Abstract
Infection with Plasmodium berghei ANKA induces cerebral malaria in susceptible mice. Brain-sequestered CD8+ T cells are responsible for this pathology. We have evaluated the role of CCR2, a chemokine receptor expressed on CD8+ T cells. Infected CCR2-deficient mice were as susceptible to cerebral malaria as wild-type mice were, and CD8+ T-cell migration to the brain was not abolished.
Cerebral malaria (CM) contributes to around 2 million deaths annually, mainly in African children. Brain sequestration of parasitized erythrocytes (PE) is thought to be responsible for this pathology (4, 18). However, though necessary, PE sequestration cannot account alone for CM, since this phenomenon has been observed in non-CM cases (25). Leukocyte sequestration has often been described within brain postcapillary venules from patients who died of CM (9, 21); however, ethical considerations limit investigation of the role of these cells in pathogenesis. In a mouse model of CM with Plasmodium berghei ANKA, characterized by paralysis, deviation of the head, ataxia, convulsions, and coma, histological studies have shown that PE and leukocytes are sequestered in brain capillaries (10, 12, 20, 22). We have recently demonstrated that recruitment of macrophages, neutrophils, and T lymphocytes to the brain is associated with the onset of the disease and that the recruited CD8+ T-cell subset is responsible for the neurological symptoms and the ensuing death (2). We postulated that a chemokine receptor(s) must be necessary for the migration of these pathogenic CD8+ T cells to the brain. We focused on one of these chemokine receptors, CCR2, since it has been shown previously to be expressed on CD8+ T cells migrating to the brain after a viral infection (19). CCR2 is a member of the seven-transmembrane G protein-coupled receptor superfamily and binds ligands such as CCL2 (MCP-1), CCL7 (MCP-3), and CCL12 (MCP-5) (29). In the mouse, CCR2 is expressed on monocytes; T cells, in particular CD8+ T cells (17); endothelial cells; and brain cells like astrocytes and microglial cells (5, 11). CCR2 has been shown elsewhere to be implicated in leukocyte adhesion, monocyte recruitment (13, 26), and dendritic cell trafficking (23).
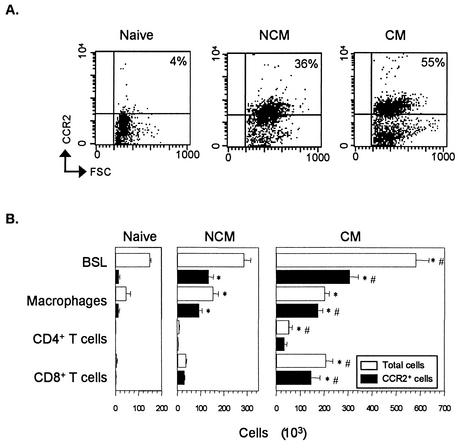
With the use of a recently described monoclonal antibody (MAb) to mouse CCR2 (17), expression of this molecule was investigated by cytofluorometry on total brain-sequestered leukocytes (BSL) and on the cell populations (macrophages and T lymphocytes) which are known to express CCR2 (17), isolated from 129/Ola × C57BL/6J F2 wild-type (WT) naive mice or P. berghei ANKA-infected WT mice with or without CM. BSL were isolated as previously described (2), and leukocyte subsets were identified with the following antibodies: biotinylated rat immunoglobulin G2b (IgG2b) MAb anti-mouse F4/80 (Tebu, Le Perray-en-Yvelines, France), hamster IgG MAb anti-mouse CD3 conjugated to phycoerythrin (clone 17A2; PharMingen), rat IgG2a antibody anti-mouse CD8α conjugated to quantum red (clone 53-6.7; Sigma), rat IgG2a MAb anti-mouse CD4 conjugated to quantum red (clone H129-19; Sigma), and purified rat antibody anti-mouse CCR2 (17). Ultravidin conjugated to phycoerythrin (Leinco Technologies Inc., St. Louis, Mo.) and goat IgG anti-rat IgG conjugated to fluorescein isothiocyanate (Polysciences, Inc., Warrington, Pa.) were used as secondary reagents. For each sample, 5,000 cells were analyzed. CCR2+ BSL were more numerous in WT mice with CM than in those without CM (NCM) or in naive mice (Fig. 1). BSL from WT mice with CM also expressed more CCR2 on their surface (mean fluorescence intensity [MFI], 57.1 ± 10.4) than did BSL from mice without CM (MFI, 30.1 ± 2.9; one-factor analysis of variance and Tukey test, P < 0.05; five mice per group) or BSL from naive mice (MFI, 25.15 ± 2; P < 0.01). Moreover, a strong and significant accumulation of CD8+ T cells expressing CCR2 was observed in the brains of CM mice but not in those from NCM or naive mice (Fig. 1B).
FIG. 1.
CCR2 is expressed on BSL. (A) Representative dot plot of BSL from CCR2 WT mice (naive, NCM, and CM) stained with an anti-CCR2 MAb versus size (forward size scatter [FSC]). Data are representative of five animals per group. (B) Number of total sequestered leukocyte subsets (white bars) and CCR2+ leukocyte subsets (black bars) from the whole brain of WT mice (naive, NCM, and CM). Samples of brain leukocyte suspension from mice infected with 106 PE and healthy mice were stained with MAbs specific for neutrophils, macrophages, and CD4+ and CD8+ T cells and for CCR2 and analyzed by flow cytometry. Absolute numbers of a given subset were calculated by multiplying the percentage of positive cells for this subset by the total number of BSL. CCR2+ cell numbers were determined by using the percentage of CCR2 positive cells within each subset multiplied by the total number of this subset. *, P < 0.05 versus CM mice (one-factor analysis of variance followed by Tukey test). #, P < 0.05 versus NCM mice. This experiment is representative of three.
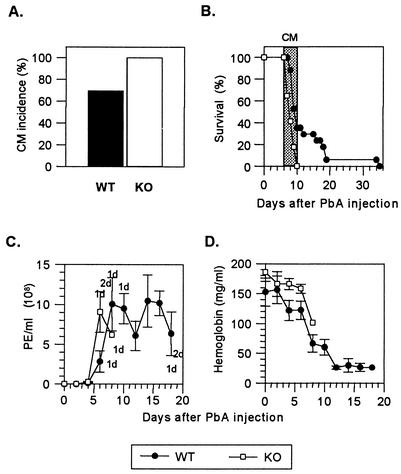
Since CCR2 is expressed on pathogenic CD8+ T cells, we next investigated susceptibility in CCR2-knockout (KO) mice (14). These mice display severe deficits in macrophage (7, 14), neutrophil (6), and T-cell (8) migration in response to either antigenic or nonantigenic challenge and an impaired type 1 cytokine response (7). CCR2-KO and WT mice were infected with 106 PE, and their parasitemia and anemia (hemoglobin levels) were determined every other day as previously described (28). All the KO mice but only 60 to 80% of WT mice developed CM and died between days 6 and 10 after infection (Fig. 2A and B). Though parasite levels were not significantly different between the two groups during the first week, the remaining WT mice died 2 weeks later (Fig. 2B) of hyperparasitemia (Fig. 2C) and anemia (Fig. 2D).
FIG. 2.
CM incidence, survival, parasite load, and hemoglobin levels after P. berghei ANKA (PbA) infection of CCR2 WT and KO mice. (A) CM incidence occurring between day 6 and day 10 in WT (n = 32) and KO (n = 27) mice infected with 106 PE. On day 10, as calculated by Fisher's exact test, P was 0.0049 between WT and KO mice. (B) Survival of WT (n = 17) and KO (n = 17) mice infected with 106 PE. Neurological signs first appear late on days 6 to 10 (shaded area), with death occurring in <24 h after their onset. (C) PE per milliliter of blood ± standard errors of the means. WT (n = 5) and KO (n = 5) mice were infected with 106 PE. Mortality is indicated at the top (KO mice) and at the bottom (WT mice) as the number of dead mice (d) on that day. The difference between WT and KO mice on day 6 was not significant. (D) Hemoglobin levels (means ± standard errors of the means) in WT (n = 5) and KO (n = 5) mice infected with 106 PE.
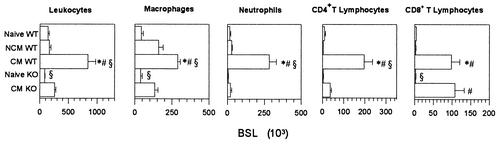
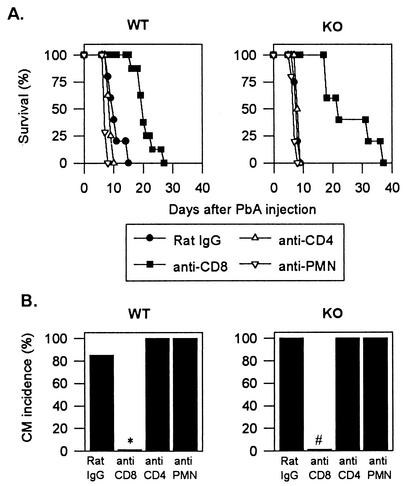
Histopathological analysis of the midbrain region of infected mice was performed as described previously (1) and revealed petechial hemorrhages and leukocyte accumulation in the capillaries of WT mice with CM, whereas these changes were not observed in infected WT mice without CM (data not shown). Brains of infected KO mice with CM showed ring hemorrhages with apparently fewer leukocytes in the capillaries than in those of WT mice with CM (data not shown). We thus quantified the total number of BSL from WT and KO mice. As shown in Fig. 3, BSL from KO mice with CM were less numerous than BSL from WT mice with CM. Nevertheless, there was a significant threefold increase in BSL number in infected KO mice compared to naive KO mice. There were eight times more BSL from WT mice with CM than from naive WT mice. NCM WT mice contained the same number of BSL as did naive WT mice (Fig. 3). BSL from the different mouse groups were further phenotyped by cytofluorometry. Macrophages were identified as F4/80+, neutrophils were identified as F4/80− and Gr-1+ (rat IgG2b MAb anti-mouse Gr-1 conjugated to fluorescein isothiocyanate, clone RB6-8C5; PharMingen), and T cells were identified as described above. We observed a significant increase in the numbers of macrophages, neutrophils, and CD4+ and CD8+ T lymphocytes (but not of other cell types) in CM WT mice compared with naive or NCM WT mice. Macrophages and CD8+ T cells, but no other cell types, increased in infected KO mice with CM compared with naive KO mice. However, the number of macrophages in KO mice with CM was significantly lower than in CM WT mice (Fig. 3). In contrast, similar numbers of CD8+ T cells, the subset responsible for CM in WT mice, were found in CM WT and CM KO mice. Depletion experiments were carried out to investigate the role of brain-sequestered CD8+ T cells in CCR2-KO mice with CM. Depletion of BSL subsets was performed at day 6, just before the onset of CM, by injecting intraperitoneally 1 mg of the following MAbs: rat IgG anti-mouse CD8 (clone 2.43; ATCC TIB 210), rat IgG anti-mouse CD4 (clone GK1.5; ATCC TIB 207), or antipolymorphonuclear cells (15). More than 98% of blood CD8+ or CD4+ T cells were depleted as verified by fluorescence-activated cell sorting (FACS) analysis. Depletion of blood neutrophils was more than 80% as verified by FACS analysis with anti-Gr-1 MAb. Purified rat IgG (Sigma) was used as a negative control. Macrophages were depleted at day 5 after P. berghei ANKA injection by intravenous injection of 0.2 ml of phosphate-buffered saline containing approximately 1 mg of dichloromethylenediphosphonate (Cl2-MDP) encapsulated in liposomes (27). More than 90% of blood F4/80+ cells were depleted as verified by FACS analysis 2 days later. All CCR2-KO mice depleted of CD4+ T cells, neutrophils, or macrophages died of CM (Fig. 4 and data not shown), whereas none of the anti-CD8-treated KO mice developed CM. Identical results were observed in infected and similarly depleted WT mice (Fig. 4 and data not shown).
FIG. 3.
Levels of whole-brain-sequestered leukocytes in CCR2-KO and WT mice after P. berghei ANKA infection. Enumeration of BSL was performed on perfused brains from KO (n = 15) and WT (n = 8) mice at the time when CM is diagnosable (days 6 to 10), NCM WT mice (days 9 to 10) (n = 6), and naive KO (n = 10) or WT (n = 11) mice. Cell numbers were determined as described for Fig. 1B. Values are expressed as means ± standard errors of the means. *, P < 0.05 (one-factor analysis of variance followed by Tukey test), significantly different from naive WT mice; #, P < 0.05, Tukey test, significantly different from NCM WT mice; and , P < 0.05, Tukey test, significantly different from CM KO mice. This experiment is representative of three.
FIG. 4.
Role of CD8+ T cells in CM in CCR2 KO mice. The effector role of CD8+ T cells was demonstrated through a series of depletion experiments with infected CCR2-KO and WT mice. The figure shows survival (A) and CM incidence (B) in infected WT or KO mice injected with the following rat antibodies: control IgG (n = 5), anti-CD8 (n = 5), anti-CD4 (n = 5), and anti-polymorphonuclear cell (PMN) (n = 5) on day 6. *, P < 0.05 (Fisher test) versus P. berghei ANKA-infected WT mice treated with control rat IgG; #, P < 0.0001 (Fisher test) versus rat IgG-treated KO mice. This experiment is representative of two.
Finally the role of cytokines was investigated, since a type 1 response, which is altered in CCR2-KO mice (7, 23, 24), has been associated elsewhere with CM development (1, 16). Both CM WT and KO mice, however, developed similar serological and cellular type 1 responses overall (data not shown).
Our results clearly show that CCR2 is not necessary for CM to occur. CCR2 deficiency was associated with a reduction in numbers of macrophages, neutrophils, and CD4+ T cells but not of CD8+ T cells. Our results further confirm that CD8+ T cells are responsible for CM death (2). It is remarkable that the pathology in WT and CCR2-KO mice was due to the sequestration of less than 105 CD8+ T cells in the vasculature of a whole brain. CCR2 has also been shown previously to be expressed on brain cells like endothelial cells, astrocytes, and microglial cells (5, 11), and signaling through this receptor may activate these cell types for chemokine and cytokine production. However, our results indicate that CCR2 signaling in these cells is not required for the development of CM. Since migration of CD8+ T cells to the brain occurred normally in CCR2 KO mice, this implies that another chemokine receptor(s) is involved in this process. We have shown recently that CCR5 deficiency results in the decrease in CM susceptibility in mice of the same genetic background (3). Preliminary results indicate that more than 80% of brain-sequestered CD8+ T cells from infected WT or CCR2 KO mice express CCR5 (data not shown). More studies are needed to determine if other chemokine receptors are involved in rodent and eventually in human CM.
Acknowledgments
We thank Georges Snounou for critical reading of the manuscript.
This work was supported in part by a grant from Junta Nacional de Investigação Cientifica e Tecnologica (JNICT) and Fondation de La Recherche Médicale to Laurent Rénia. Elodie Belnoue held a fellowship from MENRT. Fabio T. M Costa was supported by a fellowship from the CAPES foundation, Brazil. Ana Margarida Vigário held a fellowship from Junta Nacional de Investigação Cientifica e Tecnologica (JNICT), Portugal.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amani, V., A. M. Vigario, E. Belnoue, M. Marussig, L. Fonseca, D. Mazier, and L. Rénia. 2000. Involvement of IFN-γ receptor-mediated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 30:1646-1655. [DOI] [PubMed] [Google Scholar]
- 2.Belnoue, E., M. Kayibanda, A. M. Vigario, J. C. Deschemin, N. Van Rooijen, M. Viguier, G. Snounou, and L. Renia. 2002. On the pathogenic role of brain-sequestered αβ CD8+ T cells in experimental cerebral malaria. J. Immunol. 169:6369-6375. [DOI] [PubMed] [Google Scholar]
- 3.Belnoue, E., M. Kayibanda, J.-C. Deschemin, M. Viguier, M. Mack, W. A. Kuziel, and L. Renia. CCR5 deficiency decreases susceptibility to experimental cerebral malaria. Blood, in press. [DOI] [PubMed]
- 4.Berendt, A. R., G. D. H. Turner, and C. I. Newbold. 1994. Cerebral malaria: the sequestration hypothesis. Parasitol. Today 10:412-414. [DOI] [PubMed] [Google Scholar]
- 5.Berger, O., X. Gan, C. Gujuluva, A. R. Burns, G. Sulur, M. Stins, D. Way, M. Witte, M. Weinand, J. Said, K. S. Kim, D. Taub, M. C. Graves, and M. Fiala. 1999. CXC and CC chemokine receptors on coronary and brain endothelia. Mol. Med. 5:795-805. [PMC free article] [PubMed] [Google Scholar]
- 6.Blease, K., B. Mehrad, T. J. Standiford, N. W. Lukacs, J. Gosling, L. Boring, I. F. Charo, S. L. Kunkel, and C. M. Hogaboam. 2000. Enhanced pulmonary allergic responses to aspergillus in CCR2−/− mice. J. Immunol. 165:2603-2611. [DOI] [PubMed] [Google Scholar]
- 7.Boring, L., J. Gosling, S. W. Chensue, S. L. Kunkel, R. V. Farese, Jr., and H. E. Broxmeyer. 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 100:2552-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fife, B. T., G. B. Huffnagle, W. A. Kuziel, and W. J. Karpus. 2000. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 192:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grau, G. E., C. D. Mackenzie, R. A. Carr, M. Redard, G. Pizzolato, C. Allasia, C. Cataldo, T. E. Taylor, and M. E. Molyneux. 2003. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J. Infect. Dis. 187:461-466. [DOI] [PubMed] [Google Scholar]
- 10.Hearn, J., N. Rayment, D. N. Landon, D. R. Katz, and J. B. De Souza. 2000. Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect. Immun. 68:5364-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesselgesser, J., and R. Horuk. 1999. Chemokine and chemokine receptor expression in the central nervous system. J. Neurovirol. 5:13-26. [DOI] [PubMed] [Google Scholar]
- 12.Jennings, V. M., J. K. Actor, A. A. Lal, and R. L. Hunter. 1997. Cytokine profile suggesting that murine cerebral malaria is an encephalitis. Infect. Immun. 65:4883-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara, T., G. Warr, J. Loy, and R. Bravo. 1997. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J. Exp. Med. 186:1757-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuziel, W. A., S. J. Morgan, T. C. Dawson, S. Griffin, O. Smithies, K. Ley, and N. Maeda. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci. USA 94:12053-12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez, A. F., M. Strath, and C. J. Sanderson. 1984. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br. J. Haematol. 57:489-494. [DOI] [PubMed] [Google Scholar]
- 16.Lou, J., R. Lucas, and G. E. Grau. 2001. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clin. Microbiol. Rev. 14:810-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack, M., J. Cihak, C. Simonis, B. Luckow, A. E. Proudfoot, J. Plachy, H. Bruhl, M. Frink, H. J. Anders, V. Vielhauer, J. Pfistinger, M. Stangassinger, and D. Schlondorff. 2000. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166:4697-4704. [DOI] [PubMed] [Google Scholar]
- 18.MacPherson, G. G., M. J. Warrell, N. J. White, S. Looareesuwan, and D. A. Warrell. 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119:385-401. [PMC free article] [PubMed] [Google Scholar]
- 19.Nansen, A., O. Marker, C. Bartholdy, and A. R. Thomsen. 2000. CCR2+ and CCR5+ CD8+ T cells increase during viral infection and migrate to sites of infection. Eur. J. Immunol. 30:1797-1806. [DOI] [PubMed] [Google Scholar]
- 20.Neill, A. L., and N. H. Hunt. 1992. Pathology of fatal and resolving Plasmodium berghei cerebral malaria in mice. Parasitology 105:165-175. [DOI] [PubMed] [Google Scholar]
- 21.Patnaik, J. K., B. S. Das, S. K. Mishra, S. Mohanty, S. K. Satpathy, and D. Mohanty. 1994. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am. J. Trop. Med. Hyg. 51:642-647. [PubMed] [Google Scholar]
- 22.Rest, J. R. 1982. Cerebral malaria in inbred mice. I. A new model and its pathology. Trans. R. Soc. Trop. Med. Hyg. 76:410-415. [DOI] [PubMed] [Google Scholar]
- 23.Sato, N., S. K. Ahuja, M. Quinones, V. Kostecki, R. L. Reddick, P. C. Melby, W. A. Kuziel, and S. S. Ahuja. 2000. CC chemokine receptor (CCR)2 is required for Langerhans cell migration and localization of Th1 cell type (Th1)-inducing dendritic cells: absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato, N., W. A. Kuziel, P. C. Melby, R. L. Reddick, V. Kostecki, W. Zhao, N. Maeda, S. K. Ahuja, and S. S. Ahuja. 1999. Defects in the generation of IFN-γ are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1α-, or CCR2-deficient mice. J. Immunol. 163:5519-5525. [PubMed] [Google Scholar]
- 25.Silamut, K., N. H. Phu, C. Whitty, G. D. Turner, K. Louwrier, N. T. Mai, J. A. Simpson, T. T. Hien, and N. J. White. 1999. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 155:395-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traynor, T. R., W. A. Kuziel, G. B. Toews, and G. B. Huffnagle. 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J. Immunol. 164:2021-2027. [DOI] [PubMed] [Google Scholar]
- 27.Van Rooijen, N., A. Sanders, and T. K. van den Berg. 1996. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods 193:93-99. [DOI] [PubMed] [Google Scholar]
- 28.Vigario, A. M., E. Belnoue, A. Cumano, M. Marussig, F. Miltgen, I. Landau, D. Mazier, I. Gresser, and L. Renia. 2001. Inhibition of Plasmodium yoelii blood-stage malaria by interferon alpha through the inhibition of the production of its target cell, the reticulocyte. Blood 97:3966-3971. [DOI] [PubMed] [Google Scholar]
- 29.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]