Abstract
Lyme disease is a tick-borne infection that can lead to chronic, debilitating problems if not recognized or treated appropriately. Borrelia burgdorferi, the causative agent of Lyme disease, is maintained in nature by a complex enzootic cycle involving Ixodes ticks and mammalian hosts. Many previous studies support the notion that B. burgdorferi differentially expresses numerous genes and proteins to help it adapt to growth in the mammalian host. In this regard, several studies have utilized a dialysis membrane chamber (DMC) cultivation system to generate “mammalian host-adapted” spirochetes for the identification of genes selectively expressed during mammalian infection. Here, we have exploited the DMC cultivation system in conjunction with microarray technology to examine the global changes in gene expression that occur in the mammalian host. To identify genes regulated by only mammal-specific signals and not by temperature, borrelial microarrays were hybridized with cDNA generated either from organisms temperature shifted in vitro from 23°C to 37°C or from organisms cultivated by using the DMC model system. Statistical analyses of the combined data sets revealed that 125 genes were expressed at significantly different levels in the mammalian host, with almost equivalent numbers of genes being up- or down-regulated by B. burgdorferi within DMCs compared to those undergoing temperature shift. Interestingly, during DMC cultivation, the vast majority of genes identified on the plasmids were down-regulated (79%), while the differentially expressed chromosomal genes were almost entirely up-regulated (93%). Global analysis of the upstream promoter regions of differentially expressed genes revealed that several share a common motif that may be important in transcriptional regulation during mammalian infection. Among genes with known or putative functions, the cell envelope category, which includes outer membrane proteins, was found to contain the most differentially expressed genes. The combined findings have generated a subset of genes that can now be further characterized to help define their role or roles with regard to B. burgdorferi virulence and Lyme disease pathogenesis.
Lyme disease, caused by the spirochete Borrelia burgdorferi, is the most common arthropod-borne infection in the United States (39). Undiagnosed infection with B. burgdorferi often results in chronic disease, which can lead to sequelae such as carditis, arthritis, and neuritis (53). In nature, B. burgdorferi is maintained through a complex enzootic cycle involving ticks and mammalian hosts, typically small rodents (29). To perpetuate this enzootic cycle, B. burgdorferi must adapt physiologically to two dramatically different environments. Consistent with the adaptation process, several genes and the proteins they encode have been shown to be specifically up-regulated or down-regulated as this organism is transmitted from its tick vector to the mammalian host (1, 2, 18, 21, 23, 47). The best-characterized example of differential gene expression in B. burgdorferi involves two outer surface lipoproteins, OspA and OspC, which are down-regulated and up-regulated, respectively, during tick feeding (9, 23, 46, 47). In addition to OspA and OspC, recent studies have identified several borrelial molecules that are differentially expressed during tick feeding and mammalian infection, including several OspE-related, OspF-related, Elp, and Mlp lipoproteins: EppA, p35, p37, and Lp6.6 (1, 2, 7, 12, 17, 23, 28, 55-57).
Defining genes differentially expressed by human pathogens during the mammalian phase of infection should help elucidate those that are integral to pathogenesis and virulence (32, 52). For this reason, many studies over the past decade have used different methodologies to help identify bacterial antigens expressed only during mammalian infection (32, 33). Among these studies, several have been focused on identifying B. burgdorferi antigens differentially expressed in the mammalian host (1, 14, 17, 26, 49, 55). Along these lines, a rat dialysis membrane chamber (DMC) implant model has been used to generate mammalian host-adapted B. burgdorferi to help identify genes regulated by mammalian host-specific signals (1, 16, 22, 23). Here, we have used B. burgdorferi microarrays in conjunction with the DMC animal model to help identify genes regulated by mammalian host-specific factors. While a similar analysis was recently reported by Revel et al. (41), it is important to note that the prior study did not use a clonal isolate of B. burgdorferi. This is important, since it is now well recognized that uncloned isolates of B. burgdorferi contain subpopulations of organisms with various phenotypes (16). Therefore, we utilized a B. burgdorferi strain B31-MI clonal isolate for our array analyses, which revealed several genes differentially regulated by mammalian host-specific signals that were previously unrecognized.
MATERIALS AND METHODS
Strains and growth conditions.
A clonal derivative of B. burgdorferi strain B31-MI, designated B31c8, was generated by plating organisms on BSK-H (Sigma Chemical Co., St. Louis, Mo.) agar plates (3, 27, 44). Specific primers for each of the known B31 plasmids were used to confirm that the colony isolated contained all 21 borrelial plasmids (6, 16, 19). For temperature shift experiments, clone B31c8 was first cultivated in BSK-H medium supplemented with 6% rabbit serum at 23°C to the mid-logarithmic phase (5 × 107 per ml). Organisms grown at 23°C were then seeded at a concentration of 1,000 spirochetes per ml into medium prewarmed to 37°C and cultivated to the mid-logarithmic phase. Mammalian host-adapted B. burgdorferi cells were cultivated in DMCs, which were seeded with the same 23°C culture of organisms used to seed the temperature shift cultures mentioned above as previously described (1, 22). Infectivity assays using C3H/HeJ mice were performed as described in reference 1.
RNA isolation and probe generation.
Organisms were harvested at mid-logarithmic phase from three different 50-ml temperature-shifted cultures and 16 different DMC-implanted rats. Similar to prior studies with B. burgdorferi strain 297 (1), growth curves with the B31c8 clone determined that 5 × 107 bacteria per ml for 37°C temperature-shifted cultures and 7 × 106 bacteria per ml from DMC cultures corresponded to the mid-logarithmic phase. Therefore, to ensure organisms from the same stage of growth were used for the microarray analyses, mid-logarithmic-phase cultures from three different groups of DMCs and all three temperature shift cultures were pelleted by centrifugation, and RNA was isolated with TRI-REAGENTLS solution according to the manufacturer's instructions (Molecular Research Center, Inc., Cincinnati, Ohio). All contaminating genomic DNA was subsequently eliminated from the RNA preparations by incubation with 5 μl of RQ1 RNase-free DNase (Promega, Madison, Wis.) at 37°C for 2 h. DNase was inactivated by incubation at 70°C for 30 min before RNA preparations were pooled and collected by precipitation in 100% ethanol. The resulting pellets were washed in 70% ethanol, allowed to air dry, and resuspended in RNase-free water at a concentration of 1 μg/μl. cDNA probes were generated from 5 μg of each RNA preparation in reaction mixtures containing Superscript II reverse transcriptase (Invitrogen, Carlsbad, Calif.), oligonucleotides specific for the 3′ end of all annotated B. burgdorferi open reading frames (ORFs) (6, 19), and [α-33P]dATP (Amersham Pharmacia Biotech, Piscataway, N.Y.). Unincorporated radioactivity was removed from the completed cDNA synthesis reaction by using micro Bio-Spin P-30 chromatography columns (Bio-Rad Laboratories, Hercules, Calif.).
Generation of microarrays.
Nylon arrays containing B. burgdorferi amplicons were generated as described previously (37). Briefly, the published nucleotide sequence of B. burgdorferi strain B31-MI was manually curated by Sherwood Casjens (University of Utah Medical Center), which revealed 1,697 “gene features” (i.e., annotated ORFs and putative pseudogenes) that were subsequently PCR amplified with specific oligonucleotides designed by Sigma-Genosys (Houston, Tex.). Ten microliters of each amplicon was subjected to agarose gel electrophoresis and quantitated by densitometric analysis with known molar quantities of molecular size markers. Among the 1,697 gene features, 1,628 (96%) were successfully amplified. Ten nanograms of each amplicon was subsequently applied as spots in duplicate to positively charged nylon membranes by Sigma-Genosys. Following spotting, arrays were cross-linked with UV irradiation. Eight genomic DNA spots also were included on each membrane (two in each corner) for positive controls and to help facilitate array template alignment during image analysis.
Hybridization and data analysis.
Paired nylon membrane arrays were prehybridized at 60°C for 1 h in 10 ml of ExpressHyb hybridization solution (Clontech Laboratories, Inc., Palo Alto, Calif.) containing 1 mg of sheared salmon testes DNA (Sigma Chemical Co.). Radioactively labeled cDNA probes were added to the prehybridized membranes and allowed to incubate at 60°C for 16 h. The membranes were subsequently washed twice for 30 min each with a 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-1% sodium dodecyl sulfate (SDS) solution prewarmed to 60°C. This was followed by two more 30-min washes at 60°C in 0.1× SSC-0.5% SDS, before the membranes were washed one final time in 2× SSC at room temperature. Membranes were then wrapped in cellophane and exposed to a phosphor screen (Amersham Pharmacia Biotech) for 48 h. Phosphor screens were then scanned with a Storm 840 PhosphorImager (Amersham Pharmacia Biotech), and the resulting images and associated signal data were imported into ArrayVison version 6.0 (Imaging Research, St. Catharines, Ontario, Canada) to determine densities for each spot by using local background subtraction. Density values were exported into Microsoft Excel (Microsoft Corporation, Redmond, Wash.) for subsequent analyses, as previously described (10). Briefly, raw density values were first converted into a percent density value of the total array density before duplicate spots were combined and averaged. Data from three arrays (six independent spots for each ORF) were then subjected to an unpaired, two-tailed Student's t test to determine which ORFs were expressed at a significantly different level (≥1.5-fold up- or down-regulated and P < 0.001) between temperature-shifted and DMC-cultivated organisms. Density values that did not exceed 2 standard deviations above the local background densities in both conditions were removed from the final data sets. After image analysis, the radioactive probe was stripped from each membrane by incubation in 0.4 M NaOH for 30 min at 45°C followed by two washes in 200 mM Tris-HCl (pH 7.0)-0.1× SSC-0.2% SDS for 30 min at 45°C. Membranes were subsequently wrapped in cellophane and exposed to a phosphor screen overnight to ensure stripping was successful. Array experiments for both conditions were performed in triplicate, and membranes were alternated between the two conditions for hybridizations to help control for interarray variation.
QRT-PCR.
To confirm the microarray data, 25 different ORFs, including ospA, ospB, ospC, and flaB, were subjected to quantitative real-time (QRT)-PCR in triplicate for comparison to the microarray data (Table 1). Briefly, oligonucleotide primers were designed with PRIMER EXPRESS software (PE Biosystems, Foster City, Calif.). All primer pairs selected produced only one amplicon of the expected size when B. burgdorferi total genomic DNA was used as a template for PCR, indicating all primers were specific for their respective genes. Using 3′ ORF-specific primers as described above, 2 μg of total RNA isolated from three independent pools of DMC cultivated organisms or three different cultures of temperature-shifted organisms was used to generate six different sets of cDNA for the QRT-PCR analyses. For all experiments, 5 ng of cDNA was subjected to QRT-PCR with a Perkin-Elmer ABI Prism 7700 sequence detection system and the SYBR Green master mix according to the manufacturer's instructions (PE Biosystems). To ensure that there was no contaminating genomic DNA in the cDNA and to identify any possible primer dimer artifact, reaction mixtures containing cDNA generated without reverse transcriptase and reaction mixtures containing primers alone were also included as controls. Template cDNAs generated from the six different DMC or temperature-shifted environments were normalized by using the constitutively expressed flaB gene. Differential up- or down-regulation for each ORF was determined by comparing the average change in threshold cycle (ΔCt) of each ORF with three different cDNA pools for each environmental condition.
TABLE 1.
Oligonucleotides used for QRT-PCR and expression data comparing ORT-PCR to microarray data
| ORF | Forward/reverse primer (5′→3′) | Fold up-or down- regulationa
|
|
|---|---|---|---|
| QRT-PCR | Microarray | ||
| BB0147 (flaB) | ATGTTAGCAGCCTTGACGAGAAA/GATCGTACTTGCCGTCTTTGTTTT | −0.02 | 1.54 |
| BB0152 | ACTGTTGGAATTGGAACGATCAT/TTAACGCCTTTTTCAATGGCA | −4.45 | −4.68 |
| BB0232 | TTTAGATCTTTTGGTACATTTGAAGTTAGAA/AACGTGATGATCTAGGACCTTAACATACT | 0.02 | 1.58 |
| BB0237 | AATTTGCTCCTTTAATATGCTATGATGA/TTTGACCAAGAATCGTTTGAAAAA | −6.97 | 1.46 |
| BB0240 | AAGTCCCGAAATACCAGGAGAAAT/TTCTTGCTGCTGTGTAAATACCAAA | −0.97 | 1.75 |
| BB0242 | TGTTTGGCAAGCAAATAAATCAATT/TTTACCTTTTCAAAAGGTTTAGACAGTTT | −1.35 | 1.24 |
| BB0248 | TTTGCTTAAAAACGAAACCGATACTA/TCAGCAAACATTGTTTGTCTAAAGAAT | −10.30 | −2.08 |
| BB0362 | TGCAGAACCATTTGACACAAATATAC/TTCTTGGAAGGTTGATTAGCAGGT | −0.17 | −1.32 |
| BB0469 | AGCGCTAAAAGTAAGCAATATTTCAAT/AACCTAATTTAACATACTTTGCAACCAA | −0.06 | 4.24 |
| BB0565 | AGAGGAATAAGGGAGTTAACATTTCAAA/TTCATTGACATAATCTACTAAAATTCCAAGA | 0.25 | 2.48 |
| BB0690 | ATTTCTTTGTTATTCACAAAAAAACTCAAA/AATTCAGAATCATATCCAAGCATTCTT | −1.54 | −1.20 |
| BB0757 | ATAATGATGCTTGTGTTTTGCATGA/AAGCCTGGAAACATCCTTGGTA | −1.17 | −1.78 |
| BBA15 (ospA) | ATGTTAGCAGCCTTGACGAGAAA/GATCGTACTTGCCGTCTTTGTTTT | −18.92 | −5.85 |
| BBA16 (ospB) | AAATGGGAAGACAGTACTAGCACTTTAA/TGTATTGTTGTACTGTAATTGTACCATCTGTT | −31.92 | −8.66 |
| BBA52 | TCAAAAAACTCAAGACCTTCCAAAA/AATTCACTTTCTGCACCGTTAAGAT | −7.09 | −2.78 |
| BBA59 | TTGGTCGTGGGATTTTAATAGATTCTA/TGAGGCTTTTGATTGTGGGTTT | −19.85 | −5.16 |
| BBA62 | ATTATTTGTTGCTTGCGAAACTACA/TCATATCTTTATCTGTCATTGGAGCTGT | −39.44 | −11.73 |
| BBA65 | ACTGATATTTTCAATTTAGCAGAGATTGTAA/TGTTGGATTCGTATACCACCTGATATT | −2.76 | −1.41 |
| BBA73 | TTCATGGATAAAAATCGTCGATAACA/CAACCCTTATTACTTCTCCGAGAATT | −11.42 | −2.14 |
| BBB19 (ospC) | CTGATGCAAAAGAAGCCATTTTAA/TGCTTTTGACAAGACCTCTACTGATT | −0.05 | 1.61 |
| BBE19 | TAAAATAGTTGCCATTTGCTCAATTAA/TTAGAAGAACTTTATATTTTTTAGACAAGAGTGTT | −5.24 | −3.16 |
| BBJ09 (ospD) | ACAGTAGAAGCAAAAGATAAGTTAATTGATGT/TCTGCAAGCTTTGTATTGTTCGTAGT | −48.23 | −18.77 |
| BBK53 | CAAACTTTTTTTGAGAATTCGGAAA/ACGATGTTCAGACGCATATAATTTTAA | −1.32 | −1.01 |
| BBL40 | ATTACAGGGCCTGTATATGATGATTTTACT/AGCTTTCCTAATCCTTCGTCGTTA | −1.50 | −1.03 |
| BBR41 | ACAGAAAAATTACAATGGACCAATGAA/AAAGCGCACCTTCTGAAATAAGTAATA | −0.55 | −1.40 |
QRT-PCR or microarray data represent fold up- or down-regulation of each ORF in DMC-cultivated organisms compared to that in temperature-shifted organisms.
Sequence analysis.
Multiple sequence alignments and phenogram analyses were performed using the ClustalW sequence alignment program of the MacVector version 6.5.3 software package (Oxford Molecular Group, Campbell, Calif.).
RESULTS
Generation of a cloned B31-MI strain and phenotypic analysis.
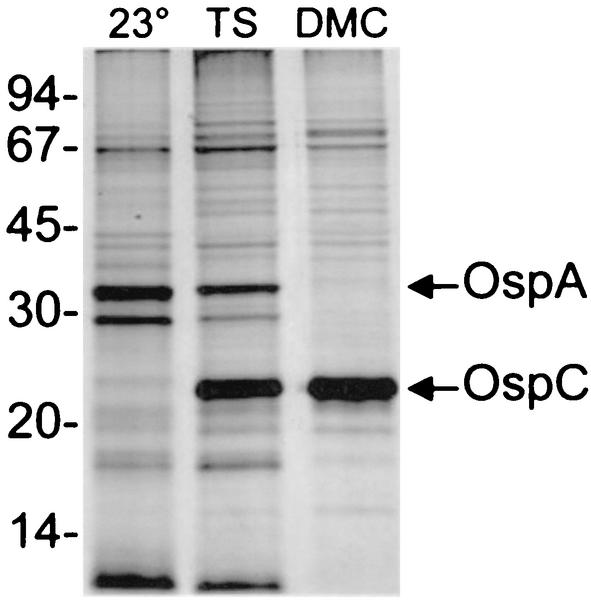
To begin a global transcriptional analysis of B. burgdorferi in a mammalian host-adapted state, we first generated a cloned isolate from the original uncloned B. burgdorferi B31-MI strain. This was done by plating organisms on solid agar BSK-H plates and subsequently isolating single colonies as previously described (16). One clone, designated B31c8, was identified that contained all known plasmids, which was confirmed by PCR screening with specific primer pairs (data not shown) (16). We next determined that clone B31c8 was fully virulent by injecting 103 organisms into C3H/HeJ mice and confirming infection by cultivating ear-punch biopsies in BSK-H media (data not shown). Additionally, since a recent study reported that clonal B31 isolates can have different phenotypes with regard to their response to environmental signals (16), we also confirmed that the B31c8 clone undergoes the expected protein expression changes after temperature shift (e.g., OspC up-regulated) and after growth in DMCs within rat peritoneal cavities (e.g., OspA down-regulated) (Fig. 1).
FIG. 1.
SDS-polyacrylamide gel electrophoresis and silver stain profiles of clonal isolate B31c8 under different cultivation conditions. Whole-cell-lysates from 107 B31c8 organisms either cultured at 23°C (lane 23°), temperature shifted from 23°C to 37°C (lane TS), or cultivated within rat DMCs (lane DMC) were separated on a 12.5% polyacrylamide gel and stained with silver. The OspA and OspC proteins are indicated. Molecular mass standards (in kilodaltons) are indicated to the left.
Identification of genes responsive to mammalian host-specific factors.
The underlying hypothesis prompting this study was that genes regulated by signals other than temperature in the mammalian host could help to identify molecules important in borrelial pathogenesis. To ensure that genes differentially expressed by temperature changes alone were excluded from the final analysis, we compared the global transcriptional profile of organisms temperature shifted from 23°C to 37°C in vitro with that of mammalian host-adapted organisms (i.e., organisms shifted from 23°C into rat DMCs). This was accomplished by spotting, hybridizing, and statistically analyzing the membrane arrays as outlined in Materials and Methods. Arrays were probed in triplicate with multiple preparations of total RNA pooled from three different temperature shift experiments and 16 different explanted DMCs. Statistical analysis of the combined data sets resulted in the identification of 125 ORFs differentially expressed in the mammalian host environment (i.e., ≥1.5-fold up- or down-regulated and P ≤ 0.001). Of the 125 ORFs identified, 58 (46%) were significantly up-regulated, while 67 (54%) were significantly down-regulated (Tables 2 and 3, respectively).
TABLE 2.
ORFs up-regulated during DMC cultivation
| ORF designation (description) | Replicon | Family | Cate- gorya | Signal eptide | Fold up- regulation |
|---|---|---|---|---|---|
| BBQ01 (conserved hypothetical protein [Borrelia burgdorferi]) | Ip56 | 55 family | HX | − | 6.78 |
| BB0125 (hypothetical protein) | Chromosome | U | − | 5.99 | |
| BBJ26 (ABC transporter, ATP-binding protein [Methanococcus jannaschii]) | lp38 | 4 family | TP | − | 5.48 |
| BBB06 (PTS system, cellobiose-specific IIB component [cetA] [Bacillus subtilis]) | cp26 | TP | − | 4.39 | |
| BBG17 (hypothetical protein) | Ip28-2 | U | − | 3.64 | |
| BBJ27 (hypothetical protein) | lp38 | U | − | 3.59 | |
| BB0489 (ribosomal protein L24 [rplX] [Escherichia coli]) | Chromosome | RP | − | 3.53 | |
| BB0569 (hypothetical protein) | Chromosome | U | − | 3.39 | |
| BB0603 (membrane-associated protein p66 [B. burgdorferi]) | Chromosome | CE | + | 3.35 | |
| BBG19 (hypothetical protein) | Ip28-2 | 117 family | U | − | 3.32 |
| BB0809 (tRNA-guanine transglycosylase [tgt] [Zymomonas mobilis]) | Chromosome | ARS | − | 3.06 | |
| BB0844 (hypothetical protein) | Chromosome | 12 family | U | + | 3.02 |
| BBQ63 (hypothetical protein, family 117 paralog, pseudogene) | Ip56 | 117 family | U | − | 2.95 |
| BB0495 (ribosomal protein S5 [rpsE] [Bacillus stearothermophilus]) | Chromosome | RP | − | 2.92 | |
| BBA64 (antigen, P35 [B. burgdorteri]) | Ip54 | 54 family | CE | + | 2.73 |
| BB0289 (flagellar assembly protein [fliH] [Borrelia burgdorferi]) | Chromosome | F | − | 2.64 | |
| BB0479 (ribosomal protein L4 [rplD] [B. burgdorferi]) | Chromosome | RP | − | 2.64 | |
| BB0298 (conserved hypothetical protein [B. burgdorferi]) | Chromosome | HX | + | 2.63 | |
| BB0290 (flagellar motor switch protein [fliG-2] [B. burgdorferi]) | Chromosome | 38 family | F | − | 2.61 |
| BB0364 (conserved hypothetical protein [Bacillus subtilis]) | Chromosome | HX | − | 2.56 | |
| BB0798 (competence protein F, putative [Haernophitus influenzae]) | Chromosome | X | + | 2.43 | |
| BB0291 (flagellar basal-body rod protein [fliF] [B. burgdorferi]) | Chromosome | F | − | 2.26 | |
| BB0450 (RNA polymerase sigma-54 factor [ntrA] [Azotobacter vinelandii]) | Chromosome | TR | − | 2.23 | |
| BB0652 (protein-export membrane protein [secD] [Escherichia coli]) | Chromosome | 136 family | PE | − | 2.18 |
| BBQ06 (conserved hypothetical protein [B. burgdorferi]) | Ip56 | 48 family | HX | − | 2.16 |
| BBG13 (hypothetical protein) | Ip28-2 | U | − | 2.14 | |
| BB0419 (response regulatory protein [rrp-1] [Synechocystis sp. strain PCC6803]) | Chromosome | 14 family | GM | − | 2.08 |
| BB0684 (carotenold biosynthesis protein, putative [Sulfolobus solfataricus]) | Chromosome | X | − | 2.02 | |
| BB0283 (flagellar hook protein [flgE] [B. burgdorferi]) | Chromosome | 78 family | F | − | 2.00 |
| BBJ51 (vtsE1 protein, authentic frameshift [vlsE1] [B. burgdorferi]) | Ip38 | 170 family | CE | + | 2.00 |
| BB0806 (hypothetical protein) | Chromosome | U | + | 1.99 | |
| BB0791 (thymidine kinase [tdk] [B. subtilis]) | Chromosome | NM | − | 1.96 | |
| BB0376 (S-adenosylmethionine synthetase [metK] [B. subtilis]) | Chromosome | IM | − | 1.94 | |
| BB0624 (hypothetical protein) | Chromosome | U | + | 1.94 | |
| BB0683 (3-hydroxy-3-methylglutaryl-CoA synthase [Arabidopsis thaliana]) | Chromosome | FM | − | 1.90 | |
| BB0393 (ribosomal protein L11 [rplK] [Thermotoga maritima]) | Chromosome | RP | − | 1.88 | |
| BBJ23 (hypothetical protein) | Ip38 | 106 family | U | + | 1.85 |
| BB0780 (ribosomal protein L27 [rpmA] [H. influenzae]) | Chromosome | RP | − | 1.83 | |
| BB0697 (conserved hypothetical protein [H. influenzae]) | Chromosome | HX | − | 1.77 | |
| BB0240 (glycerol uptake facilitator [glpF] [B. subtilis]) | Chromosome | TP | − | 1.75 | |
| BBG31 (conserved hypothetical protein [B. burgdorferi]) | Ip28-2 | 50 family | HX | − | 1.75 |
| BB0440 (ribosomal protein L34 [rpmH] [B. burgdorferi]) | Chromosome | RP | − | 1.72 | |
| BB0640 (spermidine/putrescine ABC transporter, permease protein [potC] [E. coli]) | Chromosome | 41 family | TP | − | 1.71 |
| BB0257 (cell division protein, putative [E. coli]) | Chromosome | D | − | 1.64 | |
| BB0559 (PTS system, glucose-specific IIA component [crr] [B. burgdorferi]) | Chromosome | TP | − | 1.63 | |
| BB0764 (sensory transduction histidine kinase, putative [B. subtilis]) | Chromosome | GM | − | 1.61 | |
| BB0449 (conserved hypothetical protein [E. coli]) | Chromosome | HX | − | 1.61 | |
| BBB19 (outer surface protein C (ospC) [B. burgdorferi]) | cp26 | CE | + | 1.61 | |
| BB0036 (DNA topoisomerase IV [parE] [B. burgdorferi]) | Chromosome | 30 family | R | − | 1.59 |
| BB0232 (hbbU protein [B. burgdorferi]) | Chromosome | PD | − | 1.58 | |
| BB0712 (RNA polymerase sigma-70 factor [rpoD] [B. burgdorferi]) | Chromosome | 89 family | TR | − | 1.58 |
| BBP34 (conserved hypothetical protein [B. burgdorferi]) | cp32-1 | 80 family | HX | − | 1.58 |
| BB0675 (hypothetical protein) | Chromosome | U | − | 1.56 | |
| BB0660 (GTP-binding protein [era] [E. coli]) | Chromosome | CH | − | 1.54 | |
| BB0538 (conserved hypothetical protein [B. burgdorferi]) | Chromosome | 125 family | HX | − | 1.52 |
| BB0680 (methyl-accepting chemotaxis protein [mcp-4] [E. coli]) | Chromosome | 13 family | CH | + | 1.51 |
| BBG24 (hypothetical protein) | Ip28-2 | 104 family | U | − | 1.50 |
| BBH36.2 (conserved hypothetical protein, pseudogene [B. burgdorferi]) | Ip28-3 | 102 family | HX | − | 1.50 |
ARS, amino acid biosynthesis; B, biosynthesis; CE, cell envelope; CH, chemotaxis proteins; D, division; F, flagellar biosynthesis; FM, fatty acid metabolism; GM, growth and metabolism; HE, hemolytic proteins; HS, heat shock proteins; HX, hypothetical conserved proteins; IM, intermediary metabolism; NM, nucleotide metabolism; PD, protein degradation; PE, protein export; PM, protein metabolism; R, replication; RP, ribosomal proteins; TF, translation factors; TP, transporter proteins; TR, transcriptional regulation proteins; U, hypothetical proteins; X, other.
TABLE 3.
ORFs down-regulated during DMC cultivation
| ORF designation (description) | Replicon | Family | Cate- gorya | Signal peptide | Fold down- regulation |
|---|---|---|---|---|---|
| BBJ09 (outer surface protein D [ospD] [B. burgdorferi]) | lp38 | CE | + | −18.77 | |
| BBH16 (hypothetical protein) | lp28-3 | U | + | −12.81 | |
| BB102 (conserved hypothetical protein [B. burgdorferi]) | lp28-4 | 84 family | HX | − | −11.78 |
| BBA62 (lipoprotein [B. burgdorferi]) | lp54 | CE | + | −11.73 | |
| BBI39 (hypothetical protein) | lp28-4 | 54 family | U | + | −11.56 |
| BBD18 (hypothetical protein [B. burgdorferi]) | lp17 | U | − | −11.17 | |
| BBJ41 (antigen, P35, putative [B. burgdorferi]) | lp38 | 54 family | CE | + | −10.80 |
| BBI36 (antigen, P35, putative [B. burgdorferi]) | lp28-4 | 54 family | CE | + | −9.53 |
| BBA16 (outer surface protein B [ospB] [B. burgdorferi]) | lp54 | 53 family | CE | + | −8.66 |
| BBI38 (hypothetical protein) | lp28-4 | 54 family | U | + | −7.54 |
| BBA69 (hypothetical protein) | lp54 | 54 family | U | + | −6.65 |
| BBA68 (hypothetical protein) | lp54 | 54 family | U | + | −6.57 |
| BBH29 (conserved hypothetical protein [B. burgdorferi]) | lp28-3 | 49 family | HX | − | −5.90 |
| BBA15 (outer surface protein A [ospA] [B. burgdorferi]) | lp54 | 53 family | CE | + | −5.85 |
| BBA74 (outer membrane porin [oms28] [B. burgdorferi]) | lp54 | 171 family | CE | + | −5.79 |
| BBK45 (immunogenic protein P37, putative [B. burgdorferi]) | lp36 | 75 family | CE | + | −5.68 |
| BBA38 (hypothetical protein) | lp54 | 146 family | U | − | −5.40 |
| BBA61 (conserved hypothetical protein [Borrelia garinii]) | lp54 | HX | − | −5.18 | |
| BBA59 (lipoprotein [B. burgdorferi]) | lp54 | CE | + | −5.16 | |
| BB0152 (glucosamine-6-phosphate isomerase [nagB] [Haemophilus influenzae]) | Chromosome | IM | − | −4.68 | |
| BBA40 (hypothetical protein) | lp54 | 148 family | U | − | −4.54 |
| BBA39 (hypothetical protein) | lp54 | 147 family | U | − | −4.16 |
| BBQ15 (conserved hypothetical protein [B. burgdorferi]) | lp56 | 107 family | HX | − | −4.13 |
| BBR28 (lipoprotein [lp] [B. burgdorferi]) | cp32-4 | 113 family | HX | + | −3.90 |
| BBF06 (conserved hypothetical protein [B. burgdorferi]) | lp28-1 | 57 family | HX | − | −3.75 |
| BBJ19 (conserved hypothetical protein [B. burgdorferi]) | lp38 | 62 family | HX | − | −3.74 |
| BBL29 (conserved hypothetical protein [B. burgdorferi]) | cp32-8 | 161 family | HX | − | −3.72 |
| BBK13 (conserved hypothetical protein [Synechocystis sp. strain PCC6803]) | lp36 | 40 family | HX | + | −3.70 |
| BBP29 (conserved hypothetical protein [B. burgdorferi]) | cp32-1 | 161 family | HX | − | −3.68 |
| BBK39 (hypothetical protein) | lp36 | 59 family | U | − | −3.42 |
| BBP22 (conserved hypothetical protein [B. burgdorferi]) | cp32-1 | 142 family | HX | − | −3.26 |
| BBE19 (plasmid partition protein, putative [Bacillus subtilis]) | lp25 | 32 family | HX | − | −3.16 |
| BBN27 (conserved hypothetical protein [B. burgdorferi]) | cp32-9 | 80 family | HX | − | −3.12 |
| BBA56 (hypothetical protein) | lp54 | 160 family | U | − | −3.08 |
| BBH15 (hypothetical protein) | lp28-3 | U | − | −2.87 | |
| BBR27 (conserved hypothetical protein [B. burgdorferi]) | cp32-4 | 80 family | HX | − | −2.84 |
| BBA52 (outer membrane protein [B. burgdorferi]) | lp54 | CE | + | −2.78 | |
| BBN22 (conserved hypothetical protein, authentic frameshift [B. burgdorferi]) | cp32-9 | 142 family | HX | − | −2.72 |
| BBL28 (lipoprotein [B. burgdorferi]) | cp32-8 | 113 family | CE | + | −2.62 |
| BBR31 (conserved hypothetical protein [B. burgdorferi]) | cp32-4 | 57 family | HX | − | −2.60 |
| BBS31 (conserved hypothetical protein [B. burgdorferi]) | cp32-3 | 161 family | HX | − | −2.59 |
| BBO27 (conserved hypothetical protein [B. burgdorferi]) | cp32-7 | 80 family | HX | − | −2.54 |
| BBF09 (hypothetical protein, paralogous family 71, authentic frameshift) | lp28-1 | 71 family | U | − | −2.40 |
| BBK07 (hypothetical protein) | lp36 | 59 family | U | + | −2.40 |
| BBP30 (conserved hypothetical protein [B. burgdorferi]) | cp32-1 | 57 family | HX | − | −2.33 |
| BBJ31 (hypothetical protein) | lp38 | 59 family | U | − | −2.23 |
| BBA55 (hypothetical protein) | lp54 | 159 family | U | − | −2.18 |
| BBA73 (antigen, P35, putative [B. burgdorferi]) | lp54 | 54 family | CE | + | −2.14 |
| BBJ08 (hypothetical protein) | lp38 | 12 family | U | + | −2.09 |
| BB0248 (oligoendopeptidase F [pepF] [Lactococcus lactis]) | Chromosome | PD | − | −2.08 | |
| BBS25 (conserved hypothetical protein [B. burgdorferi]) | cp32-3 | 112 family | HX | − | −2.05 |
| BBE23.2 (hypothetical protein, authentic point mutation [B. burgdorferi]) | lp25 | 32 family | U | − | −2.05 |
| BBR34 (conserved hypothetical protein [B. burgdorferi]) | cp32-4 | 49 family | HX | − | −2.05 |
| BBN21 (hypothetical protein, paralogous family 141, authentic frameshift [B. burgdorferi]) | cp32-9 | 141 family | U | − | −2.04 |
| BBJ36 (hypothetical protein) | lp38 | 92 family | U | + | −1.99 |
| BBP28 (lipoprotein [B. burgdorferi]) | cp32-1 | 113 family | CE | + | −1.94 |
| BBL15 (hypothetical protein) | cp32-8 | 156 family | U | − | −1.91 |
| BBN29 (hypothetical protein, paralogous family 161, authentic point mutation [B. burgdorferi]) | cp32-9 | 161 family | U | − | −1.82 |
| BBF26.1 (conserved hypothetical protein, pseudogene [B. burgdorferi]) | lp28-1 | 101 family | HX | − | −1.81 |
| BB0757 (ATP-dependent Clp protease proteolytic component [clpP-2] [H. influenzae]) | Chromosome | 3 family | PD | − | −1.78 |
| BBN04 (hypothetical protein) | cp32-9 | 148 family | U | − | −1.78 |
| BBO25 (conserved hypothetical protein [B. burgdorferi]) | cp32-7 | 112 family | HX | − | −1.77 |
| BBR04 (hypothetical protein) | cp32-4 | 148 family | U | − | −1.77 |
| BBP13 (hypothetical protein) | cp32-1 | 154 family | U | − | −1.75 |
| BBR25 (conserved hypothetical protein [B. burgdorferi]) | cp32-4 | 112 family | HX | − | −1.61 |
| BBQ32 (conserved hypothetical protein [B. burgdorferi]) | lp56 | 112 family | HX | − | −1.55 |
| BBS09 (conserved hypothetical protein [B. burgdorferi]) | cp32-3 | 108 family | HX | − | −1.55 |
ARS, amino acid biosynthesis; B, biosynthesis; CE, cell envelope; CH, chemotaxis proteins; D, division; F, flagellar biosynthesis; FM, fatty acid metabolism; GM, growth and metabolism; HE, hemolytic proteins; HS, heat shock proteins; HX, hypothetical conserved proteins; IM, intermediary metabolism; NM, nucleotide metabolism; PD, protein degradation; PE, protein export; PM, protein metabolism; R, replication: RP, ribosomal proteins; TF, translation factors; TP, transporter proteins; TR, transcriptional regulation proteins; U, hypothetical proteins; X, other.
Validation of microarray data by real-time PCR.
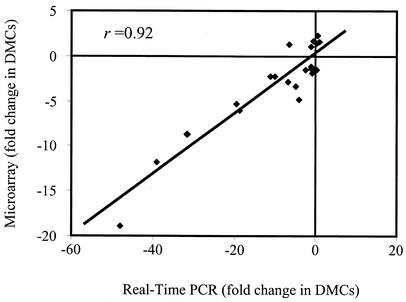
To verify that the microarray data generated in these studies could be supported by a separate experimental methodology, QRT-PCR was employed. A total of 25 genes were selected for QRT-PCR for comparison to the microarray data (Table 1), which included the well-characterized ospA, ospB, ospC, ospD, and flaB genes. As shown in Fig. 2, when the nontransformed, raw data were subjected to correlation analysis, the QRT-PCR and microarray data were found to have a correlation coefficient of r = 0.92, which strongly supported the independently generated microarray data.
FIG. 2.
Comparative analysis of QRT-PCR and microarray data. Twenty ORFs were selected at random for QRT-PCR for comparison with microarray data. Additionally, ospA, ospB, ospC, ospD, and flaB were included in the analysis. All QRT-PCRs were performed in triplicate to determine the average ΔCt for subsequent statistical analysis, which resulted in a correlation coefficient of r = 0.92.
Paralogous gene families and cross-hybridization.
A major caveat with DNA microarrays is that cross-hybridization between paralogous genes can lead to the erroneous identification of differentially expressed genes. This occurs when a gene that is not differentially expressed cross-hybridizes with transcripts from a gene that is differentially expressed under the conditions analyzed. Given the large amount of gene redundancy in the borrelial genome (6, 19, 40), it was important to determine at the outset which of the 125 significant ORFs may have been identified due to cross-hybridization with a highly similar gene or genes. As listed in Tables 2 and 3, 79 ORFs were found to belong to B. burgdorferi paralogous gene families. A detailed examination of the significant ORFs revealed that 51 of the 79 ORFs were <80% identical to their most similar paralog. Given that genes with <80% identity should not cross-hybridize under the stringent probing and washing conditions utilized (42), this would leave only 28 ORFs that could have been the result of possible cross-hybridization. Evidence that ORFs with <80% identity did not cross-hybridize also was generated experimentally. For example, paralogous family 12 contains five ORFs (bb0844, bbg01, bbk01, bbj08, and bbh37) that are between 43 and 81% identical, and significantly different patterns of expression could be identified among these paralogs. In this regard, bb0844 was found to be significantly up-regulated 3.02-fold, while bbj08 was found to be significantly down-regulated 2.09-fold during DMC cultivation. Additionally, bbj08 is 81% identical to bbh37, but bbh37 was not differentially expressed in the DMC environment. The combined findings are consistent with the notion that genes must be >80% identical for cross-hybridization to occur at a significant level. Of the remaining 28 paralogs that contained >80% identity, all were from 10 different gene families (paralogous families 54, 57, 59, 80, 112, 113, 117, 142, 148, and 161). As would be expected (5, 6), three-fourths of these (21 of 28) were encoded on the highly homologous 32-kb circular plasmids (cp32s) or the closely related 56-kb linear plasmid: lp56 is thought to have been generated by a prior recombination event between a linear plasmid and a cp32 (6). The remaining seven ORFs were encoded on lp28-1, lp28-2, lp28-4, lp38, or lp36. Future quantitative reverse transcription-PCR experiments will be required to sort out which of the remaining 28 paralogs are actually differentially expressed. In any event, this analysis suggests that a minimum of 107 ORFs (if only one paralog from each of the 10 families identified is differentially expressed) and a maximum of 125 ORFs (if all paralogs from all families are differentially expressed) are significantly up- or down-regulated during DMC cultivation.
Genomic location of ORFs responsive to mammalian host factors.
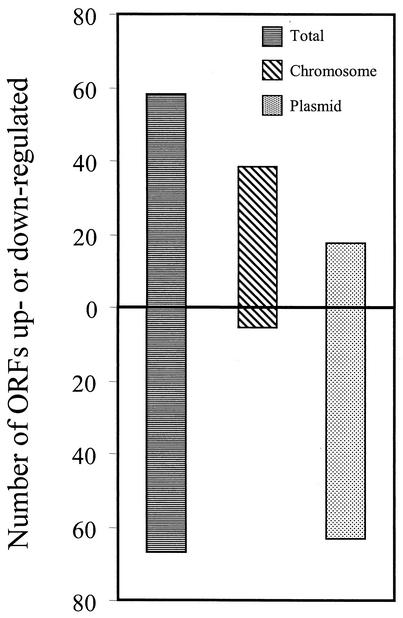
The B. burgdorferi linear chromosome consists of approximately 1 Mb of genetic information, which accounts for about two-thirds of the borrelial genome, however, only 35% (44) of the genes identified as being differentially regulated by mammalian host factors were chromosomally encoded. Thus, the overwhelming majority of the genes identified were encoded on the various borrelial plasmids. Interestingly, there was an obvious dichotomy in the overall direction of differential expression between chromosome- and plasmid-encoded genes, with 93% (41 of 44) of the differentially expressed genes encoded by the chromosome being significantly up-regulated, while 79% (64 of 81) of the plasmid-encoded genes were identified as significantly down-regulated (Fig. 3) during DMC cultivation.
FIG. 3.
Dichotomy between chromosomally and plasmid-encoded ORFs in their direction of differential expression. Of the 125 ORFs found to be differentially expressed within rat DMCs, 58 were up-regulated, and 67 were down-regulated. A total of 44 are encoded on the chromosome, 41 of which are up-regulated (93%). The plasmids contained 81 differentially expressed ORFs, 61 of which are down-regulated (79%).
Differentially expressed ORFs containing leader peptides.
The inverse relationship in expression patterns observed between the chromosomally and plasmid-encoded genes also was found with regard to genes encoding proteins containing known or putative leader peptides. This is a key group of molecules, because it includes potentially surface-exposed outer membrane proteins, which likely are important in host-pathogen interactions during disease establishment and throughout chronic infection. Of the 125 differentially expressed ORFs identified, 34 (27%) encode proteins with either signal peptidase I (SPI) or SPII cleavage sites (Tables 2 and 3). Seven are encoded on the chromosome, with all being up-regulated, while the other 27 are plasmid encoded, and almost all are down-regulated (23 of 27 [85%]). Among the down-regulated, plasmid-encoded ORFs encoding leader peptides, ospD was identified as being the most down-regulated (∼19-fold). Additionally, as would be expected from prior studies, ospA, ospB, and lp6.6 (bba62) were dramatically down-regulated in the mammalian host environment (1, 28). Besides these well-characterized lipoproteins, there were four other ORFs encoding putative lipoproteins significantly down-regulated during DMC culture, including three (bbr28, bbl28, and bbp28) that corresponded to members of the mlp lipoprotein gene family (57). Also down-regulated were two ORFs encoding genes that code for membrane-spanning proteins, oms28 (bba74) (50) and bba52, which were down-regulated approximately six- and threefold, respectively. The combined data suggest that there is an overall reduction in expression of surface-exposed molecules during DMC cultivation, which is consistent with a recent report showing that mammalian host-adapted B. burgdorferi down-regulates a majority of its lipoproteins during murine infection (31).
Distribution of plasmid-borne ORFs regulated by host factors.
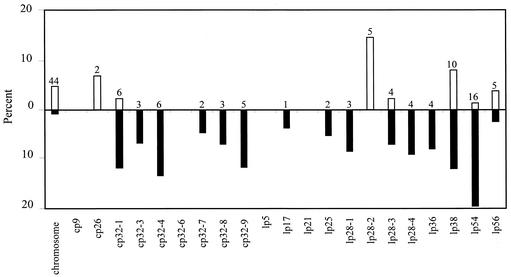
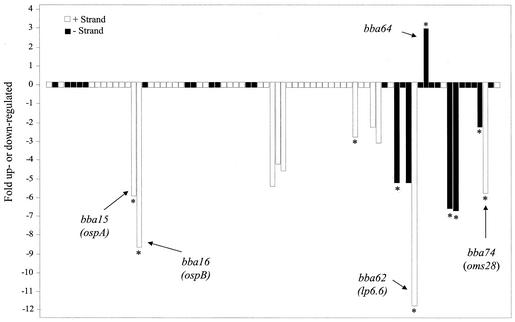
As shown in Fig. 4, 81 plasmid-encoded genes were found to be differentially regulated by host factors, with 30 (37%) being encoded either on one of six different cp32s or the related lp56. Consistent with the expression patterns noted above, almost all were significantly down-regulated (26 of 30). Conversely, of the 16 differentially expressed ORFs found on the multiple lp28s, 6 were observed to be up-regulated in the mammalian host, including all 5 of the differentially expressed ORFs encoded by lp28-2 (bbg13, bbg17, bbg19, bbg24, and bbg31). Of the 16 ORFs differentially expressed on the lp28s, none have been ascribed a functional role, although one is categorized as a cell envelope constituent (bbi36). With regard to the other plasmid elements, lp54 contained 16 differentially expressed ORFs, the most of any plasmid, with all but 1, bba64, being down-regulated in the mammalian host environment (Fig. 5). lp54 also contained the most differentially expressed ORFs encoding putative leader peptides (n = 10). Although most plasmids contained at least one differentially expressed ORF, lp5, lp21, cp9, and cp32-6 were not observed to contain any ORFs significantly regulated by host factors during DMC cultivation (Fig. 4).
FIG. 4.
Genomic distribution of ORFs differentially expressed during DMC cultivation. The total percentage of ORFs encoded by each genetic element found to be up- or down-regulated is shown. The percent up-regulated is indicated above the line (open bars), and the percent down-regulated is displayed below the line (solid bars). The total number of ORFs differentially expressed on each genetic element is noted above each bar.
FIG. 5.
Differentially expressed ORFs encoded by lp54. All 76 ORFs encoded by lp54 are displayed as boxes with bars extending up or down from specific ORFs indicating the fold up- or down-regulated during DMC cultivation. Solid and open boxes indicate gene orientations, and asterisks denote ORFs with leader peptides. Five genes discussed in the text are also labeled.
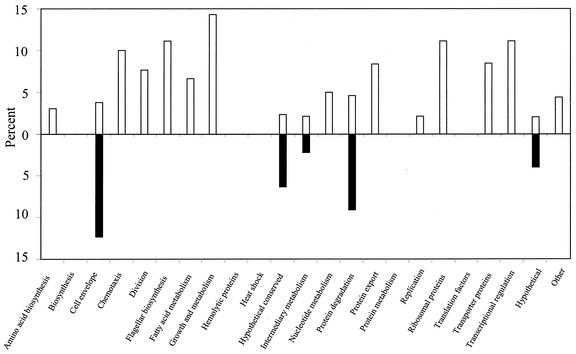
Distribution of ORFs by functional category.
As displayed in Fig. 6, among the 23 different functional categories listed for borrelial genes (19), only the hypothetical (U), hypothetical conserved (HX), cell envelope (CE), intermediary metabolism (IM), and protein degradation (PD) categories contained ORFs significantly down-regulated during DMC cultivation. Among the 67 down-regulated ORFs, only three—one from the IM category (bb0152) and two from the PD category (bb0248, and bb0757)—were not genes encoding hypothetical proteins (51 of 67 [76%]) or cell envelope constituents (13 of 67 [19%]). On the other hand, of the 58 ORFs found to be up-regulated, only 23 (40%) were grouped into genes of unknown function (U or HX), while the remaining 35 fell into 16 different functional categories. Given that a majority of the borrelial ORFs encode proteins with unknown function, it was not surprising that a majority of differentially expressed genes identified also were of unknown function and fell into the U and HX categories (74 of 125 [59%]).
FIG. 6.
ORFs differentially expressed during DMC cultivation separated by functional category. The total percentage of ORFs in each of the 23 different functional categories found to be up- or down-regulated is shown. The percent up-regulated is indicated above the line (open bars), and the percent down-regulated is displayed below the line (solid bars).
Among the currently categorized CE constituents, 17 were found to be differentially regulated by mammalian host factors (Tables 2 and 3). The number of down-regulated ORFs categorized into the CE category exceeded those found to be up-regulated by greater than threefold, (13 down-regulated and 4 up-regulated). As noted above, these included ospA, ospB, lp6.6 (bba62), and ospD. Additionally, two p35 paralogs (bbj41 and bba73) and two p37 paralogs (bbi36 and bbk45) (17); the outer membrane protein gene designated oms28 (50); bba52, which encodes a putative outer membrane protein, as annotated by the TIGR database (19); and three lipoprotein genes (bba59, bbl28, and bbp28) were among the CE constituents significantly down-regulated. Of the four up-regulated CE ORFs, only the gene encoding the membrane-associated protein p66 (Bb0603) was found to be chromosomally encoded (4, 51), the other three were plasmid encoded, including ospC (bbb19), as expected (1); bbj51 encoding a vlsE1 lipoprotein paralog (59); and a p35 paralog (bba64).
Examination of upstream regions from differentially expressed ORFs.
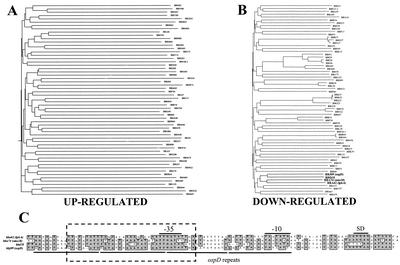
We next determined if potential cis-acting promoter/regulatory elements could be identified among the genes differentially expressed during DMC cultivation. We started this analysis by comparing the first 100 bp upstream of all identified up- or down-regulated ORFs. As shown in Fig. 7A, the homology phenogram generated from the up-regulated ORFs resulted in a tree with very deep branches, indicating none of the sequences are closely related. Consistent with this observation, no obvious sequence motifs were identified when a direct comparison of individual upstream sequences was performed. In contrast, the homology phenogram generated from the down-regulated ORFs revealed much shorter branch lengths, with several upstream sequences being grouped closely together (Fig. 7B). Inspection of the most closely related clusters revealed that many were upstream of ORFs in paralogous gene families that shared ≥90% sequence identity. However, one closely related branch consisting of four ORFs from different paralogous gene families was identified: bba62 (lp6.6), bba74 (oms28), bbd18, and bbj09 (ospD). As shown in Fig. 7C, when a multiple sequence alignment of these sequences was performed, there appeared to be a highly conserved 35-bp region shared between all four ORFs (dashed box), which also contained the putative −35 hexamer. This homologous region also encompasses a smaller 17-bp motif that is found four times in the 100 bp of ospD upstream sequence analyzed (underlined regions). Interestingly, while the upstream 100 bp of these genes were found to share an average of 61% identity, their respective structural genes averaged only 27% identity. The sequence conservation observed in these upstream sequences would suggest that there is selective pressure for the coexpression of these genes during the borrelial enzootic cycle.
FIG. 7.
Phenogram analysis of upstream regions from ORFs differentially expressed during DMC cultivation and multiple sequence alignment of selected promoters. (A and B) Phenogram analysis of the 100-bp regions just upstream of ORFs found to be up-regulated (A) or down-regulated (B). Four unrelated ORFs that were down-regulated and contained promoters that clustered together in panel B are indicated in boldface. Phenogram branch lengths are inversely proportional to the overall sequence identity between promoters. (C) Upstream sequences shown in boldface from panel B were subjected to a ClustalW multiple sequence alignment. The dashed rectangle encompasses the most highly conserved sequences that were shared by all four promoters. Solid lines below the lineup indicate the tandem repeats identified upstream of ospD. The Shine-Dalgarno ribosomal binding site (SD) and the −10 and −35 hexamers for RNA polymerase binding are indicated above the lineup.
DISCUSSION
It has been suggested that identification of genes and proteins differentially expressed by microorganisms during mammalian infection will help to elucidate the parasitic strategies used by bacterial pathogens during human disease (32). Given that many studies over the past decade have shown B. burgdorferi alters the expression of numerous molecules during mammalian infection (48), we used this spirochete as a model system for identifying genes differentially regulated in the mammalian host. By taking advantage of the rat DMC model (1), we generated mammalian host-adapted B. burgdorferi and identified a subset of genes differentially expressed in response to mammalian host signals. While we recognize that the DMC implant model has limitations, it is important to point out that the combined data strongly support our contention that genes differentially expressed by mammalian host-specific signals were preferentially identified in this study. For example, ospA and lp6.6 transcription was significantly down-regulated, and ospC transcription was significantly up-regulated, clarifying the roles of three genes previously reported to be modulated by mammalian signals (1, 23, 28, 47). Furthermore, bba64 also was found to be differentially expressed during DMC cultivation, which is a gene known to respond to environmental cues and was recently shown by Gilmore and coworkers to be expressed during mammalian infection, but not in the tick environment (20, 25). In fact, bba64 was the only gene out of 16 identified on lp54 that was significantly up-regulated. We also should note that every attempt was made to control for growth phase-related changes in gene expression. However, we cannot entirely rule out the possibility that some genes differentially expressed during DMC cultivation were identified because of differences in the growth media or conditions found between DMCs and in vitro cultures. Differences in the overall makeup of the growth media may help to explain why several ORFs related to metabolic processes, translation, motility, and cell division were identified in the microarray analysis. Although it is likely that many poorly expressed genes were missed by the global microarray screening strategy utilized, this study has provided a subset of genes that can now be inactivated by genetic methods and complemented with stable plasmid vectors in future studies to help elucidate their role or roles in the borrelial enzootic cycle and Lyme disease pathogenesis.
The array experiments revealed a dichotomy in the overall direction of expression between genes harbored on the chromosome and those harbored on plasmids. While almost all of the up-regulated ORFs were chromosomally harbored, the vast majority of down-regulated genes were harbored by the plasmids. Although this may seem to contradict previous findings suggesting that the plasmids carry proteins that play an important role in Lyme disease pathogenesis, we should note that the experimental design employed here attempted to exclude genes regulated solely by temperature. Therefore, plasmid-harbored genes that were up-regulated by both temperature shift and DMC cultivation would not have been identified, although they would be differentially expressed in relation to a 23°C environment (i.e., the tick midgut). In fact, many of the genes up-regulated by temperature also were found to be up-regulated by DMC cultivation in a similar fashion. In contrast, a subset of plasmid-encoded ORFs was found to be down-regulated during DMC cultivation in relation to temperature shift, including several ORFs that encode cell envelope proteins. This finding is consistent with the recent observation by Liang and coworkers that a majority of the borrelial surface lipoproteins are down-regulated in the mouse model of Lyme disease during chronic infection (31). While almost all of the cell envelope constituents identified were observed to be down-regulated, there was a small number of known or putative outer membrane proteins up-regulated during DMC cultivation, including p66, bba64, ospC, and a vlsE1 paralog (bbj51). Among these, it appears that the vlsE1 paralog contains a functional promoter and is transcribed, but it is highly unlikely that it is translated and exported to the borrelial surface, since it contains numerous nonsense mutations and is annotated as a nonfunctional pseudogene (19). The finding that ospC and bba64 are up-regulated during DMC cultivation is not surprising and is consistent with prior reports that these genes are differentially expressed in the mammalian host (20, 23). However, it was interesting that the outer membrane protein p66 was up-regulated during DMC cultivation. Since p66 has been shown to bind β3-chain integrins (8), which is thought to help B. burgdorferi bind platelets and megakaryocytes in the mammalian host (13), it is tempting to speculate that this protein is specifically up-regulated to aid in cell binding and dissemination of B. burgdorferi within the mammalian host during infection. This hypothesis could be tested by deleting or mutating the p66 gene in a virulent strain of B. burgdorferi and determining if this alters borrelial transmission or dissemination within the mammalian host. Experiments of this nature are now feasible, given recent advances that have made it possible to genetically manipulate at least some virulent strains of B. burgdorferi (15, 16, 24, 30).
Eleven of the 25 most down-regulated genes identified were encoded by lp54. Therefore, we hypothesized that there may have been prior recombination events within this genetic element that resulted in several lp54 genes sharing similar upstream promoter regions, which could all be regulated by a single repressor protein. While the promoter analysis did not reveal a global conservation in the upstream sequences of genes located on lp54, the promoter regions of bba62 (lp6.6) and bba74 (oms28) were found to share a 35-bp region with 60% sequence identity. Similar sequences also were found upstream of ospD (66% identity) and bbd18 (69% identity), which clustered with the bba62 and bba74 promoters in the phenogram analysis. The conserved region for all four genes was found to overlap the −35 hexamer, which is a cis-acting element known to play a key role in RNA polymerase binding. Within this 35-bp region, there also was a smaller 17-bp motif that was highly conserved between all four promoters. Interestingly, ospD, the most highly down-regulated gene identified (−18.8 fold), contained four copies of this motif within the upstream 100 bp analyzed in this study. A prior analysis of the ospD upstream region has shown that the motif identified in our analysis is actually present a total of seven times in the upstream 200-bp region of ospD (34, 36). Given the overall magnitude of the down-regulation of these four genes, combined with the conserved sequences and motifs found in all four of these promoters, it is tempting to speculate that the same trans-acting repressor protein may bind these promoters during DMC cultivation and regulate transcription. If the putative repressor binds all or a part of the 17-bp motif, it would be consistent with ospD being the most down-regulated, since it contains seven different regions that could be bound by the repressor. Experiments are currently being performed to help identify and characterize the putative repressor that regulates these genes.
The focus of the present study was to determine which ORFs are differentially expressed in the mammalian host environment (i.e., during cultivation within DMCs). At the same time, however, we realize that mammalian infection is only one part of the complex borrelial enzootic cycle and that proteins differentially expressed during transmission of B. burgdorferi from tick to mammal also are of importance. To identify ORFs important during the transmission phase of the life cycle, we recently performed a microarray study similar to the one described here. In this study, we compared the global gene expression patterns of organisms cultivated at 23°C with those of organisms temperature shifted (38), which resulted in the identification of 215 ORFs differentially expressed by temperature. A comparison of the ORFs previously shown to be regulated by temperature (38) with the ORFs identified here revealed four distinct categories of differentially expressed genes. The first category includes 95 different ORFs that are not affected by temperature (38) but are differentially expressed during DMC cultivation. Genes that fall into this category include p66, ospA, ospB, and lp6.6, as well as numerous genes encoding proteins of unknown function. The second category includes 10 genes previously identified as being up-regulated by temperature but down-regulated during DMC cultivation. This category includes outer membrane protein gene oms28 (50), a p35 paralog (bba73), and eight hypothetical genes (bba61, bbs31, bbq32, bbo25, bbr25, bbs25, bbk07, and bba69). The third distinct category contained only three genes, which are all down-regulated by temperature but up-regulated during DMC cultivation. This category includes glpF (glycerol uptake facilitator [bb0240]), crr (a glucose-specific phosphotransferase system component [bb0559]), and a hypothetical gene (bb0364). The final category comprises 17 genes that are up- or down-regulated by temperature (38) and are further up- or down-regulated during cultivation in DMCs. The genes that are synergistically up-regulated by both temperature and mammalian host factors include ospC, bba64, bbb06, and bb0376, while the synergistically down-regulated genes encode OspD, a p35 paralog (BBJ41), a putative outer membrane protein (BBA52), and 10 different hypothetical proteins. Although a majority of the genes previously found to be regulated by temperature were not identified here as being differentially expressed by mammalian host factors (76%), this was not surprising and strongly suggests that the experimental strategy we employed did exclude genes regulated only by temperature as originally intended.
Recently, a B. burgdorferi microarray study was performed by Revel et al. (41). However, it is difficult to compare our data with those reported in the prior study for several reasons. First, the prior array study used a nonclonal isolate of B. burgdorferi, which likely confounded final data interpretation due to the fact that genotypic and phenotypic variation is inherent in uncloned borrelial cultures. Second, and possibly most important, in an attempt to mimic the in vivo conditions of the tick midgut, multiple variables were changed at the same time in the prior study (i.e., temperature and pH). Therefore, it is difficult to discern which genes were regulated by mammalian host signals, which were regulated by pH, and which responded to both variables. Given the differences in experimental design, it is not surprising that <10% of the ORFs identified here as being regulated by mammalian host-specific factors also were identified by Revel et al. (41). It also must be noted that the overall concordance between the real-time and array data in this prior report was likely overestimated. This is because the real-time PCR and array data were log transformed before performing statistical comparisons of the data, which is not an accepted practice due to the possibility of drawing correlations from discordant sets of data (11, 58). When one reexamines the log-transformed and raw data from the prior analysis, the correlation coefficient is decreased from the reported value of r = 0.88 (log-transformed data analysis) to r = 0.56 (raw data analysis). Therefore, it appears that there was actually very little correlation between the microarray and real-time PCR analyses in the prior study. This likely was one of the major contributors to the discrepancies observed between our data and the prior report.
Fikrig and coworkers recently examined the differential expression of borrelial genes in engorging Ixodes scapularis ticks (35). This was accomplished by screening a cDNA library generated from B. burgdorferi strain N40 with mRNA extracted from organisms before and after a blood meal. A comparison of our data with the prior study indicated that several genes were identified as being up-regulated in both studies, which included bb0240, p66 (bb0603), and possibly bbg24 and bb0376. The latter two genes were not fully characterized in the prior study, but were identified on two differentially expressed cDNA clones encompassing ORFs bbg24 to bbg27 and bb0374 to bb0377. The similarities in gene expression found between the two data sets would be most consistent with a mammalian host factor or factors interacting with spirochetes in both the tick midgut during a blood meal and within the DMC environment. At the same time, however, there were several ORFs differentially expressed by B. burgdorferi in the midgut of engorged ticks that we did not identify during DMC cultivation. These variations could have been the result of (i) the different B. burgdorferi strains utilized in our study and the prior study (B31 and N40, respectively); and (ii) the different model systems employed. With regard to the latter point, many of the ORFs identified as differentially expressed during tick engorgement could be regulated by cues other than mammalian host factors, such as temperature, pH, or other unidentified tick molecules that would not be present in the DMC environment.
In summary, the global transcriptional profile we observed in this study for DMC-cultivated organisms revealed that numerous genes are differentially expressed by factors present only in the mammalian host. While the majority of the ORFs identified are annotated as encoding unknown or hypothetical proteins, there were many that have been grouped into functional categories on the basis of sequence similarities or known function. Excluding genes of unknown function, the functional category of genes encoding cell envelope constituents and outer membrane proteins contained the most differentially expressed ORFs. This finding is consistent with previous reports indicating that B. burgdorferi dramatically alters its surface components as it is transmitted from the tick to mammalian host. Interestingly, many of the genes encoding surface-exposed proteins were down-regulated. This also is consistent with previous findings suggesting that specific humoral or cell-mediated immune responses in the host can alter expression of specific borrelial proteins during infection (31). Our study not only has underscored this prior observation but also suggests that factors other than a directed immune response can mediate differential gene expression during infection. This is supported by the finding that differential expression of surface proteins occurred within the DMC environment, which has a molecular mass cutoff of 8,000 Da and precludes antibody from diffusing into the chamber and interacting with spirochetes. The results presented here have laid the foundation for identifying borrelial proteins that may be important in the regulation of genes during mammalian infection, which should increase our overall understanding of the strategies used by this spirochete during human infection. Finally, we identified many genes encoding proteins that are specifically up-regulated during cultivation in the mammalian host. With the rapid progress made in recent years involving genetic manipulation of B. burgdorferi (15, 24, 43, 45, 54), the roles played by these genes in B. burgdorferi virulence and Lyme disease pathogenesis can now be addressed in future studies.
Acknowledgments
This work was supported in part by grants 0030130N from the American Heart Association and RR-15564 from the National Institutes of Health to D.R.A. C.S.B. and P.S.H. were supported in part by Molecular Pathogenesis Training grant AI-07364 from NIAID.
We thank Amy Jett for expert technical assistance and Dave Dyer and Juneann Murphy for many helpful discussions. We also thank Tyrrell Conway for advice and help with statistical analysis of array data and Michael Gilmore for assistance with real-time PCR.
Editor: A. D. O'Brien
REFERENCES
- 1.Akins, D. R., K. W. Bourell, M. J. Caimano, M. V. Norgard, and J. D. Radolf. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, D. R., S. F. Porcella, T. G. Popova, D. Shevchenko, S. I. Baker, M. Li, M. V. Norgard, and J. D. Radolf. 1995. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homolog. Mol. Microbiol. 18:507-520. [DOI] [PubMed] [Google Scholar]
- 3.Bundoc, V. G., and A. G. Barbour. 1989. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect. Immun. 57:2733-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunikis, J., and A. G. Barbour. 1999. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect. Immun. 67:2874-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caimano, M. J., X. Yang, T. G. Popova, M. L. Clawson, D. R. Akins, M. V. Norgard, and J. D. Radolf. 2000. Molecular and evolutionary characterization of the cp32/18 family of supercoiled plasmids in Borrelia burgdorferi 297. Infect. Immun. 68:1574-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 7.Champion, C. I., D. R. Blanco, J. T. Skare, D. A. Haake, M. Giladi, D. Foley, J. N. Miller, and M. A. Lovett. 1994. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect. Immun. 62:2653-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coburn, J., W. Chege, L. Magoun, S. C. Bodary, and J. M. Leong. 1999. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol. Microbiol. 34:926-940. [DOI] [PubMed] [Google Scholar]
- 9.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 10.Conway, T., B. Kraus, D. L. Tucker, D. J. Smalley, A. F. Dorman, and L. McKibben. 2002. DNA array analysis in a Microsoft Windows environment. BioTechniques 32:110-119. [DOI] [PubMed] [Google Scholar]
- 11.Daniel, W. W. 1999. Biostatistics: a foundation for analysis in the health sciences. John Wiley and Sons, Inc., New York, N.Y.
- 12.Das, S., S. W. Barthold, S. S. Giles, R. R. Montgomery, S. R. Telford, and E. Fikrig. 1997. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and mammalian host. J. Clin. Investig. 99:987-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defoe, G., and J. Coburn. 2001. Delineation of Borrelia burgdorferi p66 sequences required for integrin αIIbβ3 recognition. Infect. Immun. 69:3455-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Silva, A. M., E. Fikrig, E. Hodzie, F. S. Kantor, S. R. Telford III, and S. W. Barthold. 1998. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J. Infect. Dis. 177:395-400. [DOI] [PubMed] [Google Scholar]
- 15.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 16.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 70:2139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fikrig, E., S. W. Barthold, W. Sun, W. Feng, S. R. Telford, and R. A. Flavell. 1997. Borrelia burgdorferi p35 and p37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531-539. [DOI] [PubMed] [Google Scholar]
- 18.Fikrig, E., M. Chen, S. W. Barthold, J. Anguita, W. Feng, S. R. Telford III, and R. A. Flavell. 1999. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31:281-290. [DOI] [PubMed] [Google Scholar]
- 19.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore, R. D., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 21.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, and D. R. Akins. 2002. Changes in temporal and spatial patterns of outer surface lipoprotein expression generate population heterogeneity and antigenic diversity in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 70:3468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hefty, P. S., S. E. Jolliff, M. J. Caimano, S. K. Wikel, J. D. Radolf, and D. R. Akins. 2001. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect. Immun. 69:3618-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Indest, K. J., R. Ramamoorthy, M. Sole, R. D. Gilmore, B. J. B. Johnson, and M. T. Philipp. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect. Immun. 65:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson, M., T. Elmros, and S. Bergstrom. 1995. Subcutaneous implanted chambers in different mouse strains as an animal model to study genetic stability during infection with lyme disease Borrelia. Microb. Pathog. 18:109-114. [DOI] [PubMed] [Google Scholar]
- 27.Kurtti, T. J., U. G. Munderloh, R. C. Johnson, and G. G. Ahlstrand. 1987. Colony formation and morphology in Borrelia burgdorferi. J. Clin. Microbiol. 25:2054-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahdenne, P., S. F. Porcella, K. E. Hagman, D. R. Akins, T. G. Popova, D. L. Cox, J. D. Radolf, and M. V. Norgard. 1997. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect. Immun. 65:412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587-609. [DOI] [PubMed] [Google Scholar]
- 30.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 70:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahan, M. J., D. M. Heithoff, R. L. Sinsheimer, and D. A. Low. 2000. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu. Rev. Genet. 34:139-164. [DOI] [PubMed] [Google Scholar]
- 33.Mahan, M. J., J. W. Tobias, J. M. Slauch, P. C. Hanna, R. J. Collier, and J. J. Mekalanos. 1995. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc. Natl. Acad. Sci. USA 92:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marconi, R. T., D. S. Samuels, R. K. Landry, and C. F. Garon. 1994. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J. Bacteriol. 176:4572-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narasimhan, S., F. Santiago, R. A. Koski, B. Brei, J. F. Anderson, D. Fish, and E. Fikrig. 2002. Examination of the Borrelia burgdorferi transcriptome in Ixodes scapularis during feeding. J. Bacteriol. 184:3122-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris, S. J., C. J. Carter, J. K. Howell, and A. G. Barbour. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 60:4662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ojaimi, C., C. Brooks, D. Akins, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katonah, J. Radolf, M. Caimano, J. Skare, K. Swingle, S. Sims, and I. Schwartz. 2002. Borrelia burgdorferi gene expression profiling with membrane-based arrays. Methods Enzymol. 358:165-177. [DOI] [PubMed] [Google Scholar]
- 38.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect.Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orloski, K. A., E. B. Hayes, G. L. Campbell, and D. T. Dennis. 2000. Surveillance for Lyme disease—United States, 1992-1998. Morb. Mortal. Wkly. Rep. 49:1-9. [PubMed] [Google Scholar]
- 40.Porcella, S. F., T. G. Popova, D. R. Akins, M. Li, J. D. Radolf, and M. V. Norgard. 1996. Borrelia burgdorferi supercoiled plasmids encode multicopy tandem open reading frames and a lipoprotein gene family. J. Bacteriol. 178:3293-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Samuels, D. S., K. E. Mach, and C. F. Garon. 1994. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J. Bacteriol. 176:6045-6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuels, D. S., R. T. Marconi, W. M. Huang, and C. F. Garon. 1994. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J. Bacteriol. 176:3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sartakova, M., E. Dobrikova, and F. C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seshu, J., and J. T. Skare. 2000. The many faces of Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 2:463-472. [PubMed] [Google Scholar]
- 49.Shang, E. S., X.-Y. Wu, M. A. Lovett, J. N. Miller, and D. R. Blanco. 2001. Homologous and heterologous Borrelia burgdorferi challenge of infection-derived immune rabbits using host-adapted organisms. Infect. Immun. 69:593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skare, J. T., C. I. Champion, T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1996. Porin activity of the native and recombinant outer membrane protein Oms28 of Borrelia burgdorferi. J. Bacteriol. 178:4909-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skare, J. T., T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, J. Bunikis, S. Bergström, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1997. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect. Immun. 65:3654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slauch, J. M., M. J. Mahan, and J. J. Mekalanos. 1994. In vivo expression technology for selection of bacterial genes specifically induced in host tissues. Methods Enzymol. 235:481-492. [DOI] [PubMed] [Google Scholar]
- 53.Steere, A. C. 1995. Borrelia burgdorferi (Lyme disease, Lyme borreliosis), p. 2143-2155. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, New York, N.Y.
- 54.Stewart, P., R. Thalken, J. Bono, and P. Rosa. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714-721. [DOI] [PubMed] [Google Scholar]
- 55.Suk, K., S. Das, W. Sun, B. Jwang, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1995. Borrelia burgdorferi genes selectively expressed in the infected host. Proc. Natl. Acad. Sci. USA 92:4269-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallich, R., C. Brenner, M. D. Kramer, and M. M. Simon. 1995. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect. Immun. 63:3327-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, X., T. G. Popova, K. E. Hagman, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolf, and M. V. Norgard. 1999. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zar, J. H. 1996. Biostatistical analysis. Prentice-Hall, Inc., Upper Saddle River, N.J.
- 59.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of Vmp-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]