the spinal cord were analysed. Morphological analyses of the ovaries were made to confirm the induction of PCO in the rat model used in this study.
Materials and methods
Fifty-six virgin adult cycling Sprague-Dawley rats (Möllegaard, Denmark) weighing 195–210 g, with regular 4-day oestrus cycles were divided into three groups. They were housed four to each cage at a controlled temperature of 22°C with a 12-h light/12-h dark cycle for at least 1 week before and throughout the experimental periods. The rats had free access to pelleted food and tap water. Thirty-two rats, those in the PCO groups described below, were each given a single i.m. injection of 4 mg EV (Riedeldehaen, Germany) in 0.2 ml oil to induce well-defined PCO. Twenty-four rats, those in the healthy control groups described below, received a single i.m. injection of 0.2 ml oil only. The studies were approved by the local Ethics Committee for Animals at Göteborg University.
EA Treatment
In Part I of the experiment, the rats were divided into three experimental groups: i) a healthy control group (vehicle control), (n = 8) ii) a PCO control group (n = 8), and iii) a PCO group receiving EA (n = 8). In Part II of the experiment, the rats were divided into four experimental groups: i) a healthy control group (vehicle control) (n = 8), ii) a healthy control group receiving EA (vehicle – EA) (n = 8), iii) a PCO control group (n = 8), and iiii) a PCO group receiving EA (n = 8).
All groups were anaesthetised as described below for 25 minutes, 12 times, corresponding to the EA treatment given to the groups receiving EA. EA was given during anaesthesia every second or third day beginning 2 days after the EV injection. The points chosen for stimulation were bilateral in the mm. biceps femoris and erector spinae, in somatic segments corresponding to the innervation of the ovaries. The needles (Hegu: Hegu AB, Landsbro, Sweden) were inserted to depths of 0.5–0.8 cm and then attached bilaterally to an electrical stimulator (CEFAR ACU II, Cefar, Lund, Sweden). The points were electrically stimulated with a low burst frequency of 2 Hz; each pulse has a duration of 180 μsec, a burst length of 0.1 sec and a burst frequency of 80 Hz. The intensity was adjusted until local muscle contractions were seen (1.5 mA) to reflect the activation of muscle-nerve afferents (A-delta fibres and possibly C fibres). The location and type of stimulation were the same in all rats.
Anaesthetization
In Part I, designed to measure ET-1 concentrations, all rats were anaesthetized superficially with enfluran (EFRANE®, Abbott Scandinavia, Kista, Sweden). On day 30 after i.m. injection of EV, the rats were decapitated, i.e. 1–2 days after the last EA treatment. In Part II, designed to measure concentrations of NGF protein and expression of NGF mRNA, all rats were anaesthetized superficially with an i.p. injection of a mixture of Ketamin (50 mg/kg; PARKE-DAVIS, Warner Lambert Nordic AB, Solna, Sweden) and Rompun (20 mg/kg; Bayer, Bayer AG, Leverkusen, Germany) and by inhalation of Isofluran (Baxter Medical AB, Kista, Sweden). On day 30 after i.m. injection of EV, the rats were decapitated, i.e. 1–2 days after the last EA treatment.
Radioimmunoassay of ET-1
After the rats in Part I, designed to allow measurement of ET-1, were decapitated on day 30, the ovaries, the adrenal glands, and the hypothalamus were quickly removed and dissected on dry ice, weighed, and stored at -80°C until extraction. During extraction, the frozen tissues were weighed, cut into small pieces, then boiled for 10 minutes in 10 volumes of 1 mol/l acetic acid. After homogenization with a steel rod and a vortex mixer, the samples were centrifuged at 3000 × g for 15 minutes. The supernatants were lyophilized, then diluted in 10 ml of radioimmunoassay (RIA) buffer and kept at -20°C until analysis.
ET-1 was analysed using a non-equilibrium competitive immunoassay. The RIA was based on antiserum END 4, which had been collected from a rabbit that had been immunized with ET-1 and been coupled to bovine serum albumin (BSA) using the carbodimide method. ET-1 was labelled to a specific activity of 59 Bq/fmol using the chloramine-T method and subsequently purified by reverse-phase HPLC. Briefly, the assay was carried out by incubating the calibrators and samples with the antiserum in a final dilution of 1:7 500 for 48 h at 4°C. 125I-labelled ET-1 was then added to the samples, which were incubated for a further 24 h at 4°C. Separation was performed using a donkey anti-rabbit antibody-coated cellulose suspension (Sac-cel; Labkemi, Stockholm, Sweden). Synthetic ET-1 (Peninsula laboratories, Belmont, California, USA) served as a calibrator. The buffer blank counts were subtracted from all counts before the standard curve was evaluated (RiaCalc; Wallac, Redmond, Washington, USA) and concentrations were determined. The sample blanks did not differ statistically from the buffer blank. The amount of ET-1 required to reduce the binding of the radioligand by 50% (IC50) was 7.4 pmol/l. The intra- and interassay coefficients of variation were 5% and 8% respectively at 25 pmol/l. When the cross-reactivity of antiserum END 4 to ET-1 was used as a 100% reference, the cross-reactivities were as follows: ET-2, 32%; ET-3, 120%; big ET, 6%. For methodological details see Lemne et al. (1994) [15].
Enzyme Immunoassay of NGF protein and RT-PCR-ELISA of mRNA NGF
After the rats in Part II, designed to allow measurement of NGF protein and mRNA NGF, were decapitated on day 30, the ovaries, the adrenal glands, and the dorsal part of the spinal cord were quickly removed and dissected on dry ice, weighed, and stored at -80°C until extraction.
ELISA
The samples were sonicated in extraction buffer (0.1% Triton X-100, 100 mM Tris-HCl, pH 7.2, 400 mM NaCl, 4 mM EDTA, 0.2 mM PMSF, 0.2 mM benzethonium chloride, 2 mM benzamidine, 40 U/ml aprotinin, 0.05% sodium azide, 2% BSA, and 0.5% gelatine; 1 ml/100 mg of tissue) followed by centrifugation at 10 000 × g for 30 minutes. The supernatants were used for the assay. The bioactive form of 2.5S NGF, purified from mouse submaxillary glands and prepared in the laboratory at the Institute of Neurobiology (CNR) in Rome, Italy, according to the method of Bocchini and Angeletti (1969) [16], was used as a standard. NGF was dissolved in extraction buffer and the standard curve was in a range of 1000 pg ml (-1) and 15.6 pg ml (-1). Enzyme-linked immunosorbent assays (ELISA) were performed as described by Wescamp and Otten (1987) [17] with a minor modification [18]. Specific NGF binding was assessed by use of monoclonal mouse anti β-2.5S NGF (Boehringer Mannheim), which reacts with both the 2.5S and the 7S biologically active forms of NGF. All assays were performed in triplicate, and the absorbency of the samples and standards was corrected for non-specific binding (i.e. the absorbency in a well coated with purified mouse IgG). The content of NGF in the samples was determined in relation to the NGF standard curve. Data were not corrected for recovery of NGF from samples, which was routinely above 90%. With these criteria, the limit of sensitivity of NGF ELISA averaged 0.5 pg per assay.
RT-PCR-ELISA
The concentrations of NGF mRNA were evaluated using the reverse transcriptase-polymerase chain reaction (RT-PCR) ELISA protocol, exactly as previously described by Tirassa and co-workers (2000) [19]. The total RNA was extracted from the ovaries, adrenal glands, and spinal cord by using the method of [20] as modified in the TRIzol Kit (Invitrogen AB, Lidingö, Sweden). Complementary DNA was synthesized from 1 μg of total RNA using 200 Units of M-MLV reverse transcriptase (Promega Italia, Milan, Italy) in 20 μl of total volume reaction. NGF. Glyceraldehyde-3-phosphate dehydrogenas (GAPDH) genes were co-amplified in a single-tube PCR reaction (30 cycles: 1 minute at 95°C; 1 minute at 55°C; 2 minutes at 72°C) using 5'-biotinylated specific primers to generate biotinylated PCR products detectable by digoxygenin labeled probes in an immuno-enzymatic assay. The amount of amplified products was measured at an optical density (O.D.) of 450/690 nm (O.D. 450/690) using a Dynatech ELISA Reader 5000. A GAPDH level of O.D. 450/690 was used to normalise the relative differences in sample size, differences in integrity of the individual RNA, and variations in reverse transcription efficiency. For exact methodological details and the primer/probe sequences, see Tirassa et al. [19].
Ovarian morphology
After the rats in Part II were decapitated on day 30, one ovary per rat was removed, cleaned of adherent connective fat tissue, and fixed in 4% formaldehyde buffer for at least 24 hours. Thereafter the samples were dehydrated and imbedded in paraffin. The ovaries were longitudinally sectioned, with a thickness of 4 μ, and every tenth section – six section from each ovary were taken and stained with haematoxylin-eosin. An experienced pathologist (SC), blinded to grouping analysed the follicle population under microscope. There was no intention to quantify the number of growing or atretic follicles but rather to establish whether ovulation with corpora lutea formation had occurred within the given time frame. According to morphometric (stereological) and statistical principles there are no need to perform a statistical analysis in this situation.
Statistical analyses
All data were analysed using the software package SPSS 10.0. ET-1 concentrations in the ovaries, the adrenal glands, and the hypothalamus were evaluated using the analysis of variance (ANOVA), and the groups were tested using multiple comparisons with the correction of Bonferroni. All results are reported as a mean ± standard error of mean (SEM). A p-value less than 0.05 was considered significant.
Results
ET-1 changes after EV injection and response to electro-acupuncture
Means ± SEM for ET-1 concentrations (pmol/g) in the ovaries, the adrenal glands, and the hypothalamus in all groups are presented in Table 1. These analyses were performed to determine whether ET-1 concentrations are affected in the steroid-induced rat PCO model and if repeated EA treatments modulate ET-1 concentrations. Ovarian ET-1 concentrations were significantly lower in the PCO group receiving EA than in the healthy control group (p < 0.05). The ET-1 concentrations in the hypothalamus were significantly higher in the PCO group receiving EA than in the healthy control group (p < 0.05). The ET-1 concentrations in the adrenal glands were not significantly different between the groups.
Table 1.
Endothelin-1 (ET-1) concentrations (mean ± SEM) in the ovaries, the adrenal glands, and the hypothalami in the three experimental groups the healthy control group, the polycystic ovary (PCO) control group, and the PCO group receiving electro-acupuncture (EA).
| ET-1 concentrations (pmol/g) | |||
| Healthy control | PCO Control | PCO EA | |
| Ovary | 0.44 ± 0.11 | 0.38 ± 0.14 | 0.15 ± 0.05a |
| Adrenal gland | 0.01 ± 0.01 | 0.05 ± 0.02 | 0.06 ± 0.04 |
| Hypothalamus | 0.14 ± 0.05 | 0.33 ± 0.10 | 0.71 ± 0.23a |
a p < 0.05 when the PCO group receiving EA were compared with the healthy controls.
NGF changes after EV injection and response to electro-acupuncture
Means ± SEM for NGF protein (pg/g) concentrations and NGF mRNA (O.D. 450/690) in the ovaries, the adrenal glands and the spinal cord in Table 2. These analyses were performed to determine whether NGF production is increased as previously reported [4] and, if so, whether repeated EA treatment modulates this increase [13]. Concentrations of ovarian NGF protein were significantly higher in the PCO control group than in either the healthy control group (p < 0.001) or the healthy group receiving EA (p < 0.001). Repeated EA treatments resulted in significantly lower concentrations of ovarian NGF protein in the PCO group receiving EA than in the PCO control group (p < 0.05). The ovarian concentrations of NGF protein in the healthy control group, the healthy group receiving EA, and the PCO group receiving EA, however, were similar. No significant differences in NGF concentrations in the adrenal glands or the spinal cord were found. The expressions of ovarian NGF mRNA was significantly depressed in the PCO control group compared with both the healthy control group (p < 0.05) and the healthy group receiving EA (p < 0.05). The PCO group receiving EA, however, differed from neither the healthy control group nor the healthy group receiving EA.
Table 2.
Nerve Growth Factor (NGF) protein concentrations and NGF mRNA expression (mean ± SEM) in the ovaries, the adrenal glands and the dorsal part of the spinal cord in the four experimental groups the healthy control group, the healthy group receiving electro-acupuncture (EA), the polycystic ovary (PCO) control group, and the PCO group receiving EA.
| NGF concentrations (pg/g) | ||||
| Healthy control | Healthy EA | PCO control | PCO EA | |
| Ovary | 294.85 ± 17.00 | 265.79 ± 20.99 | 479.77 ± 26.86a | 385.34 ± 12.26b |
| Adrenal gland | 102.42 ± 26.14 | 119.97 ± 51.81 | 98.82 ± 12.46 | 94.42 ± 14.32 |
| Spinal cord | 867.36 ± 65.52 | 1600.59 ± 360.03 | 1351.26 ± 220.82 | 1081.94 ± 193.72 |
| NGF mRNA expression (O.D. 450/690) | ||||
| Ovary | 1.02 ± 0.19 | 1.10 ± 0.2 | 0.35 ± 0.06c | 0.45 ± 0.15 |
| Adrenal gland | 0.41 ± 0.06 | 0.51 ± 0.07 | 0.32 ± 0.06 | 0.37 ± 0.05 |
| Spinal cord | 0.71 ± 0.09 | 1.15 ± 0.34 | 0.57 ± 0.08 | 1.12 ± 0.29 |
a p < 0.001 when the PCO control group were compared with the healthy control group and healthy group receiving EA. b p < 0.05 when the PCO group receiving EA were compared with the PCO control group. c p < 0.001 when the PCO control group were compared with the healthy control group and the healthy group receiving EA.
Ovarian morphology – day 30
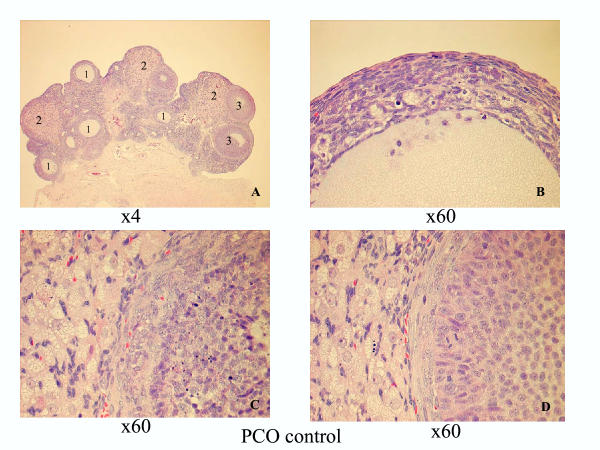
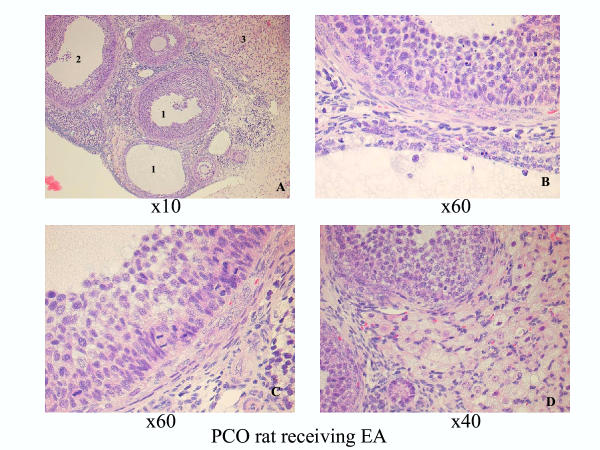
The ovaries in the healthy control group and in the healthy group receiving EA exhibited a typically normal appearance with follicles and corpora lutea in different stages of development and regression. The ovaries in both the PCO control group and in the PCO group receiving EA displayed typical PCO-like changes [4], and both the number and size of the corpora lutea in these groups were decreased compared with in the healthy groups. Typically, no young corpora lutea were present in the two PCO groups. The dominant structures were atretic follicles, regressing old corpora lutea, and a few growing "healthy" follicles in the primary, secondary, and tertiary stages (Figures 1 and 2).
Figure 1.
PCO group control group 30 days after EV injection. A. Survey view showing atretic follicles (1), regressing old corpora lutea (2), and growing "healthy" follicles (3), ×4 obj. B. Detailed view of an atretic follicle with mainly degenerated granulosa cells and macrophages, ×60 obj. C. Part of early atresia in a secondary follicle (right) and interstitial cells (right), ×60 obj. D. Part of a growing secondary "healthy" follicle (right) and interstitial cells (left), ×60 obj.
Figure 2.
PCO group receiving EA 30 days after EV injection. A. Survey view showing atretic follicles (1), a growing "healthy tertiary follicle (2), and interstitial cells (3), ×10 obj. B. Detailed view with an early atretic follicle in the upper part of the picture and a late atretic follicle near the bottom, ×60 obj. C. Part of the growing follicle with many mitotic figures among the granulosa cells, ×60 obj. D. Detailed view with parts of an interstitial gland and small healthy follicles, ×40 obj.
No substantial morphological differences were found between the groups treated with EA (healthy and PCO) and the control groups (healthy and PCO).
Discussion
The main result in the present study was that repeated treatments with low-frequency (2-Hz) EA significantly decreased ovarian concentrations of ET-1 and NGF protein in rats with steroid-induced PCO. This is an indication of lower activity in the sympathetic nerve fibres of the ovaries and is in agreement with our previous results of NGF and CRF measurements in the same rat PCO model [13,14]. In addition, the ovarian morphology confirms the validity of the present rat PCO model.
ET-1 as well as NGF has previously been shown to act directly on ovarian steroidogenesis in cows [21] and rats [7]. In addition, ET-1 concentrations in women with PCOS were found to be elevated and positively correlated with peripheral testosterone concentrations [11]. The same study, incidentally, found that long-term treatment of these women with the insulin sensitizer metformin reduced concentrations of both plasma androgen and ET-1 and improved insulin sensitivity [11]. This indicates a possible link between ET-1 and hyperinsulinaemia in women with PCOS. However, whether there is a connection between insulin secretion and PCO in the present rat model was not investigated in the present study. Furthermore, ET-1 has been found to be present in the central nervous system (CNS) of the rat, probably as a modulator of the actions of various neuropeptides [22]. ET-1 has emerged as an important modulator of stress responses in the CNS [23], as evidenced by the close relationship between ET-1 and catecholamines in stress provocations. This relationship most likely varies from region to region in the CNS. For instance, the effect that ET-1 has on sympathetic nerve activity depends on where in the CNS that ET-1 was administered [23]. In the present study, ET-1 was significantly higher in the hypothalamus following the period of EA treatments. Our findings that hypothalamic ET-1 concentrations increased while ME CRF concentrations decreased in response to repeated EA treatments suggest that there is a differential response to EA treatment.
It can be speculated that a high content of hypothalamic ET-1 may reflect two different states, i.e. either a high turnover and a high synthesis or a reduced release and, consequently an increase in concentration. CRF is another well-known modulator of stress responses and has previously been found to be elevated in the ME of rats with PCO [14]. This supports the theory that sympathetic nerve activity in the present rat PCO model was disturbed.
It has previously been shown that rats with steroid-induced PCO develop ovarian follicular cysts and that this development of cysts is preceded by an increased synthesis of NGF in the ovary [4]. These findings suggest that hyperactivation of ovarian sympathetic input caused by a single injection of EV is related to an overproduction of NGF and enhanced activity in the neurotrophic-neurogenic regulatory system and contributes to the process by which EV induces ovarian cysts and disrupts ovulation in rats [4]. That the abnormally high sympathetic activity contributes to the anovulatory state in rats with steroid-induced PCO is supported by the re-establishment of cyclicity and ovulatory capacity in rats that undergo transection of the superior ovarian nerve or are subjected to ovary-specific blockade of NGF [4,24].
In the present study, we can confirm that the steroid-induced rat PCO model is associated with increased concentrations of NGF protein and displays typical PCO-like changes in the ovarian morphology with a dominance of atretic follicles and decreased numbers and sizes of corpora lutea. In addition, we were also able to confirm our previous findings that repeated EA treatments modulate the sympathetic output in the ovaries, as evidenced by the significant decrease in concentrations of ovarian NGF protein in rats with steroid-induced PCO. Repeated EA treatment did not, however, reverse the morphological changes in the rat PCO group. This is in line with our previous results [4]. The most plausible explanation for the absence of morphological changes, might be that the EA stimulation needs to be stronger to alter the ovarian morphology.
Preliminary data from an ongoing study, investigating the most optimal intensity and frequency of EA stimulation on sympathetic outflow show that low frequency is the most optimal frequency and that the stimulation needs to be strong (3 mA). The intensity used in this study was 1.5 mA.
Surprisingly, the expression of NGF mRNA in the ovaries was significantly lower in the PCO control group than in the other three groups: the healthy control group, the healthy group receiving EA, and the PCO group receiving EA. That mRNA expression in the PCO group receiving EA did not differ from that in the healthy control group or the healthy group receiving EA is contradictory to previous findings in the same rat PCO model [4]. The most plausible explanation for this discrepancy is that the excessive concentrations of ovarian NGF protein might inhibit the expression of NGF mRNA in the ovaries. Another possible explanation is that there might be an unbalanced turnover between NGFmRNA and NGF protein, thus the NGFmRNA that is engaged in NGF protein translation is not replaced completely, or that in response to EA more NGF is produced than the quantity required. These findings need to be further investigated.
The results of ET-1 concentrations in the adrenal glands and NGF in the spinal cord and in the adrenal glands indicate a close interaction between these two systems, most likely due to the fact that the adrenal glands are part of the sympathetic nervous system.
In conclusion, repeated EA treatments significantly decrease ovarian concentrations of ET-1 and NGF protein. This supports our previous finding that EA modulates sympathetic nerve activity in the ovaries, which may be a factor in the maintenance of steroid-induced PCO. However, it remains to be elucidated whether these effects of repeated EA treatments are a result of a direct action on the ovary, a decrease in the sympathetic outflow to the ovary, or both.
Acknowledgments
Acknowledgements
We wish to thank laboratory assistant Anja Finn, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, for her invaluable help with the ET-1 analyses. This study was supported by grants from the Hjalmar Svensson foundation, Wilhelm och Martina Lundgrens Vetenskapsfond [Wilhelm and Martina Lundgren's Science Fund], the Juvenile Diabetes Research Foundation International, and Svenska Läkaresällskapet [the Swedish Medical Society].
Contributor Information
Elisabet Stener-Victorin, Email: elsv@fhs.gu.se.
Thomas Lundeberg, Email: thomas.lundeberg@fyfa.ki.se.
Stefan Cajander, Email: stefan.cajander@clm.uas.lul.se.
Luigi Aloe, Email: aloe@in.rm.cnr.it.
Luigi Manni, Email: l.manni@in.rm.cnr.it.
Urban Waldenström, Email: urban.waldenstrom@telia.com.
Per Olof Janson, Email: per-olof.janson@obgyn.gu.se.
References
- Franks S, Adams J, Mason H, Polson D. Ovulatory disorders in women with polycystic ovary syndrome. Clin Obstet Gynaecol. 1985;12:605–632. [PubMed] [Google Scholar]
- Garcia-Rudaz C, Armando I, Levin G, Escobar ME, Barontini M. Peripheral catecholamine alterations in adolescents with polycystic ovary syndrome. Clin Endocrinol. 1998;49:221–228. doi: 10.1046/j.1365-2265.1998.00511.x. [DOI] [PubMed] [Google Scholar]
- Shoupe D, Lobo RA. Evidence for altered catecholamine metabolism in polycystic ovary syndrome. Am J Obstet Gynecol. 1984;150:566–571. doi: 10.1016/s0002-9378(84)90441-1. [DOI] [PubMed] [Google Scholar]
- Lara HE, Dissen GA, Leyton V, Paredes A, Fuenzalida H, Fiedler JL, Ojeda SR. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology. 2000;141:1059–1072. doi: 10.1210/endo.141.3.7395. [DOI] [PubMed] [Google Scholar]
- Brawer JR, Naftolin F, Martin J, Sonnenschein C. Effects of a single injection of estradiol valerate on the hypothalamic arcuate nucleus and on reproductive function in the female rat. Endocrinology. 1978;103:501–512. doi: 10.1210/endo-103-2-501. [DOI] [PubMed] [Google Scholar]
- Schulster A, Farookhi R, Brawer JR. Polycystic ovarian condition in estradiol valerate-treated rats: Spontaneous changes in characteristic endocrine features. Biol Reprod. 1984;31:587–593. doi: 10.1095/biolreprod31.3.587. [DOI] [PubMed] [Google Scholar]
- Lara HE, Ferruz JL, Luza S, Bustamante DA, Borges Y, Ojeda SR. Activation of ovarian sympathetic nerves in polycystic ovary syndrome. Endocrinology. 1993;133:2690–2695. doi: 10.1210/endo.133.6.7902268. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Waldenström U, Tägnfors U, Lundeberg T, Lindstedt G, Janson PO. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2000;79:180–188. doi: 10.1034/j.1600-0412.2000.079003180.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A, Labadia A, Triguero D, Costa G. Local regulation of oviductal blood flow. Gen Pharmacol. 1996;27:1303–1310. doi: 10.1016/S0306-3623(96)00082-1. [DOI] [PubMed] [Google Scholar]
- Yoshioka S, Fujiwara H, Yamada S, Tatsumi K, Nakayama T, Higuchi T, Inoue T, Maeda M, Fujii S. Endothelin-converting enzyme-1 is expressed on human ovarian follicles and corpora lutea of menstrual cycle and early pregnancy. J Clin Endocrinol Metab. 1998;83:3943–3950. doi: 10.1210/jcem.83.11.5277. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Spina G, Kouli C, Migdalis I. Increased endothelin-1 levels in women with polycystic ovary syndrome and the beneficial effect of metformin therapy. J Clin Endocrinol Metab. 2001;86:4666–4673. doi: 10.1210/jcem.86.10.7904. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Hill DF, Costa ME, Les Dees CW, Lara HE, Ojeda SR. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinology. 1996;137:198–209. doi: 10.1210/endo.137.1.8536613. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Lundeberg T, Waldenström U, Manni L, Aloe L, Gunnarsson S, Janson PO. Effects of electro-acupuncture on nerve growth factor in rats with experimentally induced polycystic ovaries. Biol Reprod. 2000;63:1507–1513. doi: 10.1095/biolreprod63.5.1497. [DOI] [PubMed] [Google Scholar]
- Stener-Victorin E, Lundeberg T, Waldenström U, Bileviciute-Ljungar I, Janson PO. Effects of electro-acupuncture on corticotropin releasing-factor (CRF) in rats with experimentally induced polycystic ovaries. Neuropeptides. 2002;35:1–5. doi: 10.1054/npep.2002.0878. [DOI] [PubMed] [Google Scholar]
- Lemne CE, Lundeberg T, Theodorsson E, de Faire U. Increased basal concentrations of plasma endothelin in borderline hypertension. J Hypertens. 1994;12:1069–1074. [PubMed] [Google Scholar]
- Bocchini V, Angeletti PU. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969;64:787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wescamp G, Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and peripheral tissues. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- Tirassa P, Stenfors C, Lundeberg T, Aloe L. Cholecystokinin-8 regulation of NGF concentrations in adult mouse brain through a mechanism involving CCKA and CCKB receptors. Br J Pharmacol. 1998;123:1230–1236. doi: 10.1038/sj.bjp.0701718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirassa P, Manni L, Stenfors C, Lundeberg T. RT-PCR ELISA method for the analysis of neurotrophin mRNA expression in brain and peripheral tissues. J Biotechnol. 2000;84:259–272. doi: 10.1016/S0168-1656(00)00370-9. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Acosta TJ, Miyamoto A, Ozawa T, Wijayagunawardane MP, Sato K. Local release of steroid hormones, prostaglandin E2, and endothelin-1 from bovine mature follicles In vitro: effects of luteinizing hormone, endothelin-1, and cytokines. Biol Reprod. 1998;59:437–443. doi: 10.1095/biolreprod59.2.437. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Yamada H, Liu Y, Kudo M. Immunohistochemical distribution of the endothelin-converting enzyme-1 in the rat hypothalamo-pituitary axis. Neurosci Lett. 2000;284:81–84. doi: 10.1016/S0304-3940(00)00974-5. [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Morita H, Cao WH, Ling GY, Kumada M, Kimura S, Nagai R, Yazaki Y, Kuwaki T. Role of endothelin-1 in stress response in the central nervous system. Am J Physiol Regul Integr Comp Physiol. 2000;279:515–521. doi: 10.1152/ajpregu.2000.279.2.R515. [DOI] [PubMed] [Google Scholar]
- Barria A, Leyton V, Ojeda SR, Lara HE. Ovarian steroidal response to gonadotropins and beta-adrenergic stimulation is enhanced in polycystic ovary syndrome: role of sympathetic innervation. Endocrinology. 1993;133:2696–2703. doi: 10.1210/endo.133.6.8243293. [DOI] [PubMed] [Google Scholar]