Abstract
Brucella spp. are facultative intracellular pathogens that have the ability to survive and multiply in professional and nonprofessional phagocytes and cause abortion in domestic animals and undulant fever in humans. The mechanism and factors of virulence are not fully understood. To identify genes related to internalization and multiplication in host cells, Brucella abortus was mutagenized by mini-Tn5Km2 transposon that carryied the kanamycin resistance gene, 4,400 mutants were screened, and HeLa cells were infected with each mutant. Twenty-three intracellular-growth-defective mutants were screened and were characterized for internalization and intracellular growth. From these results, we divided the mutants into the following three groups: class I, no internalization and intracellular growth within HeLa cells; class II, an internalization similar to that of the wild type but with no intracellular growth; and class III, internalization twice as high as the wild type but with no intracellular growth. Sequence analysis of DNA flanking the site of transposon showed various insertion sites of bacterial genes that are virulence-associated genes, including virB genes, an ion transporter system, and biosynthesis- and metabolism-associated genes. These internalization and intracellular-growth-defective mutants in HeLa cells also showed defective intracellular growth in macrophages. These results suggest that the virulence-associated genes isolated here contributed to the intracellular growth of both nonprofessional and professional phagocytes.
Brucellosis is a major bacterial zoonosis that causes a serious debilitating disease in humans and abortion and sterility in domestic animals. The etiologic agents of brucellosis are Brucella spp., small gram-negative and facultative intracellular pathogens that can multiply within professional and nonprofessional phagocytes (9, 10). In contrast to other intracellular pathogens, Brucella species do not produce exotoxins, antiphagocytic capsules or thick cell walls, resistance forms, or fimbriae and do not show antigenic variation (16). A key aspect of the virulence of brucella is its ability to proliferate within professional and nonprofessional phagocytic host cells and thereby successfully bypasses the bactericidal effects of phagocytes, and their virulence and chronic infections are thought to be due to their ability to avoid the killing mechanisms within host cells (30, 41). The molecular mechanisms and genetic basis for intracellular survival and replication, however, are not understood completely. Some studies with nonprofessional phagocytes have shown that Brucella invades host cells and is contained within early endosome-like vacuoles. These vacuoles rapidly fuse with early autophagosomes that acquire vacuolar [H+]ATPase and lysosome-associated membrane proteins (LAMP), mature into a late autophagosome, inhibit fusion with lysosomes, and finally become a replicating vacuole normally associated with the endoplasmic reticulum (5, 11, 31, 32). The genetic basis of Brucella virulence is still poorly understood. The VirB type IV secretion system of Brucella has been identified recently (29). This operon is composed of 13 open reading frames (ORFs) that share homology with other bacterial type IV secretion systems in the intracellular trafficking of pathogens. Deletion or polar and nonpolar mutations of these ORFs were not able to replicate and survive within phagocytes (39, 44). Thus, the VirB proteins of B. abortus are thought to be constituents of the secretion apparatus.
To identify bacterial virulence genes, transposon mutagenesis is the most frequently used approach as a genetic tool (19). Various mini-Tn5 derivatives carrying standard selectable antibiotic resistance markers have been described for genetic analysis (6). Transposon insertion in any Brucella gene concerned with intracellular survival and replication may reduce the virulence of Brucella in phagocytes or experimental animals, and the identification of Brucella virulence associated genes may be easier.
We identified here several genes that encode factors required for the internalization and intracellular growth of B. abortus within professional and nonprofessional phagocytes by using mini-Tn5Km2 transposon, and we examined their characteristics. The virulence of mutants was evaluated by infecting mice with transposon mutants. Possible roles in the virulence of Brucella for the different factors identified are discussed.
MATERIALS AND METHODS
Bacterial culture and media.
All B. abortus derivatives were from 544 (ATCC 23448), smooth virulent B. abortus biovar 1 strains. B. abortus strains were maintained as frozen glycerol stocks and were cultured in brucella broth (Becton Dickinson, Sparks, Md.) or brucella broth containing 1.5% agar. Kanamycin (30 μg/ml) and nalidixic acid (25 μg/ml) were used when necessary. Escherichia coli DH5α and E. coli S-17λpir pUT mini-Tn5Km2 were used for transformation and transposon conjugation, respectively (17, 40). Each E. coli was cultured in Luria-Bertani broth or agar. If necessary, ampicillin (100 μg/ml) and kanamycin (30 μg/ml) were used. Bacterial growth rates were measured spectrophotometrically at 600 nm.
A modified version of the antibiotic agar dilution method of the National Committee for Clinical Laboratory Standards was used for determination of MIC for gentamicin (35). The MIC was defined as the lowest concentration of the antibiotic tested giving complete inhibition of bacterial growth compared with drug-free control.
Cell culture.
HeLa cells were grown at 37°C in a 5% CO2 atmosphere in Eagle minimum essential medium (MEM; Sigma, St. Louis, Mo.) containing 10% fetal bovine serum (FBS). Bone marrow-derived macrophages from female BALB/c mice were prepared by the method described previously (42). After culture in L-cell conditioned medium, the macrophages were replated for use by lifting cells in phosphate-buffered saline (PBS) on ice for 5 to 10 min, harvesting by centrifugation, and resuspension in RPMI 1640 (Sigma) containing 10% FBS. The HeLa cells or macrophages were seeded (2 × 105 to 3 × 105 per well) in 24-well tissue culture plates 1 day before infection for all assays.
Construction of mini-Tn5Km2 mutants.
To obtain a selectable marker for conjugation, mini-Tn5Km2 transposon (6) carrying the kanamycin resistance gene was introduced into pUT, a transposon donor vector, to form pUT mini-Tn5Km2. The mini-Tn5Km2-bearing plasmid pUT mini-Tn5Km2 was introduced into B. abortus from an E. coli K-12 derivative, SM17λpir (7), by conjugation, and transposon mutagenesis of B. abortus by conjugation was done as described previously (17, 27). The mutants thus obtained were purified on agar plates containing kanamycin (30 μg/ml) and were screened intracellular growth defective mutants within HeLa cells, and the mutants were kept in 20% glycerol in brucella broth at −80°C.
Infection and intracellular survival assay.
Bacterial infection and intracellular survival assay was done by using the modified method described previously (43). Briefly, B. abortus mutants were deposited onto HeLa cells grown on 96-well microtiter plates filled with MEM plus 10% FBS at a multiplicity of infection of 20, centrifuged at 150 × g for 10 min at room temperature, incubated at 37°C in 5% CO2 for 1 h, washed twice with 0.5 ml of sterile PBS, and incubated with MEM plus gentamicin (30 μg/ml) for 48 h. After incubation, the cells were washed and lysed with 0.1 ml of sterile distilled water, and 25 μl of the sample was dropped onto brucella agar and was incubated at 37°C for 48 h. After incubation, no growth samples on brucella agar were considered intracellular-growth-defective mutants and were selected. This assay was done at least three times.
DNA sequencing.
The DNA was sequenced by the following standard techniques (37). Chromosomal DNA of the mutants was digested with EcoRI, cloned to plasmid pBluescript II KS(+) (Toyobo, Tokyo, Japan), transformed into E. coli DH5α, and plated onto Luria-Bertani agar containing ampicillin (100 μg/ml) and kanamycin (30 μg/ml). Plasmid DNA was extracted by using the plasmid Mini Kit (Qiagen), and the chromosomal DNA sequence was analyzed by using the mini-Tn5Km2 transposon O′-end primer (5′-CCTCTAGAGTCGACCTGCAG-3′). The chromosomal DNA sequence database was searched by using BLASTX and BLASTN search algorithms (http://www.genome.ad.jp/and http://www.ncbi.nml.nih.gov/blast/).
Southern blot analysis.
After chromosomal DNA was extracted, it was digested for 2 h with BamHI restriction enzymes (none of which digest a DNA-specific probe for mini-Tn5Km2), separated by electrophoresis in 0.8% agarose, and transferred to positively charged nylon membranes. A 1.7-kb EcoRI and XbaI fragment that contained the kanamycin resistance gene of mini-Tn5Km2 was labeled by using a biotin nonisotopic labeling kit, and it was used as the DNA probe for Southern hybridization under stringent conditions.
Determination of efficiency of bacterial uptake and intracellular growth by cultured HeLa cells and macrophages.
To determine the uptake of bacteria and intracellular growth, HeLa cells and mouse bone marrow-derived macrophages were infected with B. abortus as described above. For analysis of bacterial uptake efficiency, both types of cells were washed once with medium after 0, 5, 15, 25, and 35 min of incubation at 37°C and then incubated with MEM or RPMI 1640 with gentamicin (30 μg/ml) for 30 min. Both types of cells were then washed three times with PBS and then lysed with distilled water. CFU were measured by serial dilutions on brucella plates. For intracellular growth efficiency, both infected cells were incubated at 37°C for 30 min, washed once with medium, incubated with MEM or RPMI 1640 plus gentamicin (30 μg/ml), and then incubated for 2, 24, and 48 h. Cell washing, lysis, and plating procedures were the same as for the analysis of the efficiency of bacterial uptake. The percent protection was calculated by dividing the number of bacteria surviving the assay by the number of bacteria in the infectious inoculum, as determined by viable counts.
LAMP-1 staining.
LAMP-1 staining was performed by using the method described previously (43). Briefly, infected macrophages were fixed in 4% periodate-lysine-paraformaldehyde-sucrose for 1 h at 37°C. All antibody-probing steps were for 1 h at 37°C. Samples were washed three times in PBS for 5 min and then permeabilized at −20°C in methanol for 10 s. After three 5-min incubations with a blocking buffer (2% goat serum in PBS), the samples were stained with anti-LAMP-1 rat monoclonal antibody 1D4B diluted 1:100 in blocking buffer. After three washes for 5 min in blocking buffer, the samples were stained with Texas red-goat anti-rat immunoglobulin G. The samples were stained with anti-B. abortus polyclonal rabbit serum and fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G in blocking buffer to identify the bacteria, placed in mounting medium, and then visualized by fluorescence microscopy. One hundred bacteria within macrophages were selected randomly, and the extent of LAMP-1 acquisition of bacteria was determined.
Bacterial adherence assay.
Bacterial adherence was assayed by a previously described method (45). Briefly, before bacterial infection HeLa cells were incubated with MEM containing cytochalasin D (500 μg/ml) for 40 min at 37°C, and bacterial infection, fixation, staining, and microscopic evaluation were done as described above for bacterial detection. One hundred HeLa cells were selected randomly, and the adherent bacteria on cells were counted.
Labeling bacterial surface proteins.
To examine the modification of bacterial surface proteins, 1-ml samples of cultured bacteria were harvested by centrifugation, washed twice with PBS, and suspended in 200 μl of PBS. Then, 10 μl of 1% of Sulfo-NHS-Biotin (Pierce, Rockford, Ill.) was added, and the mixture was placed on ice for 2 min. NHS ester reacts with the deprotonated form of the primary amine. After one wash with PBS, each sample (40 μg) was mixed with sodium dodecyl sulfate sample buffer, incubated at 100°C for 5 min before loading, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were electrotransferred to nitrocellulose membranes and were incubated with horseradish peroxidase-conjugated streptavidin. The blot was developed with Western blot detection reagents (Amersham Pharmacia Biotec).
Virulence in mice.
The virulence was determined by quantifying the survival of the strains in the spleen after 10 days. Six-week-old female BALB/c mice were infected intraperitoneally with ca. 104 CFU of brucellae in 0.1 ml of saline. Groups of five mice were infected with each strain. At 10 days postinfection the mice were sacrificed by decapitation, and their spleens were removed, weighed, and homogenized in saline. Tissue homogenates were serially diluted with PBS and were plated on brucella agar to count the number of CFU in each spleen.
RESULTS
Isolation of intracellular-growth-defective mutants.
To identify intracellular-growth-defective mutants within HeLa cells, we randomly mutagenized B. abortus with the mini-Tn5Km2 transposon, and mutants were selected on brucella plates containing antibiotics. Nonmutagenized bacteria did not grow, but mutants carrying the transposon inserted in the chromosome were resistant to antibiotics. From this result, 4,400 mutants were screened, and HeLa cells were infected with each mutant, and then intracellular-growth-defective mutants were screened (see Materials and Methods); 23 of the mutants did not survive within HeLa cells (Table 1). To confirm how many of each clone contained the transposon insertion site and whether mutants contain the transposon in chromosomal DNA, the chromosomal DNA of all mutants was extracted, digested with BamHI (no site in the transposon), and then hybridized by Southern blot analysis with a mini-Tn5Km2 probe; all mutants contained a single transposon in chromosomal DNA (data not shown).
TABLE 1.
B. abortus genes essential for intracellular growth
| Functional group and mutant | Class | Mutated genes (putative functions) | B. melitensis ORFa | B. suis ORFb |
|---|---|---|---|---|
| Transport | ||||
| K2 | III | znuA (zinc uptake system) | II0178 | II1122 |
| K13 | III | virB3 (type IV secretion system) | II0027 | II0067 |
| K26 | III | virB5 (type IV secretion system) | II0029 | II0065 |
| K40 | III | virB4 (type IV secretion system) | II0028 | II0066 |
| K46 | III | virB5 (type IV secretion system) | II0029 | II0065 |
| K50 | III | virB5 (type IV secretion system) | II0029 | II0065 |
| K51 | III | virB6 (type IV secretion system) | II0030 | II0064 |
| K52 | III | virB6 (type IV secretion system) | II0030 | II0064 |
| K55 | III | virB6 (type IV secretion system) | II0030 | II0064 |
| Amino acid synthesis, K17 | III | aspC (aminotransferase) | I0516 | I1495 |
| Sugar metabolism | ||||
| K15 | II | zwf (pentose phosphate pathway) | II0513 | II0778 |
| K18 | I | gnd (pentose phosphate pathway) | II1124 | II0111 |
| DNA/RNA metabolism | ||||
| K6 | III | purM (purines) | I1240 | I0710 |
| K9 | III | purL (purines) | I1127 | I0837 |
| K11 | III | pyrB (pyrimidines) | II0670 | II0599 |
| K19 | II | purN (purines) | I1241 | I0709 |
| K45 | III | pyrC (dihydroorotase) | II0669 | II0600 |
| K63 | III | pyrC (dihydroorotase) | I1281 | I0668 |
| W27 | II | pth (peptidyl tRNA hydrolase) | I0480 | I1536 |
| Regulation, K41 | I | spoT (stringent response) | I1296 | I0652 |
| Oxidoreduction, K54 | III | cydC (cytochrome oxidase) | II0761 | II0508 |
| Membrane structure, K23 | III | dacF (peptidoglycan synthesis) | II0350 | II0947 |
| Nicotinamide metabolism, K48 | II | pncA (pyrazinamidase) | I0545 | I1417 |
ORF of B. melitensis genome on the NCBI Entrez Genome website (http://www.ncbi.nlm.nih.gov/PMGifs/Genomes/micr.html).
ORF of B. suis genome on the NCBI Entrez Genome website (http://www.ncbi.nlm.nih.gov/PMGifs/Genomes/micr.html).
Characterization of mutants.
To investigate whether internalization into host cells of B. abortus contributes to intracellular growth, HeLa cells were infected with each mutant, and the ability of bacterial internalization was assessed. Of the 23 insertion mutants 2, K18 and K41, lost their ability to internalize almost completely, and these mutants were classified as class I strains. Four mutants—K15, K19, K48, and W27—showed the same ability to internalize as the wild-type strain, and they were classified as class II strains. The other 17 mutants showed an ability to internalize that was twice as high as the wild-type strain, and they were classified as class III (Table 1). Figure 1A and 2A show typical results of using one mutant in each class at various times of incubation. To eliminate the possibility that these results were caused by a change in sensitivity to gentamicin, the sensitivity of these mutants to gentamicin was tested. The wild-type strain and each mutant showed identical sensitivities to gentamicin.
FIG. 1.
Bacterial internalization into HeLa cells and mouse bone marrow-derived macrophages. Wild-type B. abortus (WT) or one of three representative mutants (K18, K19, and K63) was deposited onto HeLa cells (A) and macrophages (B) and then incubated at 37°C for the periods of time indicated. Bacterial internalization efficiency by both cells was determined by evaluating the protection of internalized bacteria from gentamicin killing and quantitated as described previously (see Materials and Methods). Data are the averages of triplicate samples from three identical experiments, and the error bars represent the standard deviations. Statistically significant differences between bacterial internalization of the wild type and that of mutants are indicated by asterisks (✽, P < 0.01; ✽✽, P < 0.001).
FIG. 2.
Intracellular replication of wild type and mutants within HeLa cells and mouse bone marrow-derived macrophages. HeLa cells (A) and macrophages (B) were infected with wild-type B. abortus or one of three representative mutants (K18, K19, and K63) as described in Materials and Methods. At different times of incubation, the cells were lysed, and the numbers of viable intracellular bacteria were determined. Datum points and error bars represent the mean CFU of triplicate samples from a typical experiment (performed at least four times) and their standard deviations.
The intracellular survival of Brucella spp. has been documented for several cell types. B. abortus shows a different intracellular trafficking pattern between professional and nonprofessional phagocytes (1, 11, 30). To analyze whether virulence-associated genes isolated in the present study share intracellular growth between professional and nonprofessional phagocytes, bone marrow-derived macrophages were infected with each mutant, and its internalization and intracellular growth within macrophages was examined. The results of the internalization and intracellular growth of mutants were compared to those with the wild-type strain. All class I strains, two class II strains (K15 and K19), and three class III strains (K6, K9, and K11) showed a three-fold-lower ability to internalize than the wild type and no intracellular growth within macrophages. The other 2 class II strains and 14 class III strains showed an ability to internalize similar to the wild type and no intracellular growth within macrophages. Figure 1B and 2B show typical results of using one mutant in each class after various incubation times.
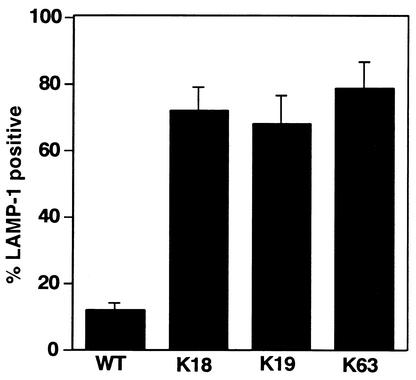
Phagosomes containing virulent B. abortus are reluctant to fuse with lysosomes, whereas dead B. abortus phagosomes colocalize with endocytic compartments in the early stage of infection in macrophages (1). To test the ability of B. abortus to target properly within macrophages early in infection, interaction of the mutants with the endocytic pathway was quantified by immunofluorescence localization of LAMP-1, a membrane protein of late endosomes and lysosomes (3, 21). As expected, most phagosomes containing the wild-type strain did not colocalize with LAMP-1 (12.3% ± 3.4% positive). In contrast, phagosomes containing a mutant in each class were frequently stained brightly by LAMP-1 antibody at 30 min of incubation (Fig. 3). This finding suggests that, in contrast to the wild-type strain, these mutants are colocalizing with either late endosomes or lysosomes.
FIG. 3.
LAMP-1 colocalization in phagosomes containing bacteria. Mouse bone marrow-derived macrophages were infected with wild-type B. abortus (WT) or one of three representative mutants (K18, K19, and K63) and then incubated for 30 min, fixed, and stained for LAMP-1. The samples were visualized by immunofluorescence microscopy. “% LAMP-1 positive” refers to the percentage of internalized bacteria that showed costaining with LAMP-1, based on observations of 100 bacteria per coverslip. Data are the averages of triplicate samples from three identical experiments, and error bars represent the standard deviations.
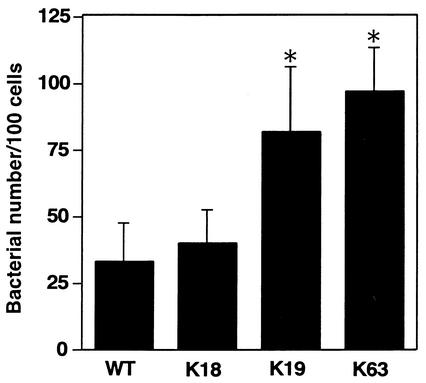
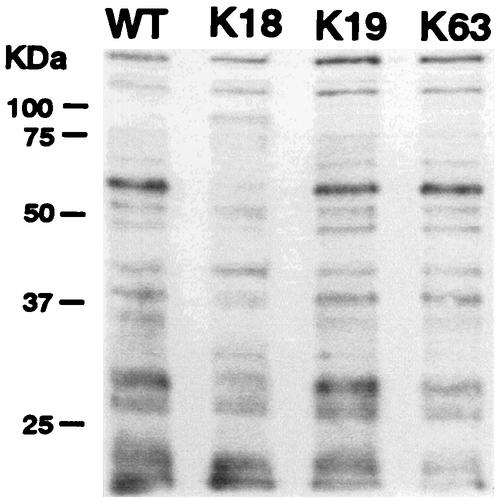
To investigate whether adherence of the mutants to HeLa cells contributes to bacterial internalization ability, the initial adherence of the mutants to HeLa cells was analyzed. Class II and III strains showed an adhesion ability twice as high as that of the wild-type strain and class I strains. Figure 4 shows typical results of using one mutant in each class after various incubation times. Biotin-labeled bacterial surface proteins of these three strains were analyzed by immunoblotting. The protein band pattern of K18 (class I) changed markedly more than other mutants (Fig. 5).
FIG. 4.
Bacterial adherence on HeLa cells. Before bacterial infection, HeLa cells were incubated with MEM containing cytochalasin D for 40 min at 37°C and then infected with wild-type B. abortus (WT) or one of three representative mutants (K18, K19, and K63), and bacteria associated with HeLa cells were scored by immunofluorescence microscopy. One hundred HeLa cells were examined per coverslip. Data are the averages of triplicate samples from three identical experiments, and error bars represent the standard deviations. Statistically significant differences between bacterial adherence of wild type and that of mutants are indicated by an asterisk (✽, P < 0.01).
FIG. 5.
Modification of surface proteins of mutants. Surface proteins of wild-type B. abortus (WT) and of three representative mutants (K18, K19, and K63) were labeled with Sulfo-NHS-Biotin and then detected by immunoblotting with horseradish peroxidase-conjugated streptavidin. The position of molecular mass markers (in kilodaltons) are shown on the left.
To determine whether this defect in internalization and intracellular replication of B. abortus correlates with an inability to establish infection in the host, we experimentally infected mice with B. abortus. Many bacteria were recovered from the spleens of mice infected with the wild-type strain at 10 days postinfection (5.4 × 106 CFU/spleen), but fewer bacteria were recovered from mice infected with class III mutant, K63 (5.5 × 101 CFU/spleen) (Fig. 6). Unexpectedly, many bacteria were recovered from the spleen of mice infected with class I mutant K18 (1.9 × 107 CFU/spleen), and class II mutant K19 showed an intermediate phenotype (6.8 × 103 CFU/spleen) (Fig. 6).
FIG. 6.
Proliferation in mice. Mice were infected intraperitoneally with wild-type B. abortus (WT) or three representative mutants (K18, K19, and K63) (104 CFU/0.1 ml). Recovery of viable bacteria from the spleen and the weights of spleens of infected mice at 10 days postinfection are shown. Error bars indicate standard deviations. Statistically significant differences between the bacterial growth of the wild type and that of mutants are indicated by asterisks (✽, P < 0.01; ✽✽, P < 0.001).
Sites of mini-Tn5Km2.
Chromosomal DNA was prepared from 23 mutants, digested with EcoRI, and cloned to plasmid pBluescript II KS(+). The resulting plasmid DNA was analyzed by using a mini-Tn5Km2 transposon O′-end primer (see Materials and Methods). An insertion site of all mutants was identified, and their gene products, ORFs, and gene location on the chromosome were characterized (Table 1). From this result, 7 genes were located on chromosome I, 14 genes were located on chromosome II, and 1 gene whose gene product is dihydroorotase was located on both chromosomes. The ORF was found by searching the BLASTX and BLASTN search algorithms. The mini-Tn5Km2 insertions of all class I and II strains were outside the VirB complex. Eight of the class III mutants had the mini-Tn5Km2 in the VirB complex (Table 1).
DISCUSSION
Our results did not identify any virulence factors such as toxins or secreted proteins able to interact with host cells, except the already-known VirB secretion system (8, 29, 39). Nineteen mutants were affected in internalization into HeLa cells under the experimental conditions used. The majority of the mutations resulting in intracellular growth defect affected genes acting in housekeeping functions, thus confirming results previously obtained by transposon mutagenesis (8, 17, 24).
In the defective internalization phenotype of class I mutants, the mini-Tn5Km2 was inserted in either the gnd and spoT gene, which had been identified as essential for intracellular growth of Brucella suis (24). 6-Phosphogluconate dehydrogenase is the third enzyme of the pentose phosphate pathway, one of two central routes of intermediary carbohydrate metabolism. Since the surface proteins of K18 changed markedly, the enzyme would affect the biosynthesis of the outer membrane that may participate in bacterial internalization. However, the survival in mice of K18 was similar to that of the wild-type strain. Although the unusual result in the present study is difficult to explain, different genes may contribute to intracellular growth in macrophages and virulence in mice. Spleen colonization profiles are generally examined for a number of weeks postinfection to evaluate the persistence of a Brucella strain in animal models. In a mouse model with wild-type Brucella, the number of bacteria in the spleen peaked at 4 days postinfection, followed by a 104-fold decrease until day 18 postinfection. Also, the weight of spleens increased and peaked by ca. 10 days postinfection. After 11 days, the splenomegaly was followed by a rapid reduction of weight (13). Based on the results of the present study, we set up the single time point of 10 days postinfection and evaluated the persistence and chronic infection in mice. One explanation of the phenotype of K18 in the mouse model is that 10 days after infection may be too early of a time point to determine whether a mutant is attenuated or not. Macrophages are widely known as cells that play an important role in inflammatory processes, as well as in the initiation, maintenance, and control of primary and secondary immune responses. The execution of these activities is mediated by complex and multifunctional processes involving macrophage secretory cytokines. Therefore, K18 may show different phenotype in cultured macrophages and in peritoneal immune cells. Another explanation is that gnd is not essential for intracellular growth in macrophages in a peritoneal environment or escape from the host immune system. Guanosine-3′,5′-bispyrophosphate (ppGpp) metabolism controls the expression of virulence by Legionella pneumophila (18). ppGpp initiates developmental programs that are critical for surviving periods of starvation in a variety of environmental microbes (20). Thus, ppGpp 3′-pyrophosphohydrolase (SpoT) would influence the expression of B. abortus virulence under intracellular conditions.
All class II genes (zwf, purN, pth, and pncA) and seven class III genes (znuA, aspC, purL, purM, pyrC, cydC, and dacF) are newly identified by our study. However, genes associated with the functional groups of amino acid synthesis, sugar metabolism, DNA/RNA metabolism, and oxidoreduction had been identified as essential for intracellular growth of B. abortus and B. suis (12, 14, 17, 24). Five enzymes in the purine or pyrimidine biosynthesis pathway encoded by purL, purM, purN, pyrB, and pyrC were identified as virulence-associated genes. These biosynthesis pathways would contribute to intracellular growth, confirming previous results on the attenuation of purE mutants from B. melitensis (12) and of purD, purF, carAB, pyrB, and pyrD from B. suis (24). The earlier works described dnaK and dnaJ encoding heat shock protein of B. suis showed different intracellular growth phenotypes (23). Also, Shigella flexneri auxotrophic mutants that harbor mutations in the aromatic amino acids (aroB, aroC, and aroD) showed different degrees of virulence, including epithelial cell invasion and intracellular growth in vitro, as well as in vivo (2). In the present study, we detected some mutants that have the same metabolic pathway but a different internalization phenotype. From these results, we speculate that each step of the metabolic pathway may contribute to internalization differently.
Several genes among class II and III mutants that participate in bacterial virulence may decrease B. abortus virulence. The insertion sites of strains K2, K15, or K54 were inside the genes: a high-affinity zinc uptake system protein that is a znuA homologue, glucose-6-phosphate-1-dehydrogenase, or a transport ATP-binding protein that is a cydC homologue, respectively. The znuA gene of Haemophilus ducreyi encodes a 32-kDa protein that is homologous to the ZnuA protein of E. coli and is part of a growing family of prokaryotic zinc transporters. The H. ducreyi znuA isogenic mutant shows a markedly decreased virulence when tested in a temperature-dependent rabbit model for experimental chancroid (25). Glucose-6-phosphate dehydrogenase encoded by the zwf gene catalyzes the first enzymatic step in the pentose phosphate cycle. This pathway provides ribose for nucleoside synthesis and reducing equivalents in the form of NADPH for reductive biosynthetic and maintenance of the cellular redox state. Salmonella enterica serovar Typhimurium zwf mutant lacking glucose-6-phosphate dehydrogenase activity increases susceptibility to reactive oxygen and nitrogen intermediates and attenuates virulence in mice (26). The ATP-binding cassette (ABC) transporters are active transport systems common in bacteria and eukaryotic cells. ABC transporters use the free energy of ATP hydrolysis to pump substances across the membrane against a concentration gradient into or out of cells (34). The cydC gene of S. flexneri encodes the ABC transporter that is homologous to the CydC protein of E. coli, which leads to defective cytochrome bd expression. The cydC mutant of S. flexneri forms markedly smaller plaques, has markedly decreased intracellular survival, and shows a 100-fold increase in lethal dose for mice compared to the wild type (46). Recently, the role of the ABC transporter in B. abortus virulence, which is homologous to Rhizobium meliloti ExsA, was described (36). Decreased survival in mice of the B. abortus ABC transporter mutant compared to the survival of the wild-type strain shows that the ABC transporter is critical for full bacterial virulence (36). The cydB and cydD genes are part of the operon cydDCAB. The cydB and cydD mutants are also defective for the intracellular growth of B. abortus and B. suis (14, 24), suggesting that functional cytochrome bd oxidase is required for growth in an intracellular environment.
Since d-alanyl-d-alanine carboxypeptidase (DacF) contributes to the maintenance of cell shape in E. coli (28), the enzyme would also contribute to the maintenance of cell shape in B. abortus and so may participate in its virulence. Consistent with this, the mtgA encoding monofunctional biosynthesis peptidoglycan transglycosylase of B. abortus is induced intracellularly (15). Thus, class III mutants increased adherence to and internalization in HeLa cells, a result that may be because of the alteration of bacterial surface due to a lack of these biosynthesis pathways. B. abortus mutants with rough lipopolysaccharide are more adherent and enter Vero cells in greater numbers than the wild type (9). These results suggest that differences in adhesiveness and invasiveness are correlated to the hydrophobicity of Brucella.
The pncA gene encoding pyrazinamidase is involved in the conversion from pyrazinamide to pyrazinoic acid. Pyrazinamide is an important antituberculosis drug, and mutation of pncA is a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis (38). Unlike most antibacterial agents, pyrazinamide, despite its remarkable in vivo activity, has no activity against M. tuberculosis in vitro except at an acidic pH (47). Presumably, pyrazinamidase would act under acidic environment. Vacuole acidification in phagocytic cells at a pH between 4.0 and 4.5 has been shown to be essential for intracellular survival during early infection by B. suis (33). Pyrazinamidase may participate in the resistance of Brucella spp. to the acidic condition of the phagosome.
Several genes in the type IV secretion system that participate in bacterial adherence may decrease B. abortus virulence. Type IV secretion systems export four types of substrates: (i) DNA conjugation intermediates; (ii) the multisubunit pertussis toxin; (iii) monomeric proteins, including primase, RecA, and the Agrobacterium tumefacience VirE and VirF proteins; (iv) and Helicobacter pylori CagA protein (4). However, the substrates of VirB secretion system of B. abortus remain unclear. Unidentified substrates may be involved in internalization into HeLa cells or macrophages.
Our earlier studies showed that bacteria move from the site of initial bacterial contact with the macrophages (22, 43). The swimming of the bacteria lasts for several minutes, with generalized plasma membrane ruffling before enclosure in macropinosomes. In contrast, contact of the virB4 mutant with the macrophages results in much smaller ruffling that is restricted to the area near the bacteria. We did not observe the process of internalization into macrophages in HeLa cells (unpublished results). Therefore, signal transduction of internalization may be different between macrophages and HeLa cells. The intracellular-growth-defective mutants in HeLa cells isolated in the present study were also defective for intracellular growth in macrophages. These results indicate that the virulence-associated genes identified in the present study, including virB genes, contribute to intracellular growth in both HeLa cells and macrophages. Presumably, a factor exists that acts on only macrophages and that may be an effector molecule secreted by the VirB complex. This effector molecule should have important roles in B. abortus virulence and needs to be studied further.
Acknowledgments
We thank Hyeng-il Cheun for critical reading of the manuscript and Toru Tobe and Ichiro Tatsuno for valuable discussions.
This study was supported, in part, by a grant from The 21st Century COE Program (A-1); the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and Scientific Research grants 12575029 and 13770129 from the Japan Society for the Promotion of Science.
Editor: D. L. Burns
REFERENCES
- 1.Arenas, G. N., A. S. Staskevich, A. Aballay, and L. S. Mayorga. 2000. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect. Immun. 68:4255-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cersini, A., A. M. Salvia, and M. L. Bernardini. 1998. Intracellular multiplication and virulence of Shigella flexneri auxotrophic mutants. Infect. Immun. 66:549-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, J. W., Y. Cha, K. U. Yuksel, R. W. Gracy, and J. T. August. 1988. Isolation and sequencing of a cDNA clone encoding lysosomal membrane glycoprotein mouse LAMP-1. J. Biol. Chem. 263:8754-8758. [PubMed] [Google Scholar]
- 4.Christie, P. J., and J. P. Vogel. 2000. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 8:354-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3:159-168. [DOI] [PubMed] [Google Scholar]
- 6.de Lorenzo, V., M. Herrero, U. Jacubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived mini-transposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 8.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 9.Detileux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Vet. Pathol. 27:317-328. [DOI] [PubMed] [Google Scholar]
- 10.Detileux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Penetration and intracellular growth of Brucella abortus in non-phagocytic cells in vitro. Infect. Immun. 58:2320-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorn, B. R., W. A. Dunn, Jr., and A. Progulske-Fox. 2002. Bacterial interactions with the autophagic pathway. Cell. Microbiol. 4:1-10. [DOI] [PubMed] [Google Scholar]
- 12.Drazek, E. S., H. H. Houng, R. M. Crawford, T. L. Hadfield, D. L. Hoover, and R. L. Warren. 1995. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect. Immun. 63:3297-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekaza, E., L. Guilloteau, J. Teyssier, J.-P. Liautard, and S. Kohler. 2000. Functional analysis of the ClpATPase ClpA of Brucella suis, and persistence of a knockout mutant in BALB/c mice. Microbiology 146:1605-1616. [DOI] [PubMed] [Google Scholar]
- 14.Endley, S., D. McMurray, and T. A. Ficht. 2001. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J. Bacteriol. 183:2454-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskra, L., A. Canavessi, M. Carey, and G. Splitter. 2001. Brucella abortus genes identified following constitutive growth and macrophage infection. Infect. Immun. 69:7736-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay, B., and S. Falkow. 1997. Common themes in microbial pathogenicity. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foulongne, V., G. Bourg, C. Cazevieille, S. Michaux-Charachon, and D. O'Callaghan. 1999. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect. Immun. 68:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer, B. K., and M. S. Swanson. 1999. Coordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 19.Hangfield, M., and R. C. Levesque. 1999. Strategies for isolation of in vivo expressed genes from bacteria. FEMS Microbiol. Rev. 23:69-91. [DOI] [PubMed] [Google Scholar]
- 20.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 12:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harter, C., and I. Mellman. 1992. Transport of the lysosomal membrane glycoprotein Igp120 (Igp-A) to lysosomes does not require appearance on the plasma membrane. J. Cell Biol. 117:311-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, S., M. Watarai, S.-I. Makino, and T. Shirahata. 2002. Membrane sorting during swimming internalization of Brucella is required for phagosome trafficking decisions. Microb. Pathog. 33:225-237. [DOI] [PubMed] [Google Scholar]
- 23.Kohler, S., E. Ekaza, J.-Y. Paquet, K. Walravens, J. Teyssier, J. Godfroid, and J.-P. Liautard. 2002. Induction of dnaK through its native heat shock promoter is necessary for intramacrophagic replication of Brucella suis. Infect. Immun. 70:1631-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohler, S., V. Foulongne, S. Ouahrani-Bettache, G. Bourg, J. Teyssier, M. Ramuz, and J. P. Liautard. 2002. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA 99:15711-15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis, D. A., J. Klesney-Tait, S. R. Lumbley, C. K. Ward, J. L. Latimer, C. A. Ison, and E. J. Hansen. 1999. Identification of the znuA-encoded periplasmic zinc transport protein of Haemophilus ducreyi. Infect. Immun. 67:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundberg, B. E., R. E. Wolf, Jr., and M. C. Dinauer, Y. Xu, and F. C. Fang. 1999. Glucose 6-phosphate dehydrogenase is required for Salmonella typhimurium virulence and resistance to reactive oxygen and nitrogen intermediates. Infect. Immun. 67:436-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michaux-Charachon, S., G. Bourg, E. Jumas-Bilak, P. Guigue-Talet, A. Allardet-Servent, D. O'Callaghan, and M. Ramuz. 1997. Genome structure and phylogeny in the genus Brucella. J. Bacteriol. 179:2344-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson, D. E., and K. D. Young. 2001. Contributions of PBP 5 and DD-carboxypeptidase penicillin binding proteins to maintenance of cell shape in Escherichia coli. J. Bacteriol. 183:3055-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 30.Pizarro-Cerda, J., E. Moreno, and J. P. Gorvel. 1999. Brucella abortus invasion and survival within professional and non-professional phagocytes, p. 201-232. In Advances in cell and molecular biology of membranes and organelles, vol. 6. JAI Press, Inc., New York, N.Y.
- 31.Pizarro-Cerda, J., E. Moreno, V. Sanguedolce, J. L. Mege, and J. P. Gorvel. 1998. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 66:2387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Grovel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porte, F., J. P. Liautard, and S. Kohler. 1999. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rolain, J., M. Maurin, and D. Raoult. 2000. Bactericidal effect of antibiotics on Bartonella and Brucella spp.: clinical implications. J. Antimicrob. Chemother. 46:811-814. [DOI] [PubMed] [Google Scholar]
- 36.Rosinha, G. M. S., D. A. Freitas, A. Miyoshi, V. Azevedo, E. Campos, S. L. Cravero, O. Rossetti, G. Splitter, and S. C. Oliveira. 2002. Identification and characterization of a Brucella abortus ATP-binding cassette transporter homolog to Rhizobium meliloti ExsA and its role in virulence and protection in mice. Infect. Immun. 70:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Scorpio, A., and Y. Zhang. 1996. Mutation in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculosis drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 39.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium tumefaciens is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatsuno, I., H. Kimura, A. Okutani, K. Kanamura, H. Abe, S. Nagai, K. Makino, H. Shinagawa, M. Yoshida, K. Sato, J. Nakamoto, T. Tobe, and C. Sasakawa. 2000. Isolation and characterization of Mini-Tn5Km2 insertion mutants of enterohemorrhagic Escherichia coli O157:H7 deficient in adherence to Caco-2 cells. Infect. Immun. 68:5943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ugalde, R. A. 1999. Intracellular lifestyle of Brucella spp. common genes with other animal pathogens, plant pathogens, and endosymbionts. Microbes Infect. 1:1211-1219. [DOI] [PubMed] [Google Scholar]
- 42.Watarai, M., H. L. Andrews, and R. R. Isberg. 2001. Formation of a fibrous structure on the surface of Legionella pneumophila associated with exposure of DotH and DotO proteins after intracellular growth. Mol. Microbiol. 39:313-329. [DOI] [PubMed] [Google Scholar]
- 43.Watarai, M., S.-I. Makino, Y. Fujii, K. Okamoto, and T. Shirahata. 2002. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell. Microbiol. 4:341-356. [DOI] [PubMed] [Google Scholar]
- 44.Watarai, M., S.-I. Makino, and T. Shirahata. 2002. An essential virulence protein of Brucella abortus, VirB4, requires an intact nucleoside-triphosphate-binding domain. Microbiology 184:1439-1446. [DOI] [PubMed] [Google Scholar]
- 45.Watarai, M., T. Tobe, M. Yoshikawa, and C. Sasakawa. 1995. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 14:2461-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Way, S. S., S. Sallustio, R. S. Magliozzo, and M. B. Goldberg. 1999. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 181:1229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]