Abstract
Alzheimer's disease (AD) is characterized by the age-related deposition of β-amyloid (Aβ) 40/42 peptide aggregates in vulnerable brain regions. Multiple levels of evidence implicate a central role for Aβ in the pathophysiology of AD. Aβ peptides are generated by the regulated cleavage of an ≈700-aa Aβ precursor protein (βAPP). Full-length βAPP can undergo proteolytic cleavage either within the Aβ domain to generate secreted sβAPPα or at the N- and C-terminal domain(s) of Aβ to generate amyloidogenic Aβ peptides. Several epidemiological studies have reported that estrogen replacement therapy protects against the development of AD in postmenopausal women. We previously reported that treating cultured neurons with 17β-estradiol reduced the secretion of Aβ40/42 peptides, suggesting that estrogen replacement therapy may protect women against the development of AD by regulating βAPP metabolism. Increasing evidence indicates that testosterone, especially bioavailable testosterone, decreases with age in older men and in postmenopausal women. We report here that treatment with testosterone increases the secretion of the nonamyloidogenic APP fragment, sβAPPα, and decreases the secretion of Aβ peptides from N2a cells and rat primary cerebrocortical neurons. These results raise the possibility that testosterone supplementation in elderly men may be protective in the treatment of AD.
Alzheimer's disease (AD) is characterized by the deposition of senile plaques and neurofibrillary tangles in vulnerable brain regions. Senile plaques are composed of aggregated β-amyloid (Aβ) 40/42(43) peptides. Evidence implicates a central role for Aβ in the pathophysiology of AD (1). Epidemiological studies have reported that estrogen replacement therapy protects against the development of AD in postmenopausal women (2, 3). We previously reported that treating cultured neurons with 17β-estradiol reduced the secretion of Aβ40/42 peptides, suggesting that estrogen replacement therapy may protect women against the development of AD by regulating Aβ precursor protein (βAPP) metabolism (4). Although it has long been established that estrogen declines during menopause in aging women, declines in testosterone in aging men, until more recently, have been more controversial. Increasing numbers of reports indicate that testosterone, especially bioavailable free testosterone, declines significantly in aging men (5, 6) and in postmenopausal women (7). Although the relative decrease in the level of testosterone in men is modest compared with those of estrogen in perimenopausal women, aging men develop clinical signs of hypogonadism, such as reduced muscle and bone mass and an increase in visceral fat. Recent studies suggest that declining testosterone levels in older men are associated with disorders similar to those associated with estrogen deficiency in postmenopausal aging women, including osteoporosis (8, 9). Testosterone supplementation in orchiectomized, aging male rats protected these animals from osteoporosis and the decrease in the rate of periosteal bone formation found in their nonsupplemented littermates (8). A decrease in serum testosterone levels has been associated with depression in aging men, with clinical improvement reported after testosterone supplementation (9). Despite reports of beneficial effects on cognition and emotional tone, there are no published data on the effects of testosterone supplementation in AD. Results from a recent small, double-blind, placebo-controlled study indicated that testosterone supplementation in older men improved both verbal and spatial memory function,‖ supporting its possible efficacy in the treatment of the cognitive dysfunction that characterizes AD. Testosterone treatment has been shown to up-regulate the p75 NGF receptor and increase NGF levels in limbic areas, especially the hippocampus (10). However, the effects of testosterone in the brain are considerably less well characterized compared with those of estrogen.
Materials and Methods
Antibodies.
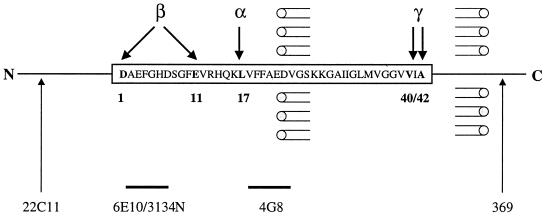
See schematic diagram for amino acid sequence of human Aβ and antibody recognition sites in Fig. 1. mAb 22C11 recognizes the N terminus of βAPP (and APLP1) (Boehringer Mannheim); mAb 6E10 (Senetek, Maryland Heights, MO) is directed at human Aβ1–10; polyclonal antibody 314N recognizes the N-terminal region of rodent Aβ; mAb 4G8 (Senetek) is directed at Aβ17–24; polyclonal antibody 369 recognizes the C terminus of βAPP.
Figure 1.
Schematic diagram of β-amyloid. The amino acid sequence of human Aβ (box), the major known sites of Aβ cleavage and of secretase actions (α, β, and γ), and the antibodies used (22C11 directed at the N-terminal region of βAPP; 6E10 directed at human Aβ1–10; 3431N directed at the N-terminal region of rodent Aβ; 4G8 directed at Aβ17–24; and 369 directed at the C-terminal region of βAPP) are shown.
Neuroblastoma (N2a) Cells.
Murine N2a cells transfected with human βAPP695 were maintained in medium containing 50% DMEM and 50% Opti-MEM, supplemented with 5% FBS, 200 μg/ml G418, and antibiotics (GIBCO/BRL), in the absence or presence of hormone. Cells were trypsinized at 0 and 7 days, and medium was replaced every 2 days. We previously reported that the effects of estrogen treatment on βAPP processing were similar when either charcoal-stripped FBS was substituted for FBS or when phenol red-free RPMI 1640 medium was substituted for DMEM/Opti-MEM (4). Standard conditions for studying N2a cells involved maintaining ≈2 × 106 cells per 60-mm dish for 10 days in the absence or presence of the indicated hormone (Sigma) followed by metabolic labeling.
Primary Neuronal Cultures.
Primary neuronal cultures were derived from the cerebral cortices of embryonic day 17 fetuses obtained from timed-pregnant Sprague–Dawley rats (Charles River Breeding Laboratories) as described previously (4, 11). Cells were plated in serum-free Neurobasal medium with N2 supplement (GIBCO) (which contains 20 nM progesterone) and 0.5 mM l-glutamine on poly-d-lysine-treated (0.1 mg/ml; Sigma) 100-mm dishes (approximately 8–10 × 106 cells per plate). Neurons were maintained with or without hormone, and medium was replaced every 2 days, as described previously (4).
Metabolic Labeling and Immunoprecipitation.
Cells were washed and incubated at 37°C for 20–30 min in methionine-free/glutamine-free DMEM (GIBCO). Cells then were pulse-labeled with 750 μCi/ml [35S]methionine (Dupont/NEN) in methionine-free medium supplemented with l-glutamine for 4 h. Cell lysates were prepared by scraping the cells in ice-cold PBS with a rubber policeman and then centrifuged briefly. The supernatant was aspirated, 100 μl of lysis buffer (0.5% deoxycholate/0.5% Nonidet P-40/5 μg/ml Trasylol/5 μg/ml leupeptin/0.25 mM PMSF) was added, and the extract was subjected to agitation, repeat centrifugation, and collection of supernatant. Samples of lysates and media were adjusted to a concentration of 0.5% SDS, and samples were heated for 1–2 min at 70–75°C. Samples were adjusted to 190 mM NaCl/50 mM Tris⋅HCl, pH 8.3/6 mM EDTA/2.5% Triton X-100. Samples of conditioned media were subjected to sequential immunoprecipitation, first with antibody 4G8 for Aβ, and then with antibody 6E10 for human and antibody 3134N for rat sβAPPα. Lysates were incubated with antibody 369 for full-length βAPP. Samples were incubated overnight with the primary antibody, followed by addition of secondary rabbit–anti-mouse antibody (Cappel) for 1 h and then addition of protein A-Sepharose beads (Pharmacia) for 2 h (all at 4°C). Samples were analyzed by 10–20% Tris-Tricine SDS/PAGE gels for Aβ and 4–12% Tris-Glycine SDS/PAGE gels for βAPP, followed by autoradiography on Kodak X-Omat AR5 film.
Immunoprecipitation–Mass Spectrometry Analyses of Aβ Release into Conditioned Medium.
Neurons were incubated in the absence or presence of 200 nM testosterone for 10 days. Serum-free medium was conditioned by cells overnight in the continued absence or presence of hormone. Aliquots of medium (5 ml) were immunoprecipitated by using antibody 4G8 and protein G/A-agarose beads. The molecular masses and concentrations of immunoprecipitated Aβ species were measured by matrix-assisted laser desorption ionization–time-of-flight–mass spectrometry analysis, as described previously (4). For analysis, Aβ12–28 and insulin (Ins2+) internal standards were added to samples.
Results
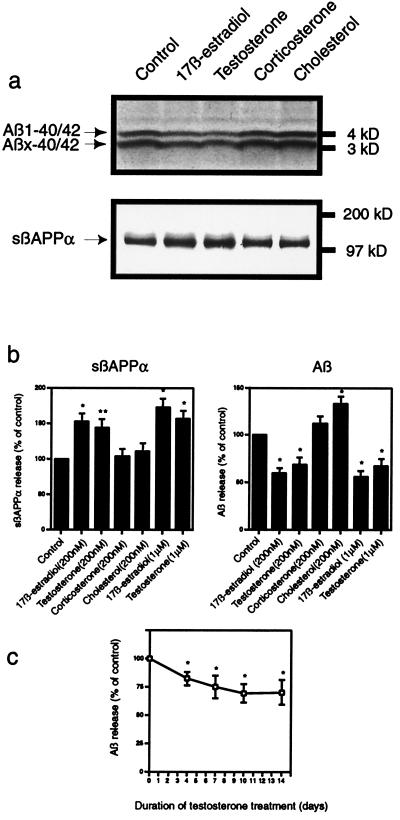
When N2a cells, which had been transfected with human wild-type βAPP695, were treated with testosterone, an increase in sβAPPα and a decrease in Aβ release were observed in the medium (Fig. 2). Treatment for 10 days with testosterone or 17β-estradiol, at 200 nM or 1 μM, stimulated secretion of sβAPPα by 50–75%. Concomitantly, these steroids reduced secretion of Aβ40/42 by 30–45%. The effect of testosterone on Aβ release was studied as a function of days of treatment and concentration of steroid. No additional effect on release could be observed either by increasing the treatment time to 14 days (Fig. 2c) or by increasing the steroid concentration to 2 μM (data not shown). The effects of testosterone were slightly less in magnitude than those of 17β-estradiol, but none of the differences observed was statistically significant.
Figure 2.
Effect of testosterone and other steroids on secretion of sβAPPα and Aβ from murine N2a neuroblastoma cells transfected with human βAPP695. (a) Representative autoradiographic analysis of sβAPPα (Lower) and Aβ (Upper). (b) Effects of steroids on the release of sβAPPα (Left) and Aβ (Right) represented as means ± SD, n = 3 (statistically different from controls by Student's t test; *, P < 0.01; **, P < 0.02). Effects of 17β-estradiol and testosterone were not statistically different from each other at either 200 nM or 1 μM. (c) Quantitation of Aβ release in response to treatment with 200 nM testosterone for 0–14 days. Data are represented as means ± SD, n = 3 (statistically different from control cells incubated for the same duration in the absence of hormone; *, P < 0.02).
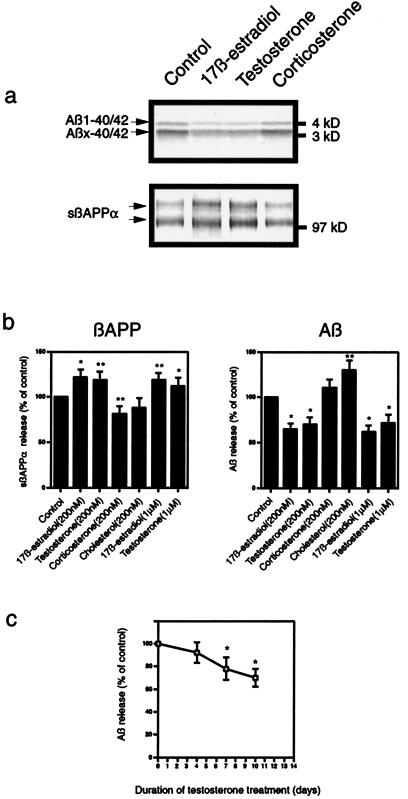
When primary neuronal cultures were treated with testosterone, an increase in sβAPPα and a decrease in Aβ release also were observed (Fig. 3). Treatment for 10 days with testosterone or 17β-estradiol, at 200 nM or 1 μM, stimulated secretion of sβAPPα by 20–30%. Concomitantly, these steroids reduced secretion of Aβ40/42 by 30–40%. The effect of testosterone on Aβ release increased with days of treatment up to 10 days (Fig. 3c). Primary neuronal cultures were not consistently viable in the absence of serum for periods longer than 10 days. Increasing the concentration of testosterone to 2 μM did not reduce Aβ release further.
Figure 3.
Effect of testosterone and other steroids on secretion of sβAPPα and Aβ from primary cultures of embryonic rat cerebrocortical neurons. (a) Representative autoradiographic analysis of sβAPPα (Lower) and Aβ (Upper). (b) Effects of steroids on the release of sβAPPα (Left) and Aβ (Right) in neurons represented as means ± SD, n = 3–4 (statistically different from control; *, P < 0.01; **, P < 0.03). Effects of 17β-estradiol and testosterone were not statistically different from each other at either 200 nM or 1 μM. (c) Quantitation of Aβ release in response to treatment with 200 nM testosterone for 0–10 days represented as means ± SD, n = 3 (statistically different from control cells incubated for the same duration in the absence of hormone; *, P < 0.03).
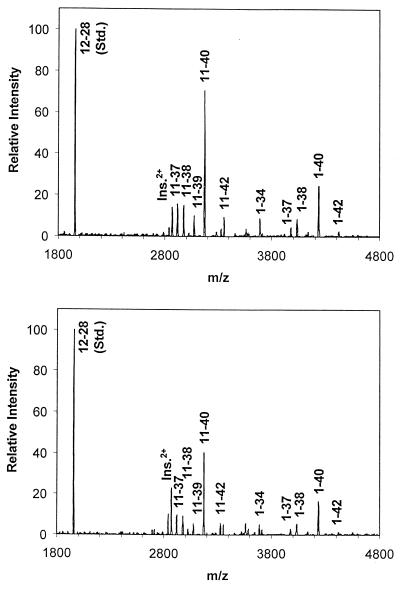
Testosterone caused reductions in the secretion of both the 4-kDa Aβ1–40/42 peptides and the 3-kDa Aβx-40/42 peptides from both N2a cells (Fig. 2a) and primary neuronal cultures (Fig. 3a). Using both radiosequencing and immunoprecipitation/mass spectrometry, we demonstrated previously in primary rat neuronal cultures that the predominant 3-kDa secreted Aβx-40/42 species are composed mainly of Aβ11–40/42 peptides and, to a lesser extent, Aβ17–40/42 peptides (11). Immunoprecipitation/mass spectrometric analysis of Aβ peptides secreted from primary neuronal cultures incubated in the presence or absence of 200 nM testosterone confirmed and expanded the observations on the testosterone-induced decrease in secretion of Aβ peptide species by neurons (Fig. 4). Testosterone treatment reduced Aβ1–40 and Aβ1–42, as well as various N- and C-terminal-truncated Aβ peptides.
Figure 4.
Immunoprecipitation/mass spectrometry analyses of Aβ release into conditioned medium. Neurons were incubated in the absence or presence of 200 nM testosterone for 10 days. Levels of Aβx-40 and Aβx-42 species measured in the presence of testosterone (Lower) were significantly lower than control (Upper) levels in each of three experiments, including the one illustrated here.
No change was seen in the level of βAPP holoprotein in either N2a cells or neurons with any of the steroids used (data not shown). To test the specificity of the effects of testosterone and estrogen on neuronal βAPP metabolism, we also examined the adrenal steroid hormone, corticosterone, and the ubiquitous sterol, cholesterol. Several previous studies have reported that cholesterol regulates sβAPPα secretion (12–15). Reported changes in Aβ release appear less clear. Studies using cultured cells demonstrated a decline in Aβ secretion under conditions that caused reductions in cholesterol (14, 16), whereas another group reported a decrease in total brain Aβ in animals fed a high cholesterol diet (13). In the present study, cholesterol treatment (200 nM) caused a 30–35% increase in Aβ40/42 secretion in both types of cultured cells but no significant effect on sβAPPα release (Figs. 2 and 3).
Levels of adrenal glucocorticoid hormones increase with aging and especially in AD, and these hormones are known for their potential deleterious effects on learning and memory (17, 18). In fact, glucocorticoid administration in experimental animals has been shown to cause memory impairments and selective cell death in the hippocampus. Interestingly, it was reported that orchiectomized, testosterone-deficient rats had an even greater propensity to exhibit glucocorticoid-induced hippocampal cell death and memory impairment, which was reversible upon testosterone treatment (19). However, no studies of potential glucocorticoid effects on βAPP metabolism have been reported. Apart from a slight decrease in sβAAPα secretion from neurons, corticosterone (200 nM) had no significant effect on sβAPPα or Aβ secretion from either cell type (Figs. 2 and 3).
Discussion
Our results indicate that neuronal cultures treated with testosterone secrete less amyloidogenic Aβ40/42 peptides and suggest that androgen supplementation for aging men, similar to estrogen replacement therapy in postmenopausal women, may be protective against the development of AD. Moreover, because testosterone also declines in postmenopausal women, estrogen replacement therapy supplemented with androgens might provide additional protection against the development of AD in women. These possible benefits must be weighed against potential deleterious effects of testosterone, including the development of prostate cancer in men and endometrial cancer in women. Our results support the biological specificity of the actions of testosterone and estrogen in altering βAPP metabolism, because equal concentrations of cholesterol and the glucocorticoid hormone, corticosterone, were not effective at reducing Aβ secretion. Given (i) the demonstrated therapeutic efficacy of estrogen in reducing the risk of dementia in women and (ii) the effects of testosterone and estrogen in reducing Aβ secretion in cell culture, it will be important to elucidate the molecular mechanisms by which these steroid hormones regulate βAPP metabolism.
Acknowledgments
We thank Bruce S. McEwen for helpful discussions, Andrew Czernik for critical reading of the manuscript, and Sacha N. Uljon for technical assistance. We thank Gopal Thinakaran and Sam Sisodia for generously providing us with N2a cells stably transfected with APPwt. This work is supported by U.S. Public Health Service Grants AG09464 (P.G.) and NS02037 (G.K.G.), the American Health Assistance Foundation (H.X.), Alzheimer's Association (G.K.G., H.X., and R.W.), and Ellison Medical Foundation (H.X. and P.G.).
Abbreviations
- Aβ
β-amyloid peptides
- βAPP
β-amyloid precursor protein
- AD
Alzheimer's disease
Footnotes
Cherrier, M. M., Craft, S., Plymate, S., Asthana, S., Matsumoto, A. & Brenner, B., Society of Endocrinology Meeting, June 25, 1998, New Orleans.
References
- 1.Selkoe D J. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- 2.Tang M X, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 3.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle D D, Metter E. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Gouras G K, Greenfield J P, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic J N, Seeger M, Relkin N R, et al. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 5.Sternbach H. Am J Psychiatry. 1998;155:1310–1318. doi: 10.1176/ajp.155.10.1310. [DOI] [PubMed] [Google Scholar]
- 6.Winters S J. Arch Fam Med. 1999;8:257–263. doi: 10.1001/archfami.8.3.257. [DOI] [PubMed] [Google Scholar]
- 7.Rako S. J Women's Health. 1998;7:825–829. doi: 10.1089/jwh.1998.7.825. [DOI] [PubMed] [Google Scholar]
- 8.Prakasam G, Yeh J K, Chen M M, Castro-Magana M, Liang C T, Aloia J F. Bone. 1999;24:491–497. doi: 10.1016/s8756-3282(99)00018-6. [DOI] [PubMed] [Google Scholar]
- 9.Tirassa P, Thiblin I, Agren G, Vigneti E, Aloe L, Stenfors C. J Neurosci Res. 1997;47:198–207. doi: 10.1002/(sici)1097-4547(19970115)47:2<198::aid-jnr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Seidman S N, Walsh B T. Am J Geriatr Psychiatry. 1999;7:8–33. [PubMed] [Google Scholar]
- 11.Gouras G K, Xu H, Jovanovic J N, Buxbaum J D, Wang R, Greengard P, Relkin N R, Gandy S. J Neurochem. 1998;71:1920–1925. doi: 10.1046/j.1471-4159.1998.71051920.x. [DOI] [PubMed] [Google Scholar]
- 12.Bodovitz S, Klein W L. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 13.Racchi M, Baetta R, Salvietti N, Ianna P, Franceschini G, Paoletti R, Fumagalli R, Govoni S, Trabucchi M, Soma M. Biochem J. 1997;322:893–898. doi: 10.1042/bj3220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti C G, Simons K. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howland D S, Trusko S P, Savage M J, Reaume A G, Lang D M, Hirsch J D, Maeda N, Siman R, Greenberg B D, Scott R W, et al. J Biol Chem. 1998;273:16576–16582. doi: 10.1074/jbc.273.26.16576. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno T, Haass C, Michikawa M, Yanagisawa K. Biochim Biophys Acta. 1998;1373:119–130. doi: 10.1016/s0005-2736(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 17.Weiner M F, Vobach S, Olsson K, Svetlik D, Risser R C. Biol Psychiatry. 1997;42:1030–1038. doi: 10.1016/s0006-3223(97)00165-0. [DOI] [PubMed] [Google Scholar]
- 18.Carlson L E, Sherwin B B, Chertkow H M. Horm Behav. 1999;35:254–263. doi: 10.1006/hbeh.1999.1518. [DOI] [PubMed] [Google Scholar]
- 19.Mizoguchi K, Kunishita T, Chui D H, Tabira T. Neurosci Lett. 1992;138:157–160. doi: 10.1016/0304-3940(92)90495-s. [DOI] [PubMed] [Google Scholar]