Abstract
Borrelia burgdorferi causes Lyme disease in humans. The genome of the sequenced type strain B31 MI consists of a linear chromosome, 12 linear plasmids, and 9 circular plasmids. Previous studies by other investigators indicated that some of these plasmids are essential for the survival of the spirochetes in vivo but not in vitro. We have studied plasmid stability during in vitro growth at 23 and 35°C, conditions that approximate the temperatures of the tick vector and the mammalian host, respectively. Starting with two clones that have all 21 plasmids, we investigated plasmid maintenance within the population and on a clonal level. After three passages (27 generations), the cultures were no longer homogeneous and some derivative clones had already lost multiple plasmids. Despite this, one of six clones analyzed after 25 passages (225 generations) retained all but one plasmid (cp9) and was able to complete the mouse-tick-mouse infectious cycle. We analyzed protein composition and regulation of gene expression of clones differing in plasmid content after serial passages. All clones tested exhibited temperature-regulated expression of several proteins, including OspC. In addition, analysis of cultures inoculated from frozen stocks suggests that freezing and/or thawing contributes to heterogeneity in the outgrowth population with respect to plasmid content. Our investigations show that in vitro propagation of a clone leads to a heterogeneous population but that virulent clones can persist through extended passage. We therefore conclude that isogenicity of clones must be confirmed irrespective of their in vitro passage history.
The spirochete Borrelia burgdorferi is a causative agent of Lyme disease (3, 28). The bacterium is maintained within an enzootic cycle between small mammals, mainly rodents, and the tick vector Ixodes spp. (7, 12). During its life cycle, the spirochete is confronted with different host environments, which require the ability to regulate gene expression in response to environmental signals (4, 5, 23, 27). The sequenced genome of the type strain B. burgdorferi B31 is organized in an unusual way compared to other bacteria, consisting of a linear chromosome and 12 linear and 9 circular plasmids (6, 9). More than 80% of the predicted open reading frames carried on the plasmids are not homologous to known sequences (6, 9). Many of the B. burgdorferi genes encoding outer surface proteins are on plasmids and are differentially expressed during the infectious cycle. Plasmids are often lost during in vitro cultivation of B. burgdorferi (2, 25). Loss of plasmids results in a heterogeneous spirochete population, which also exhibits differences in protein composition (15, 16, 25). Often, plasmid loss is accompanied by loss of infectivity in laboratory animals (21, 25, 31, 39). This suggests that the plasmids carry genes that are essential for infectivity but are not necessary for survival in artificial media (11, 17).
Loss of plasmids in the outgrowth of a putatively isogenic B. burgdorferi clone presents a problem for subsequent genetic manipulations. Here, we systematically investigated plasmid stability during in vitro propagation of two B. burgdorferi B31 MI clones in parallel and analyzed resulting phenotypes with regard to infectivity, protein profile, and temperature-dependent protein expression. We demonstrate that plasmids were lost in the population after a short period of cultivation. However, even after extended passage, the cultures still contained clones that were capable of completing the experimental mouse-tick-mouse infectious cycle. The experiments also addressed the efficacy of maintaining frozen B. burgdorferi stocks for minimization of plasmid loss. Surprisingly, the outgrowths from frozen stocks exhibited significant clonal heterogeneity, even when stocks were prepared from the primary culture of a colony. These results indicate that both in vitro passage and the standard procedures of freezing cultures and inoculating fresh cultures from frozen stocks of B. burgdorferi can exert selective pressure that can result in changes in the population with regard to plasmid content. Hence, studies in which the phenotypes of wild-type and derivative clones are compared must include confirmation that their plasmid contents are identical.
MATERIALS AND METHODS
B. burgdorferi strains and growth conditions.
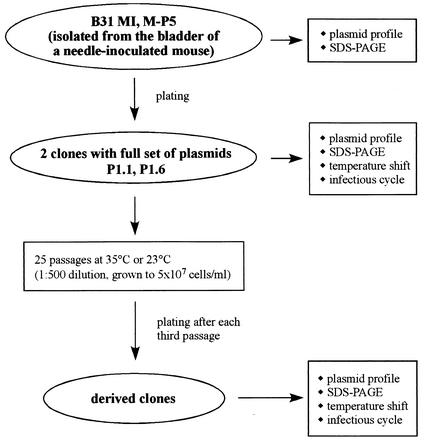
The strain B. burgdorferi B31 MI was previously described (3, 6, 9). Briefly, strain B. burgdorferi B31 was passaged four times through mice and three times in vitro. This infectious strain was designated B31 MI and was used for genomic sequencing (6, 9). A culture from this strain was passaged five times in vitro (P5) before injection into a mouse (8). Spirochetes isolated from the bladder of an infected mouse 75 days after inoculation were designated M-P5 (Fig. 1). B. burgdorferi cultures were grown in liquid Barbour-Stoenner-Kelly (BSK) II medium supplemented with gelatin and 6% rabbit serum (1). Plating was done in solid BSK medium as previously described (18).
FIG. 1.
Experimental outline.
In vitro passage of clones P1.1 and P1.6.
A 5-ml culture from a frozen stock of a B31 MI mouse reisolate (M-P5) was inoculated and grown to late exponential phase (108/ml). Dilutions of this culture were grown in solid BSK medium to obtain individual colonies, which were transferred to liquid BSK II medium (Fig. 1). Genomic DNA and frozen glycerol stocks were prepared from the primary cultures that originated from these colonies. We estimate that approximately 26 generations are required for outgrowth from a single spirochete to the late- exponential-phase culture (5 ml at 108/ml) from which frozen stocks were established. These stocks were considered passage 0 of individual clones. Two clones (P1.1 and P1.6) were shown (by PCR with plasmid-specific primers) to have all 21 plasmids previously identified in strain B31 MI (6, 8, 9). Cultures of clones P1.1 and P1.6 were inoculated from frozen glycerol stocks and grown in parallel in 5-ml cultures of liquid BSK II medium until late exponential phase (5 × 107 to 1 × 108 cells/ml) before they were passaged 1:500 into fresh medium. Each passage represents about nine generations. Cultures were vortexed extensively prior to counting, passaging, diluting, and plating to obtain accurate cell counts and to assure that colonies arose from a single bacterium. After every third passage (27 generations), the cultures were plated and clones were picked and analyzed. The cultures were subjected to 25 serial passages (225 generations) at 35 and 23°C (Fig. 1). The doubling times of P1.1 and P1.6 were about 8 h at 35°C and 21 h at 23°C and remained constant throughout the experiment. Frozen stocks were made from the cultures after each third passage. The designation of each of the picked clones consists of a number corresponding to the number of passages to which the culture was subjected before plating, the number of the parental clone from which the picked clone was derived, a letter referring to colony morphology, and a numerical designation (Table 1 and Table 2).
TABLE 1.
Plasmid content of B. burgdorferi B31 clones derived after serial passages at 35°C
| Passagea | Colony morphologyb | Plasmid(s) lostc
|
|
|---|---|---|---|
| P1.1 derived | P1.6 derived | ||
| 3 | D | cp9, lp21, lp25 | cp9 |
| E | lp28-4 | — | |
| F | cp9, lp25 | — | |
| 6 | E | cp9, lp28-4 | — |
| F | — | cp9, lp28-1, lp28-4 | |
| 9 | E | NEd | — |
| F | — | — | |
| 13 | E | — | — |
| F | lp28-4 | — | |
| 16 | D | — | — |
| E | — | — | |
| F | lp17, lp25 | — | |
| 19 | E | lp28-4 | cp9 |
| F | — | — | |
| 22 | E | — | — |
| F | lp17, lp25, lp28-2 | — | |
| 25 | E | cp9 | cp9, lp21, lp28-4 |
| F | lp28-4 | cp9, lp21 | |
One passage represents a 1:500 dilution of a late-exponential-phase culture to 1 × 105 to 5 × 105 cells/ml after ca. 3 days of growth at 35°C.
Representative clones were chosen on the basis of their differing colony morphologies. D, tiny, dense, defined border; E, small, dense center, defined border; F, large, diffuse, no defined border.
Plasmid content was assessed by PCR of individual clones derived from P1.1 and P1.6 clones after the indicated number of passages, as described in Materials and Methods. Plasmids that were not detected by PCR are listed. Dashes indicate that a PCR product was obtained for all plasmids.
NE, nonexistent. Plating of this passage resulted in colonies that all showed morphology F; no colonies with morphology E were obtained.
TABLE 2.
Plasmid content of B. burgdorferi B31 clones derived after serial passages at 23°C
| Passagea | Colony morphologyb | Plasmid(s) lostc
|
|
|---|---|---|---|
| P1.1 derived | P1.6 derived | ||
| 3 | E | lp25 | NEd |
| F | — | — | |
| 6 | E | — | — |
| F | — | — | |
| 9 | E | lp25 | cp9 |
| 13 | E | — | — |
| F | — | cp9, lp25 | |
| 16 | E | cp9, lp25 | — |
| F | cp9, lp25 | cp9, lp25 | |
| 19 | E | cp9, lp25 | — |
| F | cp9, lp25 | lp25 | |
| 22 | E | lp25 | — |
| F | cp9, lp25 | cp9, lp25 | |
| 25 | E | cp9, lp25 | cp9, lp25 |
One passage represents a 1:500 dilution of a late-exponential-phase culture to 1 × 108 cells/ml to 1 × 105 to 5 × 105 cells/ml after ca. 7 days of growth at 23°C.
Representative clones were chosen on the basis of their differing colony morphologies (Table 1).
Plasmid content was assessed by PCR of individual clones derived from P1.1 and P1.6 clones after the indicated number of passages, as described in Materials and Methods. Plasmids that were not detected by PCR are listed. Dashes indicate that a PCR product was obtained for all plasmids.
NE, nonexistent. Plating of this passage resulted in colonies that all showed morphology F; no colonies with morphology E were obtained.
PCR.
Using Wizard Genomic DNA purification kits (Promega, Madison, Wis.), total genomic Borrelia DNA was isolated from 5-ml cultures of each clone. Using 29 primer sets that amplified unique fragments of each plasmid (8), PCR was performed with 20-μl reaction mixtures (containing 50 to 100 ng of DNA). For most of the plasmids, only one primer pair was used for amplification, while for the lp56 and cp32 plasmids, two primer pairs specific for two independent open reading frames were used. Clones that showed inconclusive results with this primer set were subsequently examined with an independent primer set (17). Southern blot analyses confirmed the absence of particular plasmids in some clones. PCR conditions consisted of an initial denaturation step for 5 min at 94°C, 30 cycles with 30 s of denaturation at 94°C, 30 s of annealing at 55°C, and 1 min of extension at 72°C, and a final extension step for 7 min at 72°C. For B. burgdorferi colony screening with primer sets specific for plasmids lp25, lp28-1, and lp28-4, PCR was performed as follows: one initial denaturation step of 5 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 50°C, and 3 min at 68°C and a final extension step of 7 min at 72°C. PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide, and analyzed under UV light.
Protein profiles and temperature shift experiments.
B. burgdorferi clones were grown at 35°C either in BSK II medium with gelatin (supplemented with 6% rabbit serum) or in BSK-H medium (Sigma-Aldrich, St. Louis, Mo.). In mid-exponential phase (5 × 107 cells/ml), cultures were diluted to 5 × 105 cells/ml and incubated at 23°C. The bacteria were allowed to grow at 23°C to mid-exponential phase (5 × 107 cells/ml) before they were again diluted to 5 × 105 cells/ml and shifted to 35°C. Cultures were kept at 35°C until reaching a density of 5 × 107 cells/ml. Bacteria were harvested by centrifugation from cultures at both 23 and 35°C, washed with HN buffer (50 mM sodium chloride, 10 mM HEPES, pH 8.0), and resuspended in sample buffer (22) at 106 cells/μl.
Equal amounts of cell lysates were boiled for 5 to 10 min and separated through 12.5% polyacrylamide gels in a Bio-Rad Minicell system (Bio-Rad Laboratories, Hercules, Calif.) or in a Hoefer SE600 gel apparatus (Amersham Biosciences Corp., Piscataway, N.J.). Gels were stained with Coomassie brilliant blue R-250 (Merck AG, Darmstadt, Germany). Alternatively, using sodium phosphate buffer for the transfer, gels were blotted onto a nitrocellulose membrane as previously described (37). For staining of the Western blots, rabbit monoclonal anti-OspB antibody H4610 (kindly provided by T. Schwan, Rocky Mountain Labs, Hamilton, Mont.) was used as primary antibody at a 1:50 dilution (19, 26). Peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (whole molecule) (Sigma-Aldrich) was used as secondary antibody (1:50,000 dilution). Antibody binding was detected using enhanced chemiluminescence reagents (SuperSignal, Pierce, Rockford, Ill.).
Experimental mouse-tick-mouse cycle.
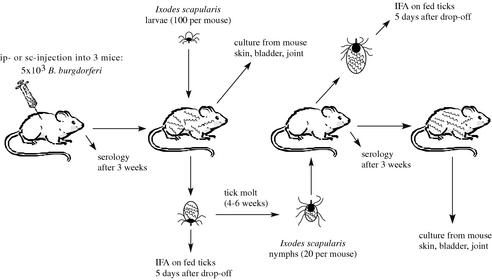
All animal experiments were conducted on protocols approved by the institution's Animal Care and Use Committee and adhered to the guidelines of the National Institutes of Health. Rocky Mountain Laboratories is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). B. burgdorferi clones were tested for infectivity in mice and ticks as shown in Fig. 2. White mice were obtained from a naïve colony at Rocky Mountain Laboratories. B. burgdorferi clones were grown in BSK II liquid medium and diluted to 2 × 104 cells/ml. A set of three adult female mice was anesthetized, and each mouse was injected with 4 × 103 bacteria intraperitoneally and 1 × 103 bacteria subcutaneously. One mouse per set was retro-orbitally bled before the injection to confirm the naïveté of the mice. After the inoculation, the mice were kept in separate cages. At 3 weeks postinoculation, the mice were anesthetized and blood was obtained from the retro-orbital sinus. The mouse sera were tested for their reactivity with P39 (BmpA) and with B. burgdorferi cell lysate to confirm infection (32, 33).
FIG. 2.
Experimental mouse-tick-mouse infectious cycle. ip, intraperitoneal injection; sc, subcutaneous injection.
Approximately 100 larval Ixodes scapularis ticks from a colony maintained at Rocky Mountain Laboratories were fed to repletion on each mouse. At 5 days after dropoff, a subset of engorged larvae was analyzed for the presence of spirochetes by indirect immunofluorescence (IFA) microscopy (see below).
After tick feeding, the mice were anesthetized, bled from the retro-orbital sinus, and sacrificed. Tissue samples from ear skin, bladder, and ankle joint synovia were grown in cultures to isolate spirochetes as previously described (34, 24, 17).
The remaining larvae were held in a humidified jar at 25°C and allowed to molt to nymphs. At 7 days or more after the molt, approximately 20 nymphs from each mouse were fed to repletion on a naïve mouse. At 5 days after dropoff, IFA microscopy was performed with a subset of engorged nymphs.
At 2 to 3 weeks after the nymphs had fed to repletion, the mice were anesthetized and retro-orbitally bled. The mouse sera were tested for reactivity with P39 and B. burgdorferi lysate. At 1 to 3 weeks later, the mice were anesthetized, bled from the retro-orbital sinus, and sacrificed. Tissue samples (from ear skin, bladder, and ankle joint synovia) were grown in cultures for detection of the presence of spirochetes.
IFA microscopy.
The assay was performed with midguts from fed ticks as previously described (29). Hyperimmune rabbit anti-B. burgdorferi Sh-2-82 antiserum (kindly provided by T. Schwan, Rocky Mountain Labs) was used as primary antibody at 1:100 dilution, and fluorescein isothiocyanate-labeled goat anti-rabbit IgG (H+L; Kierkegaard & Perry Laboratories, Gaithersburg, Md.) was used as secondary antibody (1:100 dilution). Fluorescence was detected using a Nikon Eclipse E800 light microscope equipped for epifluorescence microscopy (Nikon USA, Melville, N.Y.).
Serologic analysis.
Whole-cell lysates from B. burgdorferi cultures and Escherichia coli lysates were prepared as previously described (32). Cell lysates were separated through 12.5% polyacrylamide gels. Serologic conversion to recombinant P39 was used to assess whether the tested clone could establish an infection in mice. E. coli lysates containing the vector but lacking the gene bmpA (33) coding for P39 were used as a negative control, while B. burgdorferi lysate was used as a positive control. After electrophoresis, the gel was electroblotted onto a nitrocellulose membrane. Sera from experimentally infected mice were used as primary antibodies (1:200 dilution), and peroxidase-conjugated sheep anti-mouse IgG (whole molecule) (Sigma-Aldrich) was used as secondary antibody (1:10,000 dilution). Antibody binding was detected by enhanced chemiluminescence reagents (SuperSignal).
RESULTS
Plasmid content of clones during serial passage.
To address the issue of plasmid stability in B. burgdorferi during in vitro propagation, cultures were inoculated with two clones, P1.1 and P1.6, which contain all 21 plasmids ascribed to the parental strain B31 MI. Cultures were incubated at 23 and 35°C and plated after each third passage until passage 25 was reached (Fig. 1). Colonies appeared 14 to 18 days after plating and exhibited heterogeneous colony morphologies. Up to three representative colonies were picked (on the basis of differing colony morphologies) per plating, and their plasmid contents were analyzed by PCR with plasmid-specific primer sets (Table 1 and Table 2).
After three in vitro passages (27 generations) and one plating, the cultures were no longer homogeneous and some clones had already lost several plasmids, including cp9, lp25 and lp28-4 (Table 1 and Table 2). The presence or absence of lp25 and lp28-4 in individual clones was confirmed by Southern blot analysis (data not shown). No correlation was noted between colony morphology and plasmid content, and colony phenotype was not reproducible upon replating.
Although clones lacking multiple plasmids arose after limited in vitro passage, there was no overall trend during growth at 35°C toward rapid outgrowth of a population deficient in an increasing number of plasmids (Table 1). Of the clones examined from passages 3 through 13, 53% (9/17) retained all plasmids compared to 56% (10/18) of clones from passages 16 through 25 (Table 1). Even after 25 passages (225 generations), one of two clones examined in the P1.1-derived population still retained all of the plasmids except cp9.
A somewhat different pattern was seen during growth at 23°C. Of the clones examined from passages 3 through 13, 70% (9/13) retained all plasmids compared to only 14% (2/14) of clones from passages 16 through 25 (Table 2). In addition, the spectra of plasmids that were lost differed in cultures grown at 23 versus 35°C. Eight different plasmids were lost during growth at 35°C, whereas only two plasmids, cp9 and lp25, were lost during growth at 23°C (Table 1 and Table 2).
Analysis of cultures from frozen stocks for the presence of lp25, lp28-1, and lp28-4.
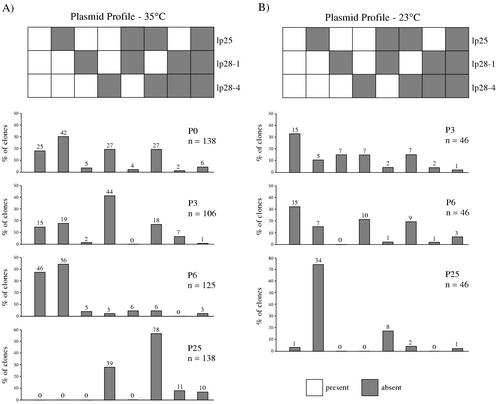
The initial analyses, while comprehensive with respect to plasmid content, were limited to a small number of clones. To obtain a more accurate assessment of plasmid loss in the population, we examined the stability of a subset of plasmids in a larger number of clones. Cultures were established from frozen stocks of clone P1.1 made after 0, 3, 6, and 25 passages at 23 and 35°C and were plated. Colonies were screened by PCR with primers specific for lp25, lp28-1, and lp28-4. We selected these plasmids because they were rapidly lost in our previous analysis and published studies implicated them as important in the mouse-tick infectious cycle (8, 11, 17). The number of colonies obtained and screened ranged from 46 to 138 per culture.
The analysis revealed several unanticipated features. First, significant heterogeneity existed in the culture derived from the passage 0 frozen stock (Table 3). Less than 20% of the clones examined from this culture retained all three plasmids. Second, the proportion of clones retaining all three plasmids was higher in the passage 6 culture than in the passage 0 culture. Finally, only a small percentage of the clones from the 23°C cultures, and none from the 35°C cultures, still contained all three plasmids by passage 25.
TABLE 3.
Percentage of colonies obtained from outgrowth of frozen stocks of P1.1 and derivative cultures that have lost one or more of the plasmids shown during in vitro propagation as assessed by colony PCR
| Temp (°C) and passagea | % of colonies that lost:
|
|||
|---|---|---|---|---|
| lp25 | lp28-1 | lp28-4 | None | |
| Passage 0 | 57 | 12 | 45 | 18 |
| 35°C | ||||
| Passage 3 | 36 | 9 | 66 | 14 |
| Passage 6 | 57 | 11 | 8 | 37 |
| Passage 25 | 64 | 15 | 100 | 0 |
| 23°C | ||||
| Passage 3 | 33 | 26 | 37 | 33 |
| Passage 6 | 44 | 11 | 50 | 33 |
| Passage 25 | 98 | 20 | 7 | 2 |
The number of colonies screened for passage 0 was 138 (the same inoculation culture was used for subsequent passages at 23 and 35°C), for passage 3 was 106 (35°C) and 46 (23°C), for passage 6 was 125 (35°C) and 46 (23°C), and for passage 25 was 138 (35°C) and 46 (23°C).
These results are surprising compared to those of the initial analysis for P1.1 and its derivatives. For example, lp28-4 was present in all 14 clones initially analyzed during continuous passage at 23°C (Table 2) but was absent in 45% of the clones in the subsequent analysis of the passage 0 culture after outgrowth from frozen stocks (Table 3). These data suggest that freezing and thawing B. burgdorferi can contribute to heterogeneity in the outgrowth population with regard to plasmid content.
The data presented in Table 3 convey the frequency with which plasmids lp25, lp28-1, and lp28-4 are lost with in vitro passage but do not provide information about the plasmid composition of the spirochete population. With the three plasmids we have monitored in this larger set of clones, eight different combinations of plasmids are possible. As shown in Fig. 3A, the plasmid profiles of individual clones were similar between passage 0 and passage 3 cultures at 35°C. The passage 6 culture at 35°C was unique, with a large proportion of clones having all three plasmids or lacking only lp25, whereas the 35°C passage 25 culture exhibited a significant shift toward clones lacking both lp25 and lp28-4. The clonal composition of the 23°C culture remained fairly stable through passage 6 but looked quite different by passage 25, with more than 70% of the clones lacking only lp25 (Fig. 3B). These differences in clonal heterogeneity among cultures might be partly due to sampling errors but presumably also reflect selective pressures exerted during in vitro passage at different temperatures and during outgrowth from frozen stocks.
FIG. 3.
Analysis of P1.1 and derivative clones from frozen stocks for detection of the presence or absence of lp25, lp28-1, and lp28-4. The number of screened colonies is shown for each graph. The eight possible combinations of plasmid contents are depicted in the diagram above the graphs. (A) Percentages of colonies from plated P1.1 and derivative cultures that lack the plasmids corresponding to those shown in the diagram at the top of the panel after 3, 6, and 25 passages at 35°C. The number of colonies with the indicated plasmid profile is shown above each column. (B) Percentages of colonies from plated P1.1-derived cultures that lack the plasmids corresponding to those shown in the diagram at the top of the panel after 3, 6, and 25 passages at 23°C. The number of colonies with the indicated plasmid profile is shown above each column.
Protein profiles and temperature-dependent regulation of protein expression.
Cell lysates were made from B. burgdorferi cultures after each third passage at 23 and 35°C and were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. No major differences in protein composition of the cultures were observed on the stained gel (data not shown). Previous studies have demonstrated that nonsense mutations in the ospB gene can arise spontaneously during in vitro propagation of B. burgdorferi (20, 21, 23, 25, 26). An 18-kDa fragment can be detected in these B. burgdorferi variants with the monoclonal antibody H4610, which recognizes the amino terminus of OspB (19, 26). We examined whether serial passage of B. burgdorferi clones P1.1 and P1.6 resulted in outgrowth of such OspB mutants and whether the temperature at which the cultures were grown influenced this. Immunoblot analysis of B. burgdorferi cultures with H4610 showed that the majority of the population in all cultures synthesized full-length OspB, irrespective of the identity of the clone of origin (P1.1 or P1.6), the temperature of propagation, or the extent of in vitro propagation (data not shown).
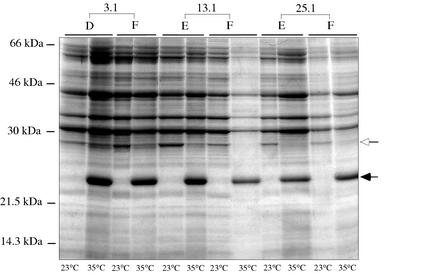
Previous studies have demonstrated that when B. burgdorferi cultures are shifted from 23 to 35°C (mimicking the temperature change that spirochetes undergo during transmission from tick vector to mammalian host), increased production of a number of outer surface proteins, including OspC, results (27, 29). Clones derived from P1.1 after passages 3, 13, and 25 at 35°C were tested for their ability to regulate protein expression in response to temperature. These clones were chosen on the basis of differences in plasmid content and the number of in vitro passages the parental clone had undergone. A protein of 24 kDa, which is also the molecular mass of OspC, was induced at 35°C relative to 23°C (Fig. 4). Another protein was synthesized at a higher level at 23°C than at 35°C (Fig. 4). For most of the clones, hybridization of a Western blot with a polyclonal antiserum to OspC (26) showed strong reactivity with the 24-kDa protein from 35°C lysates and little or no reactivity with 23°C lysates (data not shown). These results demonstrate that none of the plasmids missing in these clones (cp9, lp21, lp25, and lp28-4) was necessary for temperature-dependent expression of OspC and at least one other protein.
FIG. 4.
Comparison of sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles of P1.1-derived clones grown at different temperatures. Cell lysates were made from P1.1-derived clones grown at 23°C or after shifting them to 35°C, as indicated below each lane. Lysate from approximately 5 × 107 cells was loaded for each lane. The identities and passage numbers of clones are indicated at the top of the panel. Clone 3.1-E was missing plasmids cp9, lp21, and lp25; clone 3.1-F was missing cp9 and lp25; clone 13.1-E has a full complement of plasmids; clones 13.1-F and 25.1-F were missing lp28-4; and clone 25.1-E was missing cp9. The closed arrow on the right indicates a 24-kDa protein (OspC) that is upregulated at 35°C. The open arrow on the right indicates a 28-kDa protein that is upregulated at 23°C. Molecular masses are shown on the left.
Infectivity of clones in mice and ticks.
We compared the competence of clone P1.1 and one of its clonal derivatives (designated 25.1-E1) after 25 passages at 35°C in the experimental mouse-tick infectious cycle (Fig. 2). These clones differ in plasmid content only by the loss of cp9 from 25.1-E1. Previous studies have demonstrated that cp9 is not essential for mouse infectivity (8, 10, 11, 14, 17, 31). Both clones were infectious for mice following needle inoculation, as assessed by serologic conversion and reisolation from tissues (Table 4). In addition, both clones were acquired by larval ticks feeding on these mice and (following the molt from larvae to nymphs) were transmitted to naïve mice at the next blood meal. Finally, both P1.1 and 25.1-E1 were retained in the tick midgut in the molt from nymph to adult. These results demonstrate that both a low-passage clone and a moderately high-passage clonal derivative are infectious for mice and transmissible by ticks. Some differences were noted between these two clones in the frequency of acquisition and transmission of spirochetes by ticks; additional experiments are needed to determine whether these differences are reproducible and significant. We conclude that although in vitro propagation of B. burgdorferi results in a heterogeneous population with regard to plasmid content, clones containing all essential plasmids and capable of completing the entire mouse-tick-mouse infectious cycle can be retained after as many as 225 generations.
TABLE 4.
Infectivity of B. burgdorferi B31 in mice and ticks
| Clone | Infection route | No. of mouse sera reactive with P39/ total no. | No. of posi- tive tissues/ total no.a | No. of ticks positive by IFA/total no. |
|---|---|---|---|---|
| P1.1 | Needle inoculation | 3/3 | 4/6 | 24/24b |
| Tick bite | 3/3 | 6/9 | 9/9c | |
| 6/6d | ||||
| 25.1-E1 | Needle inoculation | 3/3 | 6/9 | 5/9b |
| Tick bite | 1/3 | 2/9 | 5/9c | |
| 3/6d |
Three tissues were analyzed per mouse.
Larval ticks.
Nymphal ticks.
Adult ticks.
DISCUSSION
In this study, we investigated the genetic and phenotypic stability of B. burgdorferi during in vitro propagation, as assessed by plasmid and protein profiles, responsiveness to temperature, and proficiency in the mouse-tick infectious cycle. We found that passaging a clone with the full complement of plasmids only three times can lead to heterogeneity with regard to the plasmid content of individual clones. Loss of plasmids cp9, lp25, lp28-1, and lp28-4 was not infrequent, as predicted from previous studies (11, 17, 21, 25). With continuous passage, however, plasmid instability did not occur to the extent anticipated. In investigations of a small number of clones after different numbers of passages (propagated at either 23 or 35°C), we found that the majority of clones retained plasmids known to be important for infectivity (such as lp25 and lp28-1) (11, 17).
Because this initial analysis was limited to a relatively small set of clones from each culture, we looked for the presence of lp25, lp28-1, and lp28-4 plasmids in a larger number of clones derived from frozen stocks made during in vitro propagation. The results we obtained from these analyses suggest that the temperature at which B. burgdorferi is grown, as well as some stage in the process of the production or use of frozen stocks, influences plasmid stability. We recognize that the analysis of individual clones we have described could also reflect a bias imparted by plating. Other investigators have reported that plating B. burgdorferi may select for clones that have lost plasmids (30). In our experience with optimized medium and plating conditions, we have obtained a plating efficiency of close to 100% (CFU compared to direct cell count of liquid culture before plating) and we have not observed a selection by solid media for clones lacking essential plasmids. We cannot extrapolate these findings to other strains and plating conditions. However, derivation of clones from isolated colonies is an unavoidable element in molecular genetic studies with this bacterium.
The in vitro phenotype observed for clone P1.1 resembles the phenotype of the previously described B clones in that they grow slowly, have full-length OspB, and are not transformable by allelic exchange. B clones were derived from strain B31 MI that had undergone five in vitro passages prior to passage through a mouse (8). The ability to transform different B. burgdorferi B31 MI clones correlates with certain phenotypes (8) and genotypes (13). To date, attempts to transform both clone P1.1 and its high-passage derivative clone, 25.1-E1, with either the shuttle vector pBSV2 (35) or by allelic exchange have not succeeded (data not shown). The question arises whether clones such as P1.1, which are not readily transformable, exhibit an altered genetic stability with in vitro propagation relative to clones with similar plasmid content that can be transformed, such as the previously described B31 MI clone A3 (8). Although we have not addressed the rate of plasmid loss as systematically in clone A3, data from a number of experiments indicate that similar spectra of plasmids are unstable in both P1.1 and A3 (K. Tilly and D. Grimm, unpublished data).
The loss of cp9, lp25, or lp28-4 did not alter the OspB phenotype or the ability to regulate protein expression in response to temperature changes. Previous studies have shown that cp9 and lp28-4 are not required for infectivity in mice (10, 11, 14, 17, 31), although a decrease in infectivity of clones that lack cp9 or lp28-4 has been described previously (11, 36, 39). Clone 25.1-E1, which was derived from clone P1.1 after 25 passages at 35°C and which lacked only cp9, was competent to infect mice by both needle inoculation and tick bite, and larval ticks acquired the strain by feeding on an infected mouse. The transmission of clone 25.1-E1 from infected nymphs to naïve mice was inefficient, however, as only one of three mice showed seroconversion to P39 and spirochetes were isolated from only that one mouse. This suggests that this high-passage clone is somewhat attenuated in infectivity relative to P1.1. In contrast, clone B31 MI A3 also lacks only cp9 yet does not exhibit reduced infectivity (8) (Tilly and Grimm, unpublished). Additional experiments are required to determine whether the apparent attenuation in infectivity of clone 25.1-E1 is reproducible and correlates with loss of cp9.
The impetus for these experiments was to determine the feasibility of genetic manipulation of B. burgdorferi through a systematic assessment of the extent to which heterogeneity arose within a B. burgdorferi population during growth of cultures. Genetic studies rely upon comparisons between isogenic wild-type and mutant clones. Transformation of B. burgdorferi is inefficient, and gene inactivation by allelic exchange requires expansion of a wild-type pathogenic clone to more than 1010 organisms to obtain the desired mutant (38). Previous studies provide ample evidence for heterogeneity after limited in vitro propagation of a B. burgdorferi clone. The loss of plasmids that are required for infectivity in mice (11, 17, 21, 25) is noteworthy. We therefore wanted to know whether the experimental manipulations necessary to generate mutants, such as growth in broth culture and colony formation on plates, inherently rendered derivative clones nonisogenic relative to their progenitor wild-type clones.
We conclude that it should be possible to generate isogenic wild-type and mutant clones, because in vitro propagation does not rapidly yield a population totally deficient in wild-type clones. However, we also note that heterogeneity in plasmid content can be detected in the primary outgrowth population from a colony; therefore, diligence with regard to demonstrating the isogenicity of wild-type and derivative mutant clones is essential for meaningful comparisons. We found an unexpected contribution to heterogeneity from the practice of maintaining frozen glycerol stocks of low-passage clones. We are investigating whether maintaining static cultures at 4°C gives rise to a more homogeneous outgrowth population. In summary, the experiments presented herein illustrate that genetic instability during in vitro propagation of B. burgdorferi is a trait that must be taken into account but does not present an insurmountable obstacle to genetic studies designed to investigate the roles of specific genes in this pathogenic bacterium.
Acknowledgments
We thank Paul Policastro for rearing and providing the Ixodes ticks; Tom Schwan for providing antibodies against B. burgdorferi and the outer surface proteins OspB and OspC; Tom Schwan, Paul Policastro, Ralph Larson, and Mike Parnell for help with animal work; Gary Hettrick and Anita Mora for graphical assistance; and Tom Schwan, Roberto Rebeil, and Olivia Steele-Mortimer for helpful comments on the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour, A. G. 1988. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J. Clin. Microbiol. 26:475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 4.Carroll, J. A., C. F. Garon, and T. G. Schwan. 1999. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll, J. A., N. El-Hage, J. C. Miller, K. Babb, and B. Stevenson. 2001. Borrelia burgdorferi RevA antigen is a surface-exposed outer membrane protein whose expression is regulated in response to environmental temperature and pH. Infect. Immun. 69:5286-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 7.Donahue, J. G., J. Piesman, and A. Spielman. 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 36:92-96. [DOI] [PubMed] [Google Scholar]
- 8.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 10.Golde, W. T., and M. C. Dolan. 1995. Variation in antigenicity and infectivity of derivatives of Borrelia burgdorferi, strain B31, maintained in the natural, zoonotic cycle compared with maintenance in culture. Infect. Immun. 63:4795-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587-609. [DOI] [PubMed] [Google Scholar]
- 13.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31 MI. Infect. Immun. 69:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris, S. J., C. J. Carter, J. K. Howell, and A. G. Barbour. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 60:4662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris, S. J., J. K. Howell, S. A. Garza, M. S. Ferdows, and A. G. Barbour. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosa, P. A., and D. Hogan. 1992. Colony formation by Borrelia burgdorferi in solid medium: clonal analysis of osp locus variants, p. 95-103. In U. G. Munderloh and T. J. Kurtti (ed.), First International Conference on Tick Borne Pathogens at the Host-Vector Interface: an agenda for research. University of Minnesota, St. Paul, Minn.
- 19.Rosa, P. A., T. Schwan, and D. Hogan. 1992. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol. Microbiol. 6:3031-3040. [DOI] [PubMed] [Google Scholar]
- 20.Sadziene, A., P. A. Rosa, P. A. Thompson, D. M. Hogan, and A. G. Barbour. 1992. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J. Exp. Med. 176:799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadziene, A., A. G. Barbour, P. A. Rosa, and D. D. Thomas. 1993. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect. Immun. 61:3590-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Schwan, T. G. 1996. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect. Agents Dis. 5:167-181. [PubMed] [Google Scholar]
- 24.Schwan, T. G., W. Burgdorfer, M. E. Schrumpf, and R. H. Karstens. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J. Clin. Microbiol. 26:893-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwan, T. G., W. Burgdorfer, and C. F. Garon. 1988. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 56:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwan, T. G., M. E. Schrumpf, R. H. Karstens, J. R. Clover, J. Wong, M. Daugherty, M. Struthers, and P. A. Rosa. 1993. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J. Clin. Microbiol. 31:3096-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwan, T. G., W. Burgdorfer, and P. A. Rosa. 1999. Borrelia, p. 746-758. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 29.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siebers, A., W. Zhong, R. Wallich, and M. M. Simon. 1999. Loss of pathogenic potential after cloning of the low-passage Borrelia burgdorferi ZS7 tick isolate: a cautionary note. Med. Microbiol. Immunol. 188:125-130. [DOI] [PubMed] [Google Scholar]
- 31.Simpson, W. J., C. F. Garon, and T. G. Schwan. 1990. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb. Pathog. 8:109-118. [DOI] [PubMed] [Google Scholar]
- 32.Simpson, W. J., W. Burgdorfer, M. E. Schrumpf, R. H. Karstens, and T. G. Schwan. 1991. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J. Clin. Microbiol. 29:236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson, W. J., W. Cieplak, M. E. Schrumpf, A. G. Barbour, and T. G. Schwan. 1994. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol. Lett. 119:382-387. [DOI] [PubMed] [Google Scholar]
- 34.Sinsky, R. J., and J. Piesman. 1989. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 27:1723-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart, P. E., R. Thalken, J. L. Bono, and P. Rosa. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714-721. [DOI] [PubMed] [Google Scholar]
- 36.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 69:3507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilly, K., S. Casjens, B. Stevenson, J. L. Bono, D. S. Samuels, D. Hogan, and P. Rosa. 1997. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol. Microbiol. 25:361-373. [DOI] [PubMed] [Google Scholar]
- 38.Tilly, K., A. F. Elias, J. L. Bono, P. Stewart, and P. Rosa. 2000. DNA exchange and insertional inactivation in spirochetes. J. Mol. Microbiol. Biotechnol. 2:433-442. [PubMed] [Google Scholar]
- 39.Xu, Y., C. Kodner, L. Coleman, and R. C. Johnson. 1996. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect. Immun. 64:3870-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]