Abstract
During the process of bloodfeeding by Anopheles stephensi, mammalian latent transforming growth factor β1 (TGF-β1) is ingested and activated rapidly in the mosquito midgut. Activation may involve heme and nitric oxide (NO), agents released in the midgut during blood digestion and catalysis of l-arginine oxidation by A. stephensi NO synthase (AsNOS). Active TGF-β1 persists in the mosquito midgut to extended times postingestion and is recognized by mosquito cells as a cytokine. In a manner analogous to the regulation of vertebrate inducible NO synthase and malaria parasite (Plasmodium) infection in mammals by TGF-β1, TGF-β1 regulates AsNOS expression and Plasmodium development in A. stephensi. Together, these observations indicate that, through conserved immunological cross talk, mammalian and mosquito immune systems interface with each other to influence the cycle of Plasmodium development.
In Anopheles, malaria parasite (Plasmodium spp.) development begins with the ingestion of blood containing sexual-stage gametocytes. Parasite fertilization commences within minutes of ingestion. Zygotes penetrate the midgut epithelium 24 to 36 h later and transform into vegetative oocysts under the basal lamina in the hemolymph-filled, open circulatory system of the mosquito. As blood digestion proceeds to completion at ∼ 48 h, parasite development continues. Oocysts grow and develop for 10 to 12 days and then release thousands of haploid sporozoites into the hemolymph. Sporozoites invade the salivary glands, where they are released into the saliva during subsequent blood feeding.
Anopheles stephensi, a primary vector of human malaria in India and the Middle East, limits malaria parasite development with the inducible synthesis of nitric oxide (NO [34]) catalyzed by A. stephensi NO synthase (AsNOS [33, 34]). Induction of AsNOS is proportional to the intensity of parasite infection and is detectable in the midgut by 6 h postinfection (14). Early induction is critical to inhibition of parasite development: dietary provision of the pan-NOS inhibitor l-N G-nitroarginine methyl ester (l-NAME), with a half-life in blood of 3 to 6 h (13), resulted in significantly higher Plasmodium infection intensities than did the inactive enantiomer d-NAME (34).
The NO-mediated defense of A. stephensi is analogous to mammalian NO-mediated inactivation of liver-invading sporozoites and blood-stage gametocytes (36, 41) and indicates that mosquitos and vertebrates share a conserved anti-Plasmodium defense. Limited information, however, is available on the regulation of AsNOS (32). In contrast, iNOS, the inducible NOS of primary importance in the mammalian arsenal against many pathogens (10), is regulated by numerous cytokines and other agents in a wide variety of mammalian cells, thereby ensuring homeostasis (10, 42). Recent studies suggest that among this diverse array of cytokines, TGF-β1 is perhaps the most potent physiological regulator of iNOS (54).
In primary cells of the vertebrate immune system (e.g., macrophages), TGF-β1 reduces iNOS expression (54), iNOS mRNA stability and translation (54), and iNOS stability and enzyme activity (42, 54). In contrast to this role as a negative regulator, TGF-β1 can upregulate iNOS expression and enzyme activity in “sentinel” cells associated with tissue immunity, including fibroblasts, chondrocytes, and epithelial cells (9, 23, 25). These latter cell types, although commonly implicated as first-line defenders of host integrity against infection (31, 50), have received less attention than cell types more commonly associated with adaptive immunity.
During parasite infection in mammalian hosts, the role of TGF-β1 in regulation of the immune response is largely dependent on timing and level of synthesis (43). Early in parasite infection, low levels of TGF-β1 appear to promote proinflammatory responses (e.g., induction of iNOS) that inhibit parasite growth, whereas later in infection, elevated levels of TGF-β1 are anti-inflammatory and serve to limit host autoimmune pathology.
In A. stephensi, the maintenance of immunological balance (e.g., regulation of AsNOS and other immune effectors) during parasite infection is poorly understood. The A. stephensi genome encodes a TGF-β superfamily member, As60A, whose expression is induced in the midgut epithelium as early as 3 h after infection with Plasmodium spp. (14, 15). Induction of As60A is correlated with periods of parasite motility and reproduction. Furthermore, expression of As60A in the A. stephensi midgut, like that of AsNOS, depends on the intensity of parasite infection (14). This finding suggests that As60A may be involved in the regulation of parasite development. Although we have not yet established that As60A regulates AsNOS in a manner similar to the regulation of iNOS by TGF-β1, As60A is the first inflammatory cytokine gene to be described from an insect.
Signaling by TGF-β superfamily members is mediated by the Smad family of proteins (3). Previous studies have demonstrated extensive cross talk among vertebrate and invertebrate (Drosophila melanogaster) TGF-β superfamily proteins and Smad signaling pathways (35). In general, activated TGF-β/activin and BMP receptor complexes recruit and phosphorylate relevant receptor Smads, which are then released to form multimeric complexes with co-Smads. Receptor Smad/co-Smad complexes translocate to the nucleus and interact with other DNA-binding proteins to regulate transcription of downstream target genes (45).
TGF-β1 is made by most mammalian cell types, especially platelets, in a biologically inactive form that can reach levels of 5,000 pg/ml in the circulation (49). Based on our understanding of the A. stephensi NO-mediated defense against Plasmodium, the regulation of iNOS and parasite infection by TGF-β1, and the demonstrated cross talk among Drosophila and vertebrate TGF-β superfamily ligands and Smads, we sought to answer the following questions. Is mammalian TGF-β1 ingested by A. stephensi during blood feeding? Could heme and NO, agents common to the mosquito midgut after feeding, participate in the activation of latent TGF-β1? Can ingested TGF-β1 regulate Plasmodium development in A. stephensi? We show here that mammalian TGF-β1 is ingested and activated in the midgut of A. stephensi and that activation may proceed through a mechanism involving heme and NO. Further, in a manner analogous to TGF-β1 regulation of human iNOS and Plasmodium development, human TGF-β1 can regulate both AsNOS expression in A. stephensi cells and parasite development in the mosquito.
MATERIALS AND METHODS
Mosquito rearing, blood feeding, midgut analyses, and Plasmodium infection.
A. stephensi Liston (Indo-Pakistan) malaria mosquitos were reared and maintained at 27°C and 75% relative humidity under a 12-h light-dark cycle. For preparation of midgut lysates, 4- to 7-day-old A. stephensi mosquitos were allowed to feed to repletion on anesthetized naive or Plasmodium berghei NK65-infected Institute of Cancer Research (ICR) mice. This protocol was reviewed and approved by the Virginia Tech Animal Care Committee in accordance with all relevant federal guidelines. Quantitative reverse transcription-PCR (RT-PCR) analysis of induction of AsNOS expression in the A. stephensi midgut epithelium was performed by using Taqman technology and the sequence detection system 7700 (PE Applied Biosystems, Foster City, Calif.) as described previously (14); six separate cohorts of blood-fed uninfected and P. berghei-infected A. stephensi were used for these assays. Differences between uninfected and P. berghei-infected A. stephensi at each time point were analyzed by using the Student t test. These and all subsequent Student t tests were performed by using Microsoft Excel 2000. To determine the concentration of nitrogen oxides (NOx) and active mammalian TGF-β1 in A. stephensi midgut lysates and whether midgut lysate components could activate latent human TGF-β1 in vitro, 50 midguts were dissected into phosphate-buffered saline (pH 7.4) [PBS; with 1% Nonidet P-40, 4 mM potassium ferricyanide or K3Fe(CN)6, 10 mM N-ethylmaleimide, and 0.1 mM diethylamine triamine pentaacetic acid [DTPA] for NOx analysis (24)], sonicated, and immediately frozen in a dry ice-ethanol bath at varied times postfeeding.
The concentration of NOx was determined essentially as described previously (37). Briefly, protein concentration was reduced in diluted midgut lysate samples by microfiltration (10- or 100-kDa Microcon; Millipore Corp., Bedford, Mass.) prior to reduction with vanadium (III)-hydrochloride at 95°C in a helium-purged, anaerobic reaction vessel; this treatment was essential to minimize sample foaming during analysis. The resulting NO was reacted with ozone for chemiluminescent detection and integration (Nitric Oxide Analyzer; Sievers Instruments, Boulder, Colo.). All values were compared to a standard curve of sodium nitrate (NaNO3) and normalized against the heme concentrations of paired sample aliquots to account for differences in blood meal volumes among mosquitoes. Heme concentrations were determined from the absorbance of the Soret band at 410 to 414 nm (2). Four separate cohorts of uninfected and P. berghei-infected A. stephensi were used for these analyses. The levels of NOx induction in midguts of P. berghei-infected A. stephensi relative to those in uninfected A. stephensi at each time point were analyzed by using the Student t test.
Concentration of active TGF-β1 in the midgut lysates was determined by using the Quantikine enzyme-linked immunosorbent assay (ELISA; R&D Systems, Inc., Minneapolis, Minn.), which detects binding to immobilized TGF-β1 type II receptors. The concentration of active TGF-β1 present in each lysate was assessed after PBS treatment for 3 h in vitro according to the manufacturer's recommendation, whereas the concentration of latent TGF-β1 in each lysate was assessed after transient acidification with 1 mM HCl (11, 56). Activation of exogenous latent TGF-β1 was assessed by ELISA after incubation of lysates with 5,000 pg or 1 μg of latent human TGF-β1/ml for 3 h at room temperature. The impact of NO on activation of exogenous latent TGF-β1 was assessed after treatment of lysates with the NO donor S-nitroso-N-acetyl-d,l-penicillamine (SNAP) alone as a control (1 mM for 3 h) or with SNAP and latent TGF-β1. Two separate cohorts of A. stephensi were used for these analyses.
To confirm that mosquito proteins did not cross-react with the anti-TGF-β1 antisera used in the Quantikine ELISA, a lysate of total proteins from immortalized ASE A. stephensi cells, which express As60A, was blotted and probed with anti-human TGF-β1. Briefly, ASE cells were washed with PBS, resuspended, lysed in loading buffer (62.5 mM Tris-HCl, pH 6.8; 2% sodium dodecyl sulfate; 25% glycerol; 0.01% bromophenol blue), and incubated at 100°C for 5 min. Approximately 22 μg of ASE cell proteins was loaded onto a 12% Tris-HCl Ready-Gel (Bio-Rad, Hercules, Calif.); 10 ng of recombinant human TGF-β1 (R&D Systems) was loaded as a positive control. After electrophoresis, separated proteins were transferred to a nitrocellulose membrane by using a semidry apparatus (Bio-Rad); poststaining of the transferred gel with Coomassie brilliant blue (48) indicated complete transfer. The nitrocellulose membrane was blocked and then incubated overnight with 1 μg of chicken anti-human TGF-β1 (R&D Systems)/ml at 4°C in blocking buffer (Tris-buffered saline [pH 7.4] with 0.1% Tween 20, 0.1% bovine serum albumin [BSA], and 10% nonfat dry milk), washed, and incubated with a 1:15,000 dilution of anti-chicken immunoglobulin G-horseradish peroxidase in blocking buffer. Antibody binding was detected by using luminol enhancer as recommended by the manufacturer (Pierce, Rockford, Ill.). To detect cross-reacting mosquito proteins, the membrane was overexposed to film for 4 h at room temperature.
For infection with Plasmodium falciparum (NF54 strain), 4- to 7-day-old female A. stephensi were allowed to feed through a 37°C water-jacketed membrane on identical aliquots of a mixture of parasites cultured in human erythrocytes with added uninfected erythrocytes (washed twice with RPMI 1640 medium) and human serum. The use of human blood components in this procedure was reviewed by the Virginia Tech Institutional Review Board and declared an exempt protocol in accordance with federal guidelines. Human serum used for mosquito feeding was stored at −70°C, thawed, and then stored at 4°C for approximately 1 week during experimental feedings. ELISA analysis of these sera revealed no detectable active human TGF-β1; latent TGF-β1 levels ranged from 3,000 to 5,000 pg/ml. For analysis of the effects of human recombinant TGF-β1 on parasite development, equivalent volumes of sterile PBS or TGF-β1 to a final concentration of 2, 200, or 2,000 pg/ml were added to the blood immediately before membrane feeding. At 7 days postinfection, A. stephensi midguts were dissected to count mature P. falciparum oocysts on the midgut epithelium. Two separate cohorts of A. stephensi were used for these assays; oocysts from 60 to 75 mosquitos from each treatment group and the PBS controls were counted. Differences between each treatment group and the PBS control group were analyzed by using the Student t test.
Activation of latent human TGF-β1 in vitro.
All experiments were conducted in siliconized plastic tubes. Carrier-free latent TGF-β1 (10 μg/ml in 1 mM HEPES; R&D Systems) was treated with Angeli's Salt (a nitroxyl anion donor; 10 μM), peroxynitrite (100 μM), or hemin (10 μM) for 30 to 60 min. Log doses of latent human TGF-β1 (6 to 2,000 pg/ml) were then prepared and assessed for TGF-β1 activity in replicated bioassays of mink lung epithelial cell (CCL-64) proliferation as described previously (55). As controls, paired plates of CCL-64 cells were treated with 10 μM hemin, 10 μM Angeli's salt, or 100 μM peroxynitrite alone and assayed as described earlier. Proliferation rates of CCL-64 cells treated with oxidants alone or with TGF-β1 prepared as described were normalized to proliferation of positive control CCL-64 cells treated with heat-activated latent TGF-β1 (11, 22). Data were analyzed by one-way analysis of variance, followed by application of the Bonferroni post hoc test by using commercially available software (SPSS, Inc., Chicago, Ill.).
Modulation of the neutralizing capacity of LAP by hemin or nitroxyl anion.
Recombinant human active TGF-β1 was added to CCL-64 cells for 20 h. Alternatively, recombinant human latency-associated peptide (LAP) was untreated or treated with 10 μM hemin or 10 μM Angeli's salt, incubated with active TGF-β1, and subsequently added to CCL-64 cells for 20 h. The cells were then assessed for proliferative capacity as described above.
Analyses of A. stephensi cell responses to human TGF-β1 in vitro.
For these assays, immortalized MSQ43 and ASE A. stephensi cell lines were maintained in modified Eagle minimum essential medium supplemented with 5% heat-inactivated fetal calf serum as described previously (E5 [20]). For analysis of change in morphology, MSQ43 cells were plated in E0 medium (no serum) and allowed to recover overnight. Duplicate plates of cells were treated with diluent (4 mM HCl, 1 mg of BSA/ml) or human TGF-β1 in diluent at 0.6, 6.0, or 30 pg/ml for 20 min or 60 min. Cells were then fixed and coverslipped for microscopic examination. At least 100 cells per treatment were scored as “round” or “spread”; the lengths (in centimeters) of the spread cells were measured on enlarged photomicrographs along the axis perpendicular to the cell body. Differences among percentages of round cells from assay replicates were analyzed by χ2 analysis with commercially available software (SPSS), while differences between mean cell lengths were analyzed by using the Student t test. For analysis of DNA synthesis, duplicate plates of ASE cells were treated with PBS or 0.08, 0.8, or 8 pg of human TGF-β1/ml in PBS for 48 h. [3H]thymidine was added to each plate to 5 μCi/ml for 30 min at room temperature. After incubation, DNA was precipitated from washed cells with 10% trichloroacetic acid and incorporated counts were measured by liquid scintillation as described previously (52). Data from three separate assays were analyzed by using the Student t test. For analysis of AsNOS expression in cultured cells, duplicate plates of ASE cells were treated with PBS or 6 pg of human TGF-β1/ml for 6, 24, or 48 h. In a second assay, ASE cells were pretreated for 1 h with PBS or with 6 pg of human TGF-β1/ml. After pretreatment, cells were exposed for 48 h to 1,000 heat-killed Micrococcus luteus or Escherichia coli organisms per ASE cell as an immune stimulus (21). Total RNA was isolated from cells postassay by using TRIzol (Invitrogen Life Technologies, Carlsbad, Calif.) reagent. Quantitative RT-PCR of AsNOS expression was performed as described previously (14). Data from replicated assays were analyzed by using the Student t test.
RESULTS
A. stephensi midgut: induction of AsNOS expression, synthesis of NO, and persistence of active mammalian TGF-β1 in a blood-rich environment.
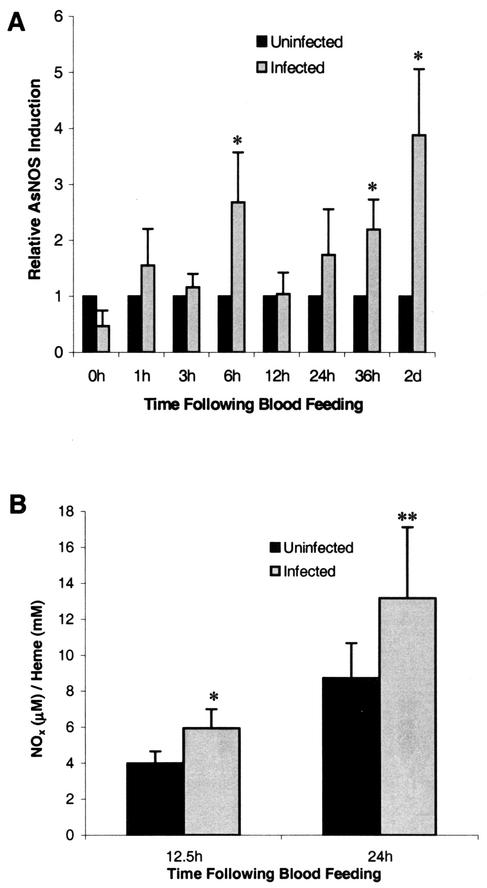
Induction of AsNOS expression in the midgut epithelium of A. stephensi after P. berghei infection was measured by using quantitative RT-PCR as described previously (14). As early as 6 h postinfection, induction of AsNOS expression in the midgut was significant relative to expression in uninfected mosquitos (Fig. 1A). In contrast, AsNOS induction at 12 and 24 h postinfection was not significant (Fig. 1A). By 36 h postinfection, however, significant induction was again noted, and this difference extended through 48 h postinfection (Fig. 1A). These data corroborate and expand previous observations demonstrating a biphasic induction pattern of AsNOS (34) and establish that induction of AsNOS expression in the midgut begins as early as 6 h postinfection.
FIG. 1.
Induction of AsNOS expression and levels of nitrogen oxides (NOx) in the A. stephensi midgut after infection with Plasmodium. (A) Induction levels of AsNOS expression in midguts dissected from six cohorts of uninfected and P. berghei-infected A. stephensi mosquitoes were determined by using quantitative RT-PCR as described previously (14). At each time point, expression levels were divided by expression in uninfected insects to show relative AsNOS induction in P. berghei-infected A. stephensi mosquitoes. Significantly enhanced levels of AsNOS expression in P. berghei-infected A. stephensi mosquitoes relative to uninfected A. stephensi are indicated with an asterisk (∗, P < 0.05). (B) Levels of NOx in midgut blood lysates from four cohorts of P. berghei-infected and uninfected A. stephensi mosquitoes were determined essentially as described previously (37); the values obtained were compared to a standard curve of NaNO3 and normalized against lysate heme concentration to account for variation in blood meal volumes among mosquitoes. Data were analyzed by using the Student t test; values are means ± the standard errors (SE). At 12.5 and 24 h postfeeding, NOx levels in the midguts of P. berghei-infected A. stephensi mosquitoes were induced relative to those in uninfected A. stephensi midguts (∗ and ∗∗, P = 0.049 and P = 0.069, respectively).
To determine whether inducible AsNOS expression was correlated with elevated output of NO in the midgut environment, we measured the concentration of NO derived from NOx in lysates of midgut blood from four separate cohorts of A. stephensi as described previously (37). In these assays, NOx would be expected to include nitrate, nitrite, and S-nitrosothiols (37), which are predominant biological products of induced NOS activity. Based on significant AsNOS induction at 6 h (Fig. 1A), we selected 12.5- and 24-h time points for these analyses. At both 12.5 and 24 h postinfection, heme-normalized NOx levels were elevated in midgut blood lysates from infected compared to uninfected mosquitos (P = 0.049 and 0.069, respectively; Fig. 1B). The mean percentage increase at 12.5 h was 59.5% (95% confidence interval [CI] −0.8 to 119.8), and at 24 h it was 50.8% (95% CI −56.6 to 158.2). Sample variation was, therefore, quite high, but less than the sample variation reported for human plasma (51, 58). Based on a mean midgut heme concentration of 12.7 ± 0.4 mM (uninfected and infected midgut lysates were not significantly different), total NOx levels in uninfected midguts would be equivalent to ca. 51 μM at 12.5 h and 110 μM at 24 h, whereas infected midgut levels would be equivalent to ca. 75 μM at 12.5 h and 168 μM at 24 h. Values in excess of 75 μM are consistent with inflammatory levels of nitrite and/or nitrate reported in human serum under conditions of sepsis (51, 58) and, therefore, suggest that the NO-mediated response of A. stephensi to Plasmodium infection is indeed an inflammatory response. These data are also consistent with our detection of elevated NADPH-dependent diaphorase activity in infected versus uninfected A. stephensi midguts at 24 h and extend our observations demonstrating elevated circulating nitrite or nitrate levels in infected A. stephensi at 7 to 14 days postinfection (34). Further, when taken together with data indicating that the host ICR mouse strain does not synthesize inducible NO in response to parasite infection (40), our data suggest that AsNOS induction beginning at 6 h drives the synthesis of significantly elevated levels of NOx in the midgut of P. berghei-infected A. stephensi within hours of infection.
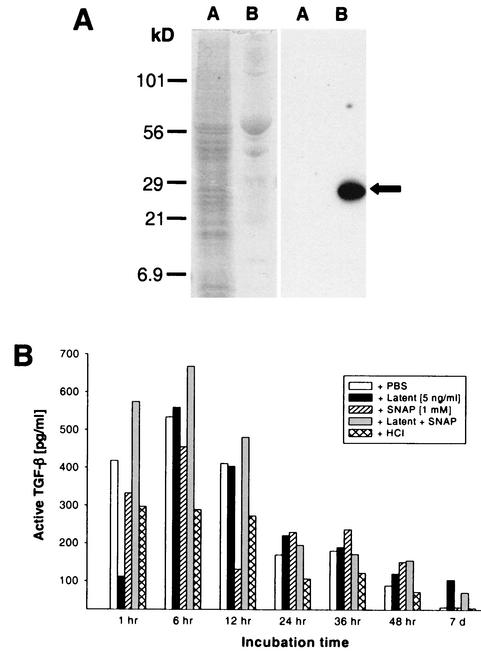
We hypothesized that, in addition to NO, mammalian TGF-β1 may be present in the A. stephensi midgut. Specifically, we predicted that A. stephensi ingests circulating mammalian TGF-β1 during blood feeding and that this cytokine may be active in the insect midgut environment. To determine the concentration of active mammalian TGF-β1 in A. stephensi midgut lysates and whether midgut lysate components could activate added latent human TGF-β1 in vitro, midguts from blood-fed mosquitoes at varied times postfeeding were assessed for the presence of active mammalian TGF-β1 by using Quantikine ELISA. Mosquito proteins do not cross-react with the anti-TGF-β1 antisera used in the ELISA (Fig. 2A) and, therefore, would not be expected to contribute to signal output from these assays.
FIG. 2.
Presence of active mammalian TGF-β1, as well as activation of latent TGF-β1 in the presence of NO, in A. stephensi midguts. (A) Mosquito cell proteins did not cross-react with anti-TGF-β1 used in the Quantikine ELISA. In the left panel, 22 μg of mosquito cell proteins (lane A) and 10 ng of human TGF-β1 (lane B) are shown after polyacrylamide gel electrophoresis and Coomassie brilliant blue staining. Identical protein samples transferred to nitrocellulose (right panel) were incubated with anti-human TGF-β1 as described in the text. Note the strong detection of human TGF-β1 in lane B (arrow) and the lack of cross-reacting mosquito proteins in lane A. (B) Lysates of mosquito midguts (50 per lysate) were prepared at various times after blood feeding (“incubation time”) indicated on the x axis. The lysates were treated as described in the boxed legend and in Materials and Methods. Active TGF-β1 was quantified in the samples by using the Quantikine ELISA.
Midgut lysates prepared from mosquitoes 1 to 48 h after blood feeding and treated ex vivo with PBS (control) appeared to contain active TGF-β1 at levels that ranged from 100 to 530 pg/ml (Fig. 2B). Active TGF-β1 was almost undetectable at 7 days (Fig. 2B). In comparison, TGF-β1 is present at 3,000 to 5,000 pg/ml, with 100% in the latent form, in platelet-poor plasma (26) from both healthy and P. berghei-infected host ICR mice (data not shown). Accordingly, recombinant human latent TGF-β1 was added to midgut lysates to a final concentration of 5,000 pg/ml in order to determine whether latent TGF-β1 could be activated in vitro by midgut lysates. Little active TGF-β1 above that already present in the midgut lysates was detected (Fig. 2B), suggesting that activating substances had been depleted.
Nitric oxide can activate latent TGF-β1 (55) and is present at high levels in the Anopheles midgut (Fig. 1B) (34). Accordingly, we examined whether NO provided by a chemical donor would enhance activation of latent TGF-β1 by midgut lysates. To test this hypothesis, lysates were treated with SNAP as a control or with SNAP and latent human TGF-β1. Based on our experience with SNAP (61, 62), we expect that the effective dose of NO delivered in 3 h was 100 μM, within the range of total NOx concentrations measured in the midgut lysates (see above). The levels of active TGF-β1 in lysates treated with SNAP only were similar to or lower than those in the lysates treated only with PBS (Fig. 2B). These findings with SNAP were consistent with earlier observations that NO does not alter antiproliferative effects of active TGF-β1 on CCL-64 cells (55) and, hence, the receptor-binding properties of active TGF-β1.
Simultaneous treatment of A. stephensi midgut lysates with both latent TGF-β1 and SNAP (Fig. 2B) led to the nearly uniform presence of higher levels of active TGF-β1 than in lysates treated with latent TGF-β1 alone; this effect was most pronounced in the 1- to 12-h lysates. At the 7-day time point, the presence of active TGF-β1 after treatment with latent TGF-β1 and SNAP verified that substance(s) with the capacity to activate latent TGF-β1 in the presence of NO were still present in the lysate, despite the complete lack of digestate at this time and the almost undetectable active TGF-β1 in the PBS-treated lysate (Fig. 2B).
As a final control, lysates of A. stephensi midgut blood (pH 7.5 to 7.8 in vivo [8]) were transiently acidified with 1 mM HCl (11, 56) in order to examine whether any residual latent TGF-β1 was present in the lysates (Fig. 2B). Acidification, however, did not result in any increase in detectable active TGF-β1. Thus, all TGF-β1 present in A. stephensi midguts within 1 h after blood feeding was active.
To verify that the capacity of the midgut lysates to activate added latent TGF-β1 was not restricted by availability of the growth factor, midgut lysates from a second cohort of blood-fed A. stephensi were subjected to the same analyses except that latent TGF-β1 was added to a final concentration of 1 μg/ml, a level 200 times that provided in the previous assays. Results from this second set of assays showed similar levels of active TGF-β1 (330 to 900 pg/ml) from 1 to 48 h after PBS treatment (data not shown). In addition, we observed similar trends of activation in the presence of added latent TGF-β1 and SNAP, at levels that ranged from three to five times that observed in the presence of 5,000 pg of added latent TGF-β1/ml under identical conditions. Because active TGF-β1 levels were not 200-fold greater than the levels observed in previous assays, we concluded that our results accurately reflected the activation capacity of A. stephensi midgut lysates.
Activation of latent TGF-β1 in vitro by the prooxidants heme and nitroxyl anion but not by peroxynitrite.
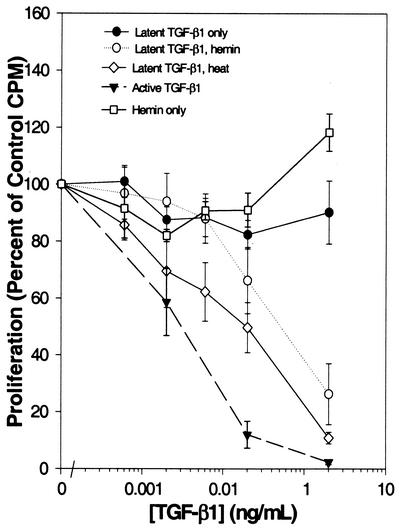
The studies described above showed that factor(s) present in the midgut of A. stephensi can activate latent TGF-β1 in synergy with NO. Heme, which can catalyze the synthesis of oxygen free radicals (4), is released into the midgut and polymerized into hematin during blood digestion. However, polymerization is not instantaneous, nor does it completely abolish radical chemistry (19). Elevated NO levels can lead to the activation of latent TGF-β1 by nitrosation of the LAP portion of latent TGF-β1 (55), whereas oxygen free radicals can activate cell-free latent TGF-β1 directly (5). Subsequently, we hypothesized that, in the redox-active Anopheles midgut, heme and NO may participate in activating latent TGF-β1.
To test this hypothesis, as well as to examine the role of reaction products of NO on the function of TGF-β1, we completed a series of in vitro assays. The mink lung epithelial cell line CCL-64 is highly sensitive to growth suppression by TGF-β1 (17). Latent human TGF-β1 at doses ranging from 6 to 2,000 pg/ml did not suppress the proliferation of CCL-64 cells, in contrast to the suppression of CCL-64 proliferation upon treatment with the same concentrations of active TGF-β1 (Fig. 3). When latent TGF-β1 was activated with heat, the 50% inhibitory concentration for the suppression of proliferation of CCL-64 cells was 0.4 ± 0.1 ng/ml (n = 6) (Fig. 3). The addition of 10 μM hemin to latent TGF-β1 for 30 min activated this cytokine (50% inhibitory concentration = 0.4 ± 0.2 ng/ml [n = 5]) to levels comparable to that observed with heat; hemin alone did not affect the proliferation of CCL-64 cells (Fig. 3).
FIG. 3.
Activation of latent TGF-β1 by hemin. CCL-64 cells were incubated with the indicated concentrations of latent TGF-β1 that was untreated, treated with 10 μM hemin, or heated. Alternatively, CCL-64 cells were incubated either with the indicated concentrations of active TGF-β1 or with dilutions of the hemin stock solution identical to those used to treat latent TGF-β1. Proliferation of CCL-64 cells was assessed by uptake of [3H]thymidine. Values are the means ± the SE of three to seven separate experiments.
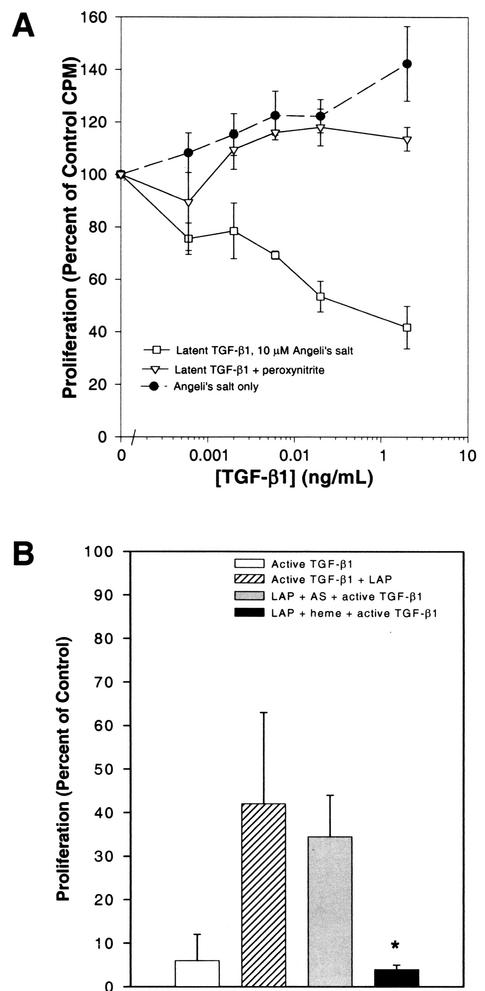
In the redox-active mosquito midgut, the effects of endogenously produced NO may be enhanced through the formation of redox products of NO, such as nitroxyl anion and peroxynitrite, two reactants commonly associated with the cellular synthesis of NO (57). In the environment of the midgut, nitroxyl anion and/or peroxynitrite could function to activate latent TGF-β1 directly. To test this hypothesis, latent TGF-β1 was treated with Angeli's salt, a nitroxyl anion chemical donor, or reagent peroxynitrite prior to analysis in CCL-64 cell proliferation assays. Our results demonstrated that, whereas Angeli's salt activated latent TGF-β1 directly, peroxynitrite did not (Fig. 4A). Angeli's salt alone, as a control, did not affect the proliferation of CCL-64 cells (Fig. 4A).
FIG. 4.
Activation of latent TGF-β1 by nitroxyl anion. (A) CCL-64 cells were incubated with the indicated concentrations of latent TGF-β1 that was treated with 10 μM Angeli's salt (nitroxyl anion chemical donor) or 100 μM peroxynitrite. Alternatively, CCL-64 cells were incubated with dilutions of the Angeli's salt stock solution identical to those used to treat latent TGF-β1. These assays were performed simultaneously with those represented in Fig. 3. Hence, data from active and latent TGF-β1 only controls are identical to those represented in Fig. 3. (B) Recombinant active human TGF-β1 (□) was added to CCL-64 cells for 20 h. Alternatively, recombinant human LAP was either left untreated (▨) or treated with 10 μM Angeli's salt (AS) ( ), or 10 μM hemin (▪); incubated with active TGF-β1; and subsequently added to CCL-64 cells for 20 h. ∗, P < 0.05 versus incubation of active TGF-β1 with untreated LAP. The proliferation of CCL-64 cells was assessed by measuring the uptake of [3H]thymidine. Values are means ± the SE of two to seven separate experiments.
We next sought to examine the mechanism by which hemin activated latent TGF-β1. One possibility was that LAP, which binds noncovalently to the active TGF-β1 dimer and maintains TGF-β1 in the latent state, was modified by redox-active hemin such that it could no longer bind to active TGF-β1, reminiscent of inactivation of LAP through S nitrosation (55). Preincubation of LAP with 10 μM hemin significantly abrogated the capacity of LAP to neutralize active TGF-β1 (P < 0.05 versus incubation of active TGF-β1 with untreated LAP; Fig. 4B); however, nitroxyl anion did not appear to cause such an effect (Fig. 4B). The presence of an agent such as heme may disrupt the interaction between LAP and active TGF-β1, permitting the S nitrosation of LAP. At high levels of NO, such as those present in the mosquito midgut (Fig. 1), S nitrosation of dissociated LAP should occur readily and prevent rebinding to and inactivation of TGF-β1 (55). Nitroxyl anion appears to activate latent TGF-β1 by a mechanism distinct from that of NO (55) or hemin (Fig. 3). These observations suggest that NO in the presence of heme may lead to sustained activation of latent TGF-β1 in the Anopheles midgut.
A. stephensi cells recognize human TGF-β1 as an immunomodulatory cytokine in vitro.
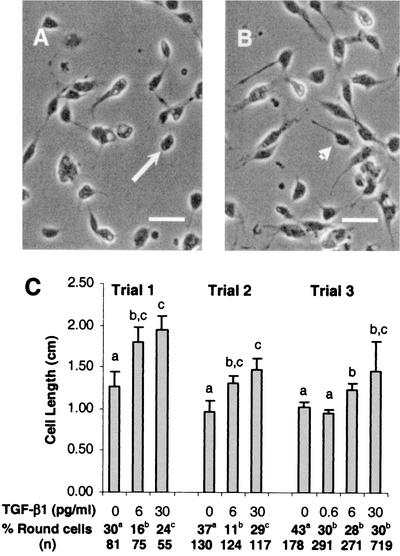
Based on our findings that mammalian TGF-β1 is activated and persists in the A. stephensi midgut to extended times after blood feeding, we sought to determine whether mammalian TGF-β1 could be recognized by A. stephensi cells in vitro. Three assays based on cell morphology, cell proliferation, and gene expression were used to assess the responses of A. stephensi cells to active TGF-β1. In the first assay, A. stephensi MSQ43 cells treated with human TGF-β1 compared to control cells showed significantly enhanced spreading (Fig. 5A and B), a behavior consistent with actin-dependent immune responsiveness of immortalized mosquito cells (30) and other insect cells (29).
FIG. 5.
Human TGF-β1 alters the morphology of A. stephensi MSQ43 cells in vitro. (A) Typical morphology of MSQ43 cells treated with diluent (4 mM HCl, 1 mgof BSA/ml) as a control for 20 or 60 min. Note the predominance of rounded cells (arrow) compared to spread cells with filopodia. (B) Typical morphology of MSQ43 cells treated with 6 or 30 pg of human TGF-β1/ml in diluent for 20 or 60 min. Note the predominance of spread cells with filopodia (arrowhead) compared to rounded cells. (C) Lengths of spread MSQ43 cells and percentages of round cells were determined from enlarged photomicrographs of treated and control cells from three trials. In trial 1, cells were treated as shown for 20 min; cell lengths are means ± the SE for n = 10 cells. In trials 2 and 3, cells were treated as shown for 60 min. Cell lengths for trial 2 are means ± the SE for n = 10 cells, whereas cell lengths for trial 3 means ± the SE for n = 40 to 80 cells. Differences between mean cell lengths within a single trial were analyzed by using the Student t test; significant differences among treatments within a trial are indicated by different lowercase letters. Differences among percentages of round cells within a single trial were analyzed by χ2; significant differences among treatments within a trial are indicated by different lowercase letters.
At doses of 6 or 30 pg of TGF-β1/ml, the mean spread cell length was significantly increased (n = 3) compared to control, diluent-treated cells (Fig. 5C). Significant decreases in the percentages of round MSQ43 cells were observed at 20 min at a dose of 6 pg/ml of TGF-β1 (trial 1; Fig. 5C) or at 60 min at a dose of 0.6 pg/ml (trial 3; Fig. 5C), indicating that 0.6 pg/ml was sufficient to cause the observed changes. These effective doses are similar to those reported for both vertebrate and invertebrate cells (38, 44, 60).
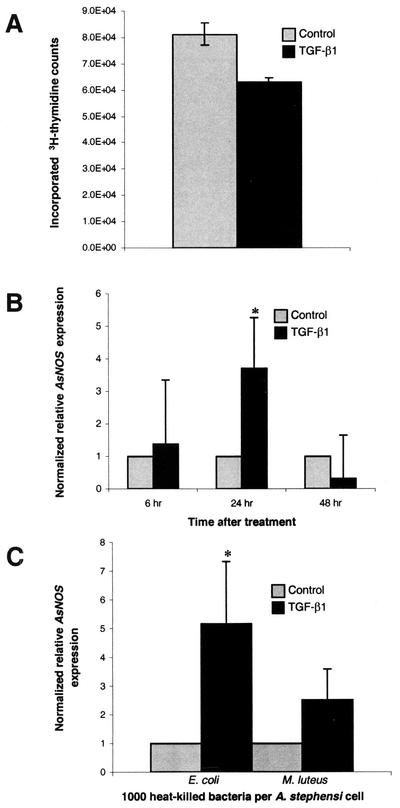
At a dose of 8 pg of human TGF-β1/ml, [3H]thymidine incorporation into A. stephensi cell DNA was reduced by nearly 25% compared to PBS-treated controls (P = 0.03; Fig. 6A). No significant differences were observed between controls and cells treated with 0.08 or 0.8 pg of human TGF-β1/ml (data not shown). Although a 25% change may be modest, similar patterns of [3H]thymidine incorporation have been observed after TGF-β1 treatment of human cardiac fibroblasts (20% decrease [1]) and rat costochondral chondrocytes (30% increase [47]).
FIG. 6.
A. stephensi cells recognize human TGF-β1 as an immunomodulatory cytokine in vitro. (A) At 48 h, human TGF-β1 at 8 pg/ml significantly reduced [3H]thymidine incorporation into A. stephensi ASE cells compared to PBS-treated controls (P = 0.03). Data were analyzed by using the Student t test; values are means ± the SE of three separate experiments. (B) At 24 h, AsNOS expression in ASE cells was significantly induced by human TGF-β1 at 6 pg/ml compared to PBS-treated controls (∗, P = 0.05). Induction levels of AsNOS expression were determined by using quantitative RT-PCR as described previously (14). PBS and TGF-β1 mean valuess within each time point were divided by the PBS mean value to show relative AsNOS induction in TGF-β1-treated cells. Data were analyzed by using the Student t test; values are means ± the SE of nine separate experiments. (C) Pretreatment with human TGF-β1 at 6 pg/ml significantly enhanced AsNOS induction in response to heat-killed E. coli (∗, P = 0.05). ASE cells were pretreated with PBS or TGF-β1 for 1 h prior to a 48-h exposure to heat-killed E. coli or M. luteus. AsNOS expression was analyzed, and mean values are illustrated as described in panel B. Data from five separate experiments were analyzed by using the Student t test.
Finally, we sought to determine whether human TGF-β1 could modulate AsNOS expression in a manner analogous to its effect on iNOS expression. To address this question, we used two assays. In the first assay, duplicate plates of ASE cells were treated with PBS or with 6 pg of human TGF-β1/ml for 6, 24, or 48 h. In the second assay, cells were pretreated for 1 h with PBS or 6 pg of human TGF-β1/ml. After pretreatment, cells were exposed for 48 h to 1,000 heat-killed M. luteus or E. coli, immune stimuli (21) that induce AsNOS expression.
In the first assay, no significant effects of human TGF-β1 on AsNOS expression were observed at 6 and 48 h (Fig. 6B). However, AsNOS expression was induced ∼3.7-fold (P = 0.05) in ASE cells at 24 h after exposure to human TGF-β1 (95% CI = 0.13 to 7.67; Fig. 6B). Further, pretreatment of ASE cells with human TGF-β1 increased AsNOS induction in the presence of immune stimuli, with a statistically significant effect noted for E. coli (Fig. 6C). Taken together, our data indicate that A. stephensi cells recognize human TGF-β1 as an immunomodulatory cytokine and that, in contrast to iNOS (54), AsNOS is induced by TGF-β1.
Human TGF-β1 alters Plasmodium development in A. stephensi.
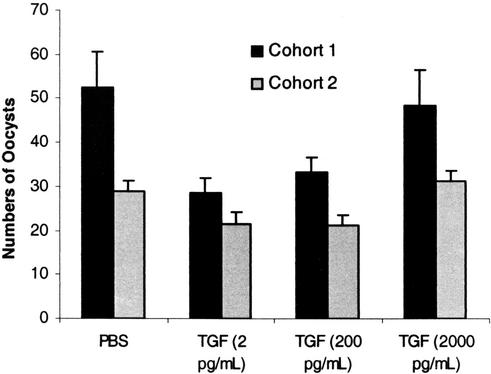
We next examined whether human TGF-β1 could alter Plasmodium development in vivo in the mosquito host. To address this question, two cohorts of A. stephensi were allowed to feed on an artificial meal containing P. falciparum-infected erythrocytes, human serum, and additional washed uninfected erythrocytes that was supplemented with equivalent volumes of PBS or 2, 200, or 2,000 pg of human TGF-β1/ml. Native α2-macroglobulin is the primary carrier of TGF-β1 in circulation (16). However, TGF-β1 is likely to be complexed exclusively to a conformationally altered form of α2-macroglobulin in stored serum (A. Kurdowska, personal communication), such as that used in our feeding assays. Consistent with the negative effects of conformationally altered α2-macroglobulin on bioavailability (28) and physiological function of TGF-β1 (59), latent TGF-β1 in the artificial meal did not appear to be activated in the A. stephensi midgut (results not shown). In contrast, recombinant active TGF-β1 added immediately prior to feeding at doses that bracket the levels of active growth factor detected in midgut lysates (Fig. 2B) would be freely available to exert a distinguishable biological effect. At 7 days postfeeding, mosquitos from each treatment were dissected to count the mature oocysts as an indicator of intensity of parasite infection.
Our results clearly demonstrated that active human TGF-β1 alters Plasmodium development in A. stephensi (Fig. 7). A dose of 2 pg of TGF-β1/ml reduced the number of P. falciparum oocysts by 46 and 26%, respectively, compared to the controls (P = 0.004 for cohort 1 and P = 0.03 for cohort 2). Similarly, 200 pg of TGF-β1/ml reduced oocyst numbers by 37 and 27%, respectively, compared to controls (P = 0.02 for cohort 1 and P = 0.03 for cohort 2). In contrast, the highest concentration of TGF-β1 (2,000 pg/ml) had no effect on the oocyst numbers in either cohort compared to the controls.
FIG. 7.
Human TGF-β1 significantly alters P. falciparum development in A. stephensi. Two cohorts of mosquitos were fed P. falciparum-infected blood with PBS or TGF-β1 at 2, 200, or 2,000 pg/ml in PBS. To determine the intensity of infection, parasite oocysts were counted from 60 to 75 mosquitos per group; data from cohorts 1 and 2 were not combined because mean infections were not equivalent. Data were analyzed by using the Student t test; values are means ± the SE. For cohorts 1 and 2, human TGF-β1 at 2 pg/ml reduced the number of P. falciparum oocysts by 46 and 26%, respectively, compared to the controls (P = 0.004 for cohort 1 and P = 0.03 for cohort 2). Similarly, 200 pg of TGF-β1/ml reduced oocyst numbers by 37 and 27%, respectively, compared to controls (P = 0.02 for cohort 1 and P = 0.03 for cohort 2). In contrast, the highest concentration of human TGF-β1 (2,000 pg/ml) had no effect on oocyst numbers in either cohort compared to the controls.
DISCUSSION
Although the impacts of TGF-β1 on the host immune response to parasite infection have not yet been fully elucidated, this cytokine is crucial for maintaining an immunological balance between parasite clearance and inflammation (43). This is the first study, however, to document the transfer of TGF-β1 from a mammalian host to an invertebrate, with consequent biological effects. Our data demonstrate that (i) mammalian TGF-β1 ingested by A. stephensi is activated in the midgut and persists during the 48 h process of blood digestion, (ii) the blood-filled A. stephensi midgut possesses factor(s) that can activate latent mammalian TGF-β1 in synergy with NO, (iii) products associated with blood digestion and NO synthesis that are either known or presumed present in the redox-active mosquito midgut can activate latent TGF-β1 in vitro, (iv) A. stephensi cells recognize mammalian TGF-β1 in vitro as an immunomodulatory cytokine, and (v) human TGF-β1 can alter development of P. falciparum in A. stephensi.
Thus, we have demonstrated that ingested mammalian blood is not only a source of nutrients for mosquito reproduction but also a source of cell signaling factors that can communicate with immunocompetent cells of the insect host. Further, these observations establish that a cytokine of critical importance to Plasmodium development in the vertebrate host can also influence parasite development in the mosquito host. Based on cross talk among TGF-β/activin and BMP ligands and Smad signaling pathways of Drosophila and human cells, we propose that mammalian TGF-β1 signals Anopheles cells through a conserved, endogenous TGF-β/activin pathway.
Intriguingly, our data indicate that murine TGF-β1, which circulates exclusively in the latent form, is activated in the mosquito midgut within 1 h of feeding (Fig. 2B). If we assume a concentration of 3,000 to 5,000 pg of latent TGF-β1/ml in ingested blood, at least 10% is activated in the midgut in this time period. What factors could contribute to this rapid and significant activation? Our in vitro data indicate that both heme (hemin) and nitroxyl anion are possible candidates (Fig. 3 and 4). Within 20 min of feeding, erythrocyte hemolysis frees 1 to 10% of the total ingested hemoglobin in the midgut lumen (12). Activation of at least one Anopheles trypsin occurs immediately after fluid ingestion (39) and contributes to a peak of proteolytic activity at 24 h that decreases the protein content by 80% at 36 h (7). The globins are dissociated from heme and digested, whereas the heme groups are polymerized to insoluble hematin under oxygenated, slightly alkaline conditions (6). Digestion, therefore, could liberate heme from hemoglobin soon after feeding in sufficient quantities to activate latent TGF-β1. Activation could be potentiated by redox products of NO: induction of AsNOS expression at 6 h postfeeding (Fig. 1A) appears to yield enhanced levels of NO by as early as 12.5 h postfeeding in the midguts of Plasmodium-infected A. stephensi (Fig. 1B). Although we do not yet know the stimulus for early AsNOS induction, which occurs when parasites are active in the blood bolus, later induction of AsNOS likely occurs in response to parasite invasion of the midgut epithelium (34). This, in turn, could serve to maintain or augment levels of redox products of NO in the A. stephensi midgut.
Levels of active TGF-β1 decline with time in the A. stephensi midgut (Fig. 2B), presumably due in part to the physical process of blood digestion. We argue, however, that active TGF-β1 is present in sufficient quantities throughout digestion to impact the physiology of A. stephensi. Further, we propose that the physiological effects of active TGF-β1 persist through 48 h after feeding, a critical period of Plasmodium development that spans fertilization, ookinete development, and midgut invasion.
Based on our observations with cultured cells, we hypothesize that the effects of mammalian TGF-β1 on Plasmodium development occur, in part, through induction of AsNOS expression in the A. stephensi midgut. Although this hypothesis remains to be tested, we propose that the induction of AsNOS expression in A. stephensi cells by human TGF-β1 is analogous to induction of iNOS by TGF-β1 in mammalian “sentinel” immune cells, including fibroblasts, chondrocytes, and immunocompetent retinal epithelial cells (9, 23, 25). These cell types are commonly implicated as first-line defenders of host integrity against infection (31, 50), functioning as critical components of the mammalian innate immune system. By comparison, insect immunity is based solely on cellular and humoral defenses that are innate (53), with tissue barriers such as the midgut serving an important role in defense against ingested and invading microorganisms. Hence, it is not unexpected to find that immortalized Anopheles cells, which possess gene expression patterns similar to circulating hemocytes of the insect cellular immune system (18), exhibit changes in proliferation and gene expression after TGF-β1 treatment that are reminiscent of those exhibited by mammalian innate immune cells.
Our data clearly demonstrate that mammalian TGF-β1 alters the development of P. falciparum in A. stephensi, a natural and important vector of this parasite in India and parts of the Middle East. Although the magnitude of the effect of TGF-β1 appears to be dependent on parasite infection intensity, which can vary significantly from cohort to cohort, the qualitative effects on Plasmodium development are nonetheless consistent. The phenomenon of inhibition of parasite growth at lower TGF-β1 doses and the lack of inhibition at the highest dose of TGF-β1 is reminiscent of the pro- and anti-inflammatory roles of TGF-β1 in balancing parasite infection and inflammation in the mammalian host. We propose that other mammalian cytokines important to Plasmodium infection, including interleukin-1, tumor necrosis factor alpha, and alpha and gamma interferon (46), may function in this capacity as well. Experiments to confirm that AsNOS is induced in vivo in response to low doses of TGF-β1 and repressed at high doses of TGF-β1, as well as examining the roles of these other cytokines, are currently under way.
The impact of mammalian immunity on parasite transmission by Anopheles has potentially significant practical implications for malaria control. A current global effort is directed toward the development and release of transgenic Anopheles that are resistant to parasite infection (27). Expression of transgenes and activity of transgene products targeted to the Anopheles midgut may be influenced by mammalian factors that remain active in the midgut environment after ingestion. There exists a panoply of growth factors, cytokines, and other signaling factors whose plasma concentrations are influenced by nutritional and diseased states of the mammalian host. Accordingly, prudent experimental designs would include assessments of parasite development in transgenic Anopheles after exposure to a variety of hosts, maintained under the variety of physiological conditions and stresses typical of regions where this type of infection is endemic.
Acknowledgments
This work was supported by Public Health Service grants AI41027 and AI50663 from the National Institute of Allergy and Infectious Diseases and by a grant from the Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust.
S.L. and T.M.L.P. acknowledge the generous support and guidance provided by Andrew J. Gow (Children's Hospital of Philadelphia, University of Pennsylvania) for NOx assays and data analysis. We are also grateful to Megan Dowler and Jackie L. Williams (Walter Reed Army Institute of Research) for assistance with infections of A. stephensi with P. falciparum and to Binnie Betton (University of Pittsburgh) for assistance with the TGF-β1 Quantikine ELISAs (R&D Systems).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Agocha, A., H. W. Lee, and M. Eghbali-Webb. 1997. Hypoxia regulates basal and induced DNA synthesis and collagen type I production in human cardiac fibroblasts: effects of transforming growth factor-β1, thyroid hormone, angiotensin II, and basic fibroblast growth factor. J. Mol. Cell Cardiol. 29:2233-2244. [DOI] [PubMed] [Google Scholar]
- 2.Antonini, E., and M. Brunori. 1971. Hemoglobin and myoglobin in their reactions with ligands. Elsevier Press, New York, N.Y.
- 3.Attisano, L., and S. Tuen Lee-Hoeflich. 2001. The Smads. Genome Biol. 2:3010.1-3010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balla, G., G. M. Vercellotti, U. Muller-Eberhard, J. Eaton, and H. S. Jacob. 1991. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab. Investig. 64:648-655. [PubMed] [Google Scholar]
- 5.Barcellos-Hoff, M. H., and T. A. Dix. 1996. Redox mediated activation of latent transforming growth factor-β1. Mol. Endocrinol. 10:1077-1083. [DOI] [PubMed] [Google Scholar]
- 6.Berner, R., W. Rudin, and H. Hecker. 1983. Peritrophic membranes and proteolytic activtity in the midgut of the malaria mosquito, Anopheles stephensi (Liston) (Insecta:Diptera) under normal and experimental conditions. J. Ultrastruct. Res. 83:195-204. [DOI] [PubMed] [Google Scholar]
- 7.Billingsley, P. F., and H. Hecker. 1991. Blood digestion in the mosquito Anopheles stephensi (Liston) (Diptera:Culicidae): activity and distribution of trypsin, aminopeptidase, and α-glucosidase in the midgut. J. Med. Entomol. 28:865-887. [DOI] [PubMed] [Google Scholar]
- 8.Billker, O., A. J. Miller, and R. E. Sinden. 2000. Determination of mosquito bloodmeal pH in situ by ion-selective microelectrode measurement: implications for the regulation of malarial gametogenesis. Parasitology 120:547-551. [DOI] [PubMed] [Google Scholar]
- 9.Blanco, F. J., Y. Geng, and M. Lotz, M. 1995. Differentiation-dependent effects of IL-1 and TGF-beta on human articular chondrocyte proliferation are related to inducible nitric oxide synthase expression. J. Immunol. 154:4018-4026. [PubMed] [Google Scholar]
- 10.Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907-916. [DOI] [PubMed] [Google Scholar]
- 11.Brown, P. D., L. M. Wakefield, A. D. Levinson, and M. B. Sporn. 1990. Physiocochemical activation of recombinant latent transforming growth factor-betas 1, 2, and 3. Growth Factors 3:35-43. [DOI] [PubMed] [Google Scholar]
- 12.Chege, G. M. M., and J. C. Beier. 1998. Blood acquisition and processing by three Anopheles (Diptera:Culicidae) species with different innate susceptibilities to Plasmodium falciparum. J. Med. Entomol. 35:319-323. [DOI] [PubMed] [Google Scholar]
- 13.Conner, E. M., S. Aiko, M. Fernandez, H. D. Battarbee, L. Gray, and M. B. Grisham. 2000. Duration of the hemodynamic effects of NG-nitro-l-arginine methyl ester in vivo. Nitric Oxide 4:85-93. [DOI] [PubMed] [Google Scholar]
- 14.Crampton, A. L., and S. Luckhart. 2001. The role of As60A, a TGF-β homolog, in Anopheles stephensi innate immunity against Plasmodium infection. Infect. Genet. Evol. 1:131-141. [DOI] [PubMed] [Google Scholar]
- 15.Crampton, A. L., and S. Luckhart. 2001. Isolation and characterization of As60A, a transforming growth factor-β gene, from the malaria vector Anopheles stephensi. Cytokine 13:65-74. [DOI] [PubMed] [Google Scholar]
- 16.Crookston, K. P., D. J. Webb, J. Lamarre, and S. L. Gonias. 1993. Binding of platelet-derived growth factor-BB and transforming growth factor-β1 to α2-macroglobulin in vitro and in vivo: comparison of receptor-recognized and non-recognized α2-macroglobulin conformations. Biochem. J. 293:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danielpour, D., and M. B. Sporn. 1990. Differential inhibition of transforming growth factor-β1 and -β2 activity by α2-macroglobulin. J. Biol. Chem. 265:6973-6977. [PubMed] [Google Scholar]
- 18.Dimopoulos, G., G. K. Christophides, S. Meister, J. Schultz, K. P. White, C. Barillas-Mury, and F. C. Kafatos. 2002. Genome expression analysis of Anopheles gambiae: responses to injury, bacterial challenge, and malaria infection. Proc. Natl. Acad. Sci. USA 99:8814-8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dix, T. A., R. Fontana, A. Panthani, and L. J. Marnett, L. J. 1985. Hematin-catalyzed epoxidation of 7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene by polyunsaturated fatty acid hydroperoxides. J. Biol. Chem. 260:5358-5365. [PubMed] [Google Scholar]
- 20.Fallon, A. M., and V. Stollar. 1987. The biochemistry and genetics of mosquito cells in culture. Adv. Cell Culture 5:97-137. [Google Scholar]
- 21.Fallon, A. M., and D. Sun. 2001. Exploration of mosquito immunity using cells in culture. Insect Biochem. Mol. Biol. 31:263-278. [DOI] [PubMed] [Google Scholar]
- 22.Flaumenhaft, R., S. Kojima, M. Abe, and D. B. Rifkin. 1993. Activation of latent transforming growth factor β. Adv. Pharmacol. 24:51-76. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert, R. S., and H. R. Herschman. 1993. Transforming growth factor beta differentially modulates the inducible nitric oxide synthase gene in distinct cell types. Biochem. Biophys. Res. Commun. 195:380-384. [DOI] [PubMed] [Google Scholar]
- 24.Gladwin, M. T., X. Wang, C. D. Reiter, B. K. Yang, E. X. Vivas, C. Bonaventura, and A. N. Schechter. 2002. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J. Biol. Chem. 277:27818-27828. [DOI] [PubMed] [Google Scholar]
- 25.Goureau, O., D. Hicks, and Y. Courtois. 1994. Human retinal pigmented epithelial cells produce nitric oxide in response to cytokines. Biochem. Biophys. Res. Commun. 198:120-126. [DOI] [PubMed] [Google Scholar]
- 26.Grainger, D. J., D. E. Mosedale, J. C. Metcalfe, P. L. Weissberg, and P. R. Kemp. 1995. Active and acid-activatable TGF-beta in human sera, platelets and plasma. Clin. Chim. Acta 235:11-31. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman, S. L., G. M. Subramanian, F. H. Collins, and J. C. Venter. 2002. Plasmodium, human and Anopheles genomics and malaria. Nature 415:702-709. [DOI] [PubMed] [Google Scholar]
- 28.LaMarre, J., M. A. Hayes, G. K. Wollenberg, I. Hussaini, S. W. Hall, and S. L. Gonias. 1991. An α2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-β1 in mice. J. Clin. Investig. 87:39-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavine, M., and M. Strand, M. 2002. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 32:1295-1309. [DOI] [PubMed] [Google Scholar]
- 30.Levashina, E. A., L. F. Moita, S. Blandin, G. Vriend, M. Lagueux, and F. C. Kafatos. 2001. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104:709-718. [DOI] [PubMed] [Google Scholar]
- 31.Lo, D., L. Feng, L. Li, M. J. Carson, M. Crowley, M. Pauza, A. Nguyen, and C. R. Reilly. 1999. Integrating innate and adaptive immunity in the whole animal. Immunol. Rev. 169:225-239. [DOI] [PubMed] [Google Scholar]
- 32.Luckhart, S., and K. Li. 2001. Transcriptional complexity of the Anopheles stephensi nitric oxide synthase gene. Insect Biochem. Mol. Biol. 31:249-256. [DOI] [PubMed] [Google Scholar]
- 33.Luckhart, S., and R. Rosenberg. 1999. Gene structure and polymorphism of an invertebrate nitric oxide synthase gene. Gene 232:25-34. [DOI] [PubMed] [Google Scholar]
- 34.Luckhart, S., Y. Vodovotz, L. Cui, and R. Rosenberg. 1998. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA 95:5700-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massague, J. 1998. TGF-beta signal transduction. Annu. Rev. Biochem. 67:753-791. [DOI] [PubMed] [Google Scholar]
- 36.Mellouk, S., S. L. Hoffman, Z. Liu, P. de la Vega, T. R. Billiar, and A. K. Nüssler. 1994. Nitric oxide-mediated antiplasmodial activity in human and murine hepatocytes induced by gamma interferon and the parasite itself: enhancement by exogenous tetrahydrobiopterin. Infect. Immun. 62:4043-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranda, K. M., M. G. Espey, and D. A. Wink. 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62-71. [DOI] [PubMed] [Google Scholar]
- 38.Moulin, V., G. Castilloux, F. A. Auger, D. Garrel, M. D. O'Connor-McCourt, and L. Germain. 1998. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp. Cell Res. 238:283-293. [DOI] [PubMed] [Google Scholar]
- 39.Müller, H. M., F. Catteruccia, J. Vizioli, A. della Torre, and A. Crisanti. 1995. Constitutive and blood meal-induced trypsin genes in Anopheles gambiae. Exp. Parasitol. 81:371-385. [DOI] [PubMed] [Google Scholar]
- 40.Murata, K., F. Takano, S. Fushiya, and Y. Oshima. 1999. Potentiation by febrifugine of host defense in mice against Plasmodium berghei NK65. Biochem. Pharmacol. 58:1593-1601. [DOI] [PubMed] [Google Scholar]
- 41.Naotunne, T. S., N. D. Karunaweera, K. N. Mendis, and R. Carter. 1993. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunology 78:555-562. [PMC free article] [PubMed] [Google Scholar]
- 42.Nathan, C., and Q.-W. Xie. 1994. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 269:13725-13728. [PubMed] [Google Scholar]
- 43.Omer, F. M., J. A. L. Kurtzhals, and E. M. Riley. 2000. Maintaining the immunological balance in parasitic infections: a role for TGF-β? Parasitol. Today 16:18-23. [DOI] [PubMed] [Google Scholar]
- 44.Ottaviani, E., D. Sassi, and D. Kletsas. 1997. PDGF- and TGF-β-induced changes in cell shape of invertebrate immunocytes: effect of calcium entry blockers. Eur. J. Cell Biol. 74:336-341. [PubMed] [Google Scholar]
- 45.Piek, E., C. H. Heldin, and P. ten Dijke. 1999. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 13:2105-2124. [PubMed] [Google Scholar]
- 46.Richards, A. L. 1997. Tumour necrosis factor and associated cytokines in the host's response to malaria. Int. J. Parasitol. 27:1251-1263. [DOI] [PubMed] [Google Scholar]
- 47.Rosado, E., Z. Schwartz, V. L. Sylvia, D. D. Dean, and B. D. Boyan. 2002. Transforming growth factor-β1 regulation of growth zone chondrocytes is mediated by multiple interacting pathways. Biochim. Biophys. Acta 1590:1-15. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., and D. W. Russell. (ed.). 2002. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Sanderson, N., V. Factor, P. Nagy, J. Kopp, P. Kondaiah, L. Wakefield, A. B. Roberts, M. B. Sporn, and S. S. Thorgeirsson. 1995. Hepatic expression of mature transforming growth factor-beta1 in transgenic mice results in multiple tissue lesions. Proc. Natl. Acad. Sci. USA 92:2572-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith, R. S., T. J. Smith, T. M. Blieden, and R. P. Phipps. 1997. Fibroblasts as sentinel cells: synthesis of chemokines and regulation of inflammation. Am. J. Pathol. 151:317-322. [PMC free article] [PubMed] [Google Scholar]
- 51.Spack, L., P. L. Havens, and O. W. Griffith. 1997. Measurements of total plasma nitrite and nitrate in pediatric patients with the systemic inflammatory response syndrome. Crit. Care Med. 25:1071-1078. [DOI] [PubMed] [Google Scholar]
- 52.Stein, G. S., J. L. Stein, J. B. Lian, T. J. Last, T. Owen, and L. McCabe. 1994. Synchronization of normal diploid and transformed cells, p. 282-287. In J. E. Celis (ed.), Cell biology: a laboratory handbook. Academic Press, Inc., San Diego, Calif.
- 53.Tzou, P., E. De Gregorio, and B. Lemaitre. 2002. How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 5:102-110. [DOI] [PubMed] [Google Scholar]
- 54.Vodovotz, Y. 1997. Control of nitric oxide production by transforming growth factor-β1: mechanistic insights and potential relevance to human disease. Nitric Oxide Biol. Chem. 1:3-17. [DOI] [PubMed] [Google Scholar]
- 55.Vodovotz, Y., L. Chesler, H. Chong, S. J. Kim, J. T. Simpson, W. DeGraff, G. W. Cox, A. B. Roberts, D. A. Wink, and M. H. Barcellos-Hoff, M. H. 1999. Regulation of transforming growth factor-β1 by nitric oxide. Cancer Res. 59:2142-2149. [PubMed] [Google Scholar]
- 56.Wakefield, L. M., D. M. Smith, S. Broz, M. Jackson, A. D. Levinson, and M. B. Sporn. 1989. Recombinant TGF-β1 is synthesized as a two-component latent complex that shares some structural features with the native platelet latent TGF-β1 complex. Growth Factors 1:203-218. [DOI] [PubMed] [Google Scholar]
- 57.Wink, D. A., M. Feelisch, Y. Vodovotz, J. Fukuto, and M. B. Grisham. 1999. The chemical biology of nitric oxide, p. 245-291. In C. A. Colton and D. L. Gilbert (ed.), Reactive oxygen species in biological systems: an interdisciplinary approach. Kluwer Academic/Plenum Publishing, New York, N.Y.
- 58.Wong, H. R., J. A. Carcillo, G. Burckart, N. Shah, and J. E. Janosky. 1995. Increased serum nitrite and nitrate concentrations in children with the sepsis syndrome. Crit. Care Med. 23:835-842. [DOI] [PubMed] [Google Scholar]
- 59.Wu, S. M., D. D. Patel, and S. V. Pizzo. 1998. Oxidized α2-macroglobulin (α2M) differentially regulates receptor binding by cytokines/growth factors: implications for tissue injury and repair mechanisms in inflammation. J. Immunol. 161:4356-4365. [PubMed] [Google Scholar]
- 60.Yokozeki, M., K. Moriyama, H. Shimokawa, and T. Kuroda. 1997. Transforming growth factor-β1 modulates myofibroblastic phenotype of rat palatal fibroblasts in vitro. Exp. Cell Res. 231:328-336. [DOI] [PubMed] [Google Scholar]
- 61.Zamora, R., L. Alarcon, Y. Vodovotz, B. Betten, P. K. Kim, K. F. Gibson, and T. R. Billiar. 2001. Nitric oxide suppresses the expression of Bcl-2 binding protein BNIP3 in hepatocytes. J. Biol. Chem. 276:46887-46895. [DOI] [PubMed] [Google Scholar]
- 62.Zamora, R., Y. Vodovotz, L. Alarcon, B. Betten, P. A. Loughran, K. S. Aulak, D. J. Stuehr, K. F. Gibson, and T. R. Billiar. 2001. Nitric oxide from the inducible nitric oxide synthase (iNOS) increases the expression of cytochrome P450 2E1 in iNOS-null hepatocytes in the absence of inflammatory stimuli. Arch. Biochem. Biophys. 390:287-294. [DOI] [PubMed] [Google Scholar]