Abstract
Toxin B (TcdB), a major Clostridium difficile virulence factor, glucosylates and inactivates the small GTP-binding proteins Rho, Rac, and Cdc42. In the present study we provide evidence that enzymatically inactive fragments of the TcdB enzymatic domain are effective intracellular inhibitors of native TcdB. Site-directed and deletion mutants of the TcdB enzymatic region (residues 1 to 556), lacking receptor binding and cell entry domains, were analyzed for attenuation of glucosyltransferase and glucosylhydrolase activity. Five of six derivatives from TcdB1-556 were found to be devoid of enzymatic activity. In order to facilitate cell entry, mutants were genetically fused to lfn, which encodes the protective antigen binding region of anthrax toxin lethal factor and mediates the cell entry of heterologous proteins. In line with reduced enzymatic activity, the mutants also lacked cytotoxicity. Remarkably, pretreatment or cotreatment of cells with four of the mutants provided protection against the cytotoxic effects of native TcdB. Furthermore, a CHO cell line expressing enzymatically active TcdB1-556 was also protected by the mutant-derived inhibitors, suggesting that inhibition occurred at an intracellular location. Protection also was afforded by the inhibitor to cells treated with Clostridium sordellii lethal toxin (TcsL), which uses the same cosubstrate as TcdB but shares Rac only as a common substrate target. Finally, the inhibitor did not provide protection against Clostridium novyi alpha-toxin (Τcnα), which shares similar substrates with TcdB yet uses a different cosubstrate. This is the first report to demonstrate that the potential exists to inhibit toxins at their intracellular site of action by using inactive mutants.
Clostridium difficile is the leading cause of hospital-acquired diarrhea and pseudomembranous colitis, a multifactorial disease involving steps in colonization, adherence, inflammation, and cellular intoxication (20). Present treatments for pseudomembranous colitis include the use of antibiotics and supportive therapy. Other, more novel treatments have recently been reported and include adjunctive treatment with Saccharomyces boulardii (12). Therapeutics that target major virulence factors of C. difficile, such as toxin A (TcdA) or toxin B (TcdB), may also provide promising new treatments since their neutralization could alleviate major cell-specific cytotoxic activities.
TcdA and TcdB are large clostridial toxins (LCTs) produced by C. difficile and are involved in development of pseudomembranous colitis. Through a series of studies, both toxins have been shown to contribute to the pathologies associated with disease (22). To promote these changes, TcdB glucosylates isoforms of small GTPases Rho, Rac, and Cdc42 within the effector binding region at residue threonine-37 (Rho) or threonine-35 (Rac and Cdc42) (18). The physiological impact of TcdB's activity includes disruption of tight junctions and increased epithelial permeability, as well as actin condensation and cell death (13, 14, 21).
The primary structure of TcdB is divided into enzymatic, translocation, and receptor binding domains, although detailed analyses of regions outside the enzymatic domain have not been carried out to date. The enzymatic domain of TcdB is encompassed by the protein's 546 amino-terminal amino acids. Less well defined translocation and receptor binding regions of TcdB are contained within the middle and carboxy-terminal regions of the protein (7). Enzymatic activity appears to require all of the 546 amino-terminal residues since amino- or carboxy-terminal deletions of this fragment decrease activity (30). Within the enzymatic region, trytophan-102 has been shown elsewhere to be essential for UDP-glucose binding (8). A conserved DXD motif within LCTs is also essential for glucosyltransferase activity (9). Other studies, involving analysis of chimeras of the enzymatic domains of TcdB and Clostridium sordellii lethal toxin (TcsL), suggest that residues 364 to 516 confer substrate specificity (17).
Steps in cell entry by TcdB have been broadly defined, yet events subsequent to entry are not well understood. For example, while we have a profile of the time course for TcdB cell entry (6, 25), very little is known about postentry events that lead to glucosylation. In the cytosol, TcdB is capable of glucosylating multiple substrates, but whether inactivation of Rho, Rac, and Cdc42 in combination is necessary for complete intoxication, or if other substrates are targeted, is not known. Giry et al. found that overexpression of Rho isoforms protects cells from TcdB (15), suggesting that inactivation of all substrates may not be necessary for cellular intoxication. Interestingly, Rho has also been shown elsewhere to regulate the suppression of apoptosis (16), so it is not entirely clear whether overexpression of Rho is protective at the substrate inactivation level or prevents events downstream of glucosylation. Recent studies from our laboratory suggest that TcdB is capable of triggering both caspase-dependent and caspase-independent apoptosis, possibly through multiple pathways (23). Collectively, these studies suggest that successful therapies directed towards inhibition of TcdB may involve blocking the toxin's access to substrate. The concept of using inactive derivatives of TcdB's enzymatic domain to inhibit wild-type toxin, by blocking access to substrate or cosubstrate, has not been investigated. Yet, such a rational approach could provide a novel method of treatment of pseudomembranous colitis as well as other diseases.
During analysis of the TcdB enzymatic domain, we discovered a set of mutants unable to modify substrate, yet capable of blocking TcdB cytopathic effects (CPEs). Herein we describe generation and analyses of these mutants and demonstrate that these proteins are potent intracellular inhibitors of TcdB. Results from these experiments indicate that a toxin derivative can be used to effectively block the activity of the native toxin within the cell. This inhibitory activity also suggests a new paradigm for a therapeutic approach to treating toxin-based diseases.
MATERIALS AND METHODS
Tissue culture, bacterial strains, and chemical reagents.
CCL-2 human cervical adenocarcinoma cells (HeLa; American Type Culture Collection, Manassas, Va.) were grown in supplemented RPMI 1640 (RP-10) medium (28) with 10% fetal bovine serum at 37°C in a humid atmosphere with 7% CO2. Stably transfected CHO cells expressing TcdB1-556 were generated and maintained as previously described (23). C. difficile strain VPI 10463, C. sordellii strain 9714, and Clostridium novyi strain NCTC 538 were obtained from the American Type Culture Collection and used as sources of culture supernatant, genomic DNA, TcdB, TcsL, and Tcnα. All reagents were of molecular biology grade and were purchased from Sigma Chemical Co., St. Louis, Mo., unless otherwise noted.
Construction of recombinant LFnTcdB fusions.
The region coding for the enzymatic domain of TcdB (TcdB1-556; nucleotides 1 to 1668) was genetically fused to lfn and cloned, expressed, and purified in Escherichia coli as previously described (27). By a similar approach, four other fusions of LFnTcdB were also constructed. Briefly, fragments encoding regions TcdB1-500 (nucleotides 1 to 1500), TcdB1-420 (nucleotides 1 to 1260), TcdB1-170 (nucleotides 1 to 510), and TcdB35-556 (nucleotides 103 to 1668) were PCR amplified and cloned into the BamHI site of pABII (27) to make the plasmids pLMS201, pLMS202, pLMS204, and pLMS301, respectively. Plasmids were transformed into E. coli XL1-Blue (Stratagene), and candidate clones were sequenced and then transformed into E. coli BL-21 Star (Invitrogen) for expression.
Site-directed mutants were generated by using Pfu Turbo DNA polymerase and the QuickChange mutagenesis approach (Stratagene). Oligonucleotides for generation of TcdB1-556C365S were GTTTACTATTAAATTGCTAGAATATGAGTCTTTCACAG (sense) and CTGTGAAGACTCATATTCTAGCAATTTAATAGTAAAAC (antisense), for TcdB1-556C365W they were GTTTTACTATTAAATTGCTACCTATGAGTCTTTCACAG (sense) and CTGTGAAAGACTCATATTGGAGCAATTTAATAGTAAAAC (antisense), and for TcdB1-556W102A they were AAAAATTTACATTTTGTTGCTATTGGAGGTCAA (sense) and TTGACCTCCAATAGCAACAAAATGTAAATTTTT (antisense). Mutants were selected in E. coli XL1-Blue and confirmed by sequencing, followed by transformation into E. coli BL-21 Star for expression.
Purification of recombinant proteins and LCTs.
Expression of LFnTcdB fusions was induced with 0.2 mM isopropyl-β-d-thiogalactopyranoside in log-phase (optical density at 600 nm, 0.8) cultures at 16°C. Cells were harvested by centrifugation at 8,700 × g; resuspended in binding buffer (5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9) supplemented with a protease inhibitor cocktail containing 4-(2-aminoethyl)benzenesulfonyl fluoride, phosphoramidone, pepstatin A, bestatin, and E-64; and lysed by sonication. LFnTcdB fusion proteins were isolated by using nickel 900 cartridges according to the instructions of the manufacturer (Novagen). As a second purification step, proteins were fractionated on a high-resolution anion-exchange (Mono-Q) column (Amersham Pharmacia). Recombinant protective antigen (PA) was isolated from E. coli BL-21 harboring the plasmid pSRB/ET-15b-PA (a generous gift from Steven Blanke) as previously described (31). TcdB, TcsL, and Tcnα were purified as previously described (25). Recombinant clones of RhoA, Rac1, and Cdc42 (a generous gift of Alan Hall) were expressed and purified as previously described (27).
Glucosylhydrolase-glucosylation assays.
Glucosylation reactions were carried out as previously described (27). Glucosylhydrolase assays were carried out in a reaction mix containing 50 mM HEPES, 100 mM KCl, 1 mM MnCl2, 1 mM MgCl2, 100 μg of bovine serum albumin/ml, 0.2 mM GDP, 40 μM UDP-[14C]glucose (303 Ci/mol; ICN Pharmaceuticals), 100 μM UDP-glucose, and 3 pmol of TcdB or 10 pmol of each fusion protein. The assay mixture was allowed to incubate overnight at 37°C, and similarly to a previously described protocol (11), the cleaved glucose was separated with AG1-X2 anion-exchange resin and counted in a liquid scintillation counter. To determine the effects of TcdB1-500 on in vitro glucosylation by TcdB, a standard glucosylation assay was performed (as described above) with the addition of a 10-fold molar excess of TcdB1-500. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and exposed to film, and the relative levels of glucose incorporation were determined by densitometry with NIH Image version 1.62 software.
Assay for CPEs and inhibitor assays.
To determine the cytopathic activity of each fusion and site-directed mutant, HeLa cells were plated in 96-well microtiter plates (3 × 104 cells/well) and allowed to incubate overnight. The following day the cells were treated with 30 pmol of each fusion plus 8.5 pmol of PA and observed for 48 h for signs of CPEs. For inhibition assays, HeLa cells were plated as before and treated with 4 pmol of the appropriate LFnTcdB fusion plus 8.5 pmol of PA in a final volume of 100 μl. At the same point the cells were cotreated with 80 fmol of TcdB and observed for CPEs. In a second competition assay, 30 pmol of TcdB1-500 plus 8.5 pmol of PA was added to cells in a final volume of 100 μl and allowed to incubate for 30 min, at which point 20 fmol of TcdB was added to the cells. Following the initial treatment, 30 pmol of TcdB1-500 and 8.5 pmol of PA were added every 30 min for the first 90 min and every hour thereafter up to 12 h. The cells were observed for CPEs for an additional 18 h. Similar competition assays were carried out with 2 pmol of TcsL or Tcnα. For inhibition assays with TcsL, cells were subjected to a brief acid pulse, which enhances cytotoxic activity for this toxin. For TcsL competition, cells were pretreated with TcdB1-500 and PA for 30 min, at which point they were treated with TcsL via an acid pulse as previously described (24). The cells were then amended with 30 pmol of TcdB1-500-8.5 pmol of PA every 30 min up to 12 h and monitored for 16 h.
CPEs for these assays were determined by visualization of rounded cells or condensation of actin by using rhodamine phalloidin staining as previously described (23). Percent CPEs were calculated by counting a minimum of 100 cells in three fields from each sample. Fully rounded cells were scored positive for CPE, and the final percent CPE was calculated as % rounded cellstest − % rounded cellscontrol, wherein the control sample is cells treated with medium alone. Cell viability assays were carried out with WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] (Dojindo Laboratories, Gaithersburg, Md.) according to the manufacturer's instructions. The following formula was used to determine percent viability [(Atest − Abackground)/(Acontrol − Abackground) × 100], wherein A is absorbance at 405 nm, background is treatment with medium alone, and control is treatment with 1% SDS.
Statistical analysis.
Results were analyzed with the statistical software component of Excel 2001. Sample variations are reported as standard deviations from the means, and significance was confirmed by Student's t test (P < 0.05).
RESULTS
Enzymatic and cytopathic activities of mutants.
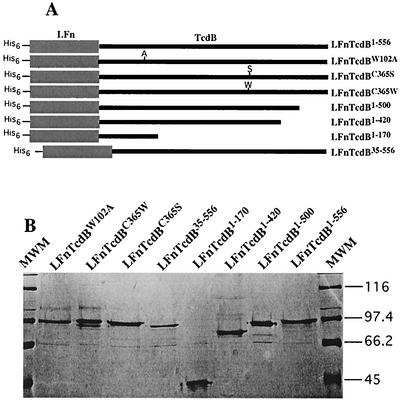
As summarized in Fig. 1, four deletion and three site-directed mutants in the TcdB enzymatic domain were constructed, cloned as fusions with lfn, and then expressed and isolated from E. coli. The nomenclature for each of these mutants is summarized in Fig. 1A. One site-directed mutant, TcdBW102A, has been previously characterized and served as a control in cytotoxicity and enzymatic assays (8). Pilot experiments suggested that TcdB1-556 could be inactivated by N-ethylmaleimide (data not shown), indicating a role for the sole cysteine in enzymatic activity; thus, site-directed mutants TcdBC365S and TcdBC365W were produced. Amino-terminal and carboxy-terminal deletions were also generated in an attempt to better define the enzymatic domain. Since these mutants lacked receptor binding and translocation domains, the fragments were fused to the cell entry proteins of anthrax lethal toxin. This anthrax toxin derivative consists of anthrax PA and a truncated form of anthrax lethal factor (LFn) to which heterologous fusions are made. PA-LFn has been used by several groups for the cytosolic delivery of a variety of proteins (2-5, 19), and we previously used this system to deliver TcdB1-556 to cultured mammalian cells (27). With this delivery system, the fusions were tested for cytopathic activity, and only LFnTcdB1-556 and LFnTcdBC365S were cytotoxic (data not shown).
FIG. 1.
LFnTcdB deletion and site-directed mutants used in this study. (A) Overview of deletion and site-directed mutants. Deletion mutants were generated by PCR, cloned in frame with lfn in pET15b, expressed in E. coli BL-21, and subsequently purified by Ni2+ affinity chromatography. Site-directed mutants were generated by the QuickChange method, by using complementary mutation-carrying oligonucleotides and pLMS200 as template. (B) SDS-PAGE analysis of His-tagged fusions. Approximately 10 μg of each isolated fusion protein was subjected to SDS-PAGE analysis. Samples and corresponding lanes are labeled within the figure. MWM, molecular weight markers; weights are shown in thousands at right.
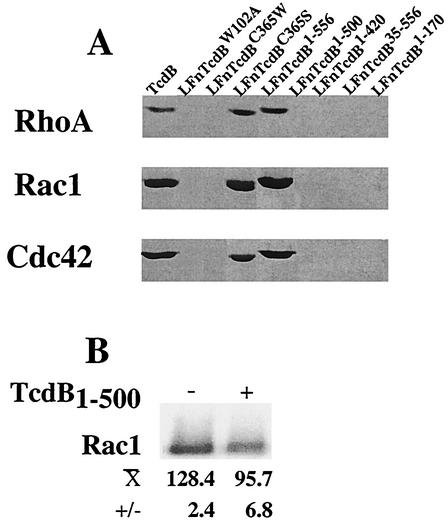
To determine if the lack of cytotoxicity was due to attenuation of enzymatic activity, mutants were tested for glucosylation of RhoA, Rac1, or Cdc42. As shown in Fig. 2, onlyLFnTcdB1-556 and LFnTcdBC365S glucosylated substrate. In line with earlier reports carboxy-terminal deletions and LFnTcdBW102A were unable to glucosylate RhoA, Rac1, or Cdc42. The remainder of the site-directed and deletion mutants were also deficient in glucosylation. Furthermore, this loss of activity was maintained across all of the shared substrates since all inactive mutants showed similar declines in glucosylation of RhoA, Rac1, and Cdc42.
FIG. 2.
Glucosylation activity of deletion and site-directed mutants on RhoA, Rac1, and Cdc42 and in vitro inhibition of Rac glucosylation. (A) Each mutant and TcdB were tested for glucosylation activity on recombinant substrates GST-RhoA, GST-Rac1, and GST-Cdc42, with UDP-[14C]glucose as cosubstrate. Following a 2-h incubation, the reaction mix was resolved by SDS-PAGE and exposed to film for 48 h. Samples and corresponding lanes are labeled within the figure. (B) LFnTcdB1-500 was included in a standard assay for TcdB-mediated glucosylation of Rac to determine if the deletion mutant attenuated modification of substrate. Following coincubation in a standard glucosylation assay, relative levels of [14C]glucose incorporation were determined by densitometry.
Each mutant was also analyzed for glucosylhydrolase activity by using radiolabeled UDP-glucose in the absence of substrate, to determine if this could account for the inability to modify substrate targets. Fusions were incubated with UDP-[14C]glucose, the liberated sugar was separated by anion-exchange chromatography, and radioactivity was determined. As shown in Table 1, even with an extended (16-h) incubation glucosylhydrolase activity was significantly reduced for all enzymatically inactive mutants. Thus, the absence of substrate modification by these mutants could be accounted for, at least in part, by defective hydrolase activity.
TABLE 1.
UDP-glucosylhydrolase activity of TcdB mutantsa
| Mutant | % Hydrolysis |
|---|---|
| TcdB | 27.7 ± 0.45 |
| TcdB1-556 | 6.9 ± 1.38 |
| TcdB1-500 | 0.6 ± 0.53 |
| TcdB1-420 | 0.2 ± 0.51 |
| TcdB1-170 | 0.7 ± 0.6 |
| TcdB35-556 | 0.0 ± 0.08 |
| TcdBC365S | 6.2 ± 3.1 |
| TcdBC365W | 1.23 ± 0.51 |
| TcdBW102A | 1.02 ± 0.1 |
Glucosylhydrolase assays were carried out in a reaction mix containing UDP-[14C]glucose plus TcdB or mutant, and liberated [14C]glucose was then determined as a percentage of the total counts prior to treatment. The data represent the means of triplicate samples.
The TcdB mutant as an inhibitor of in vitro glucosylation.
Although the TcdB mutants were devoid of glucosylhydrolase activity, the potential for substrate interactions or other, yet undefined activities seemed present. For this reason, one of the mutants (LFnTcdB1-500) was assayed for the ability to inhibit substrate glucosylation by native toxin in an in vitro assay. As shown in Fig. 2B, addition of a 10-fold excess of LFnTcdB1-500 to the standard glucosylation reaction mixture resulted in an approximately 25% reduction in the level of Rac1 glucosylation by TcdB. This result suggested that defective mutants might be capable of protecting cells from wild-type TcdB, by reducing the toxin's ability to modify substrate.
TcdB mutants as inhibitors of TcdB CPEs.
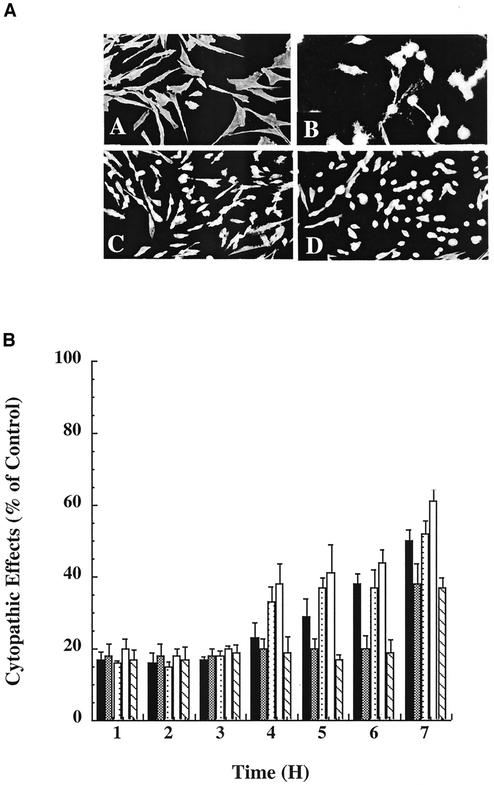
Since the inactive mutants could be effectively delivered to the cytosol of cells via the PA-LFn system, we were presented with the unique opportunity to examine the effects that these mutants might have when administered in combination with wild-type TcdB. Thus, HeLa cells were treated with TcdB in the presence or absence of PA plus each attenuated mutant. As shown in Fig. 3, PA-delivered LFnTcdB1-500, LFnTcdB1-420,LFnTcdBW102A, LFnTcdBC365W, or LFnTcdB35-556 attenuated TcdB CPEs, suggesting that the mutants had an antagonistic impact on TcdB intoxication. The inhibitor effects were dependent on the presence of inactive enzymatic domain mutants, since PA-LFn alone did not inhibit TcdB.
FIG. 3.
Inhibition of TcdB cytopathic effects by TcdB mutants. HeLa cells were cotreated with TcdB and each TcdB fusion plus PA. The cells were monitored for 7 h, and cytopathic effects were determined by visualization. (A) Representative samples of actin condensation and cell rounding in the inhibitor assay: A, phosphate-buffered saline control; B, TcdB control; C, PA-LFnTcdB1-500 plus TcdB; D, PA-LFn plus TcdB. (B) Summary of inhibitors capable of blocking TcdB CPEs. Symbols: solid bars, LFnTcdB1-420; open bars, LFnTcdBW102A; lightly stippled bars, LFnTcdBC365W; checkered bars, LFnTcdB33-556; hatched bars, LFnTcdB1-500.
It was clear from the results shown in Fig. 3B that, approximately 7 h after delivery of inhibitory fragments to the TcdB-treated cells, the protective effect began to decrease. This observation suggested that the inhibitory effect of the enzymatically inactive mutants has a limited lifetime. To address this possibility, the initial competition was set up as before and the inhibitor (LFnTcdB1-500) was added to the cells at 1-h intervals during the course of the assay. As shown in Fig. 4, by this approach more than 50% of the cells demonstrated no CPEs and appeared to be protected from the wild-type toxin during the course of the assay (>30 h). Hence, continued administration of the inhibitor maintained the protective effect against TcdB. Continued addition of the inhibitor after 12 h did not improve or change the inhibition of TcdB, suggesting that TcdB had lost activity or that the accumulated inhibitor was in sufficient excess so that its protective effect was extended.
FIG. 4.
Sustained inhibition by supplemental treatments with LFnTcdB1-500-PA. HeLa cells were cotreated with TcdB and LFnTcdB1-500-PA in the test assay. Control assays included TcdB in the absence of inhibitor or LFnTcdB1-500 and PA in the absence of TcdB. In the test sample, LFnTcdB1-500-PA was added to the cells at 1-h intervals for 12 h. The cells were then monitored for 30 h and visualized for CPEs. Samples and corresponding lines are labeled within the figure.
Inactive mutants protect CHO cells expressing TcdB1-556.
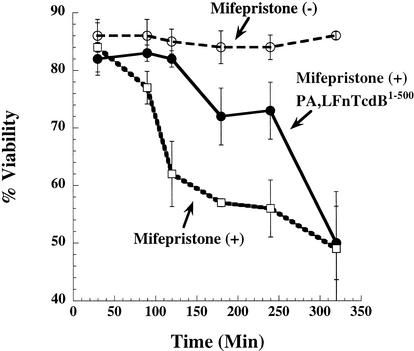
The fact that the TcdB inhibitors lack native translocation and receptor binding domains suggested that inhibition occurred within the cytosol. However, inhibition at the cell surface could not be formally excluded since cell surface-interacting regions of TcdB have not been fully elucidated. To determine if inhibition of TcdB was occurring within the cytosol, a CHO cell line capable of inducible expression of TcdB1-556 was generated. A tightly regulated expression system, pSwitch, was selected which allows expression only in the presence of the hormone mifepristone. GeneSwitch-CHOpGene/TcdB1-556 cells showed early toxic effects, such as rounding, at around 4 h following the addition of mifepristone and were no longer viable by 24 h. To test the inhibitor on these cells, mifepristone was added to the cells and inhibitor was added 2 h later and subsequently every 30 min for an additional 3 h. As shown in Fig. 5, mifepristone-treated GeneSwitch-CHOpGene/TcdB1-556 cells were protected from the effects of TcdB1-556 when PA-LFnTcdB1-500 was added at 2 h following induction. The inhibitor clearly slows the cytopathic activity of these cells following induction. Cells eventually show CPEs similar to control since the cell continues to express TcdB1-556. These results demonstrate that the inhibitor is capable of blocking TcdB intoxication at a site within the cell.
FIG. 5.
Protection of CHO cells expressing TcdB1-556. GeneSwitch-CHOpGene/TcdB1-556 cells were induced with mifepristone in the presence or absence of LFnTcdB1-500 plus PA. Cells were then observed for rounding and CPEs at the indicated time points. Samples and corresponding lines are labeled within the figure.
Inhibition of intoxication by C. sordellii lethal toxin (TcsL).
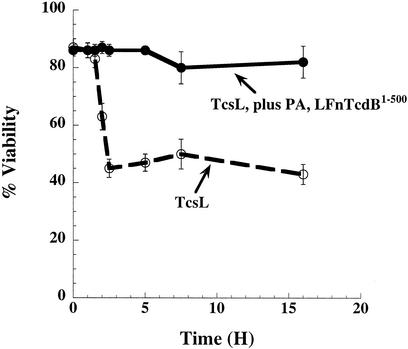
The inhibitory activity of the TcdB mutants could be due to competition for either substrate or cosubstrate. To discriminate between these two possibilities, we analyzed the ability of TcdB1-500 to protect cells from either TcsL or C. novyi alpha-toxin (Tcnα). Like TcdB, TcsL uses UDP-glucose as a substrate for glycosylation of small GTPases; however, TcsL modifies a different set of substrates (Ras, Ral, Rap, and Rac) and shares only Rac as a common substrate with TcdB. Conversely, Tcnα targets the same substrates as does TcdB yet utilizes UDP-N-acetylglucosamine as a cosubstrate. We tested the TcdB inhibitor's ability to block TcsL intoxication. In recent work some of us reported that acid pH enhances TcsL entry (24), so the initial treatment with TcsL was carried out by providing an extracellular acid pulse to TcsL. In this assay cells were pretreated with the inhibitor, then acid pulse treated with TcsL, and subsequently treated with additional inhibitor during the time course of the assay. As can be seen in Fig. 6, PA plus LFnTcdB1-500 was also able to block the activity of TcsL. Similar to results with TcdB, the inhibitor was capable of reducing TcsL's CPEs by almost 50%. Finally, LFnTcdB1-500 was analyzed for inhibition of Tcnα. Unlike the findings with TcdB and TcsL, the inhibitor had no impact on Tcnα cytotoxicity (data not shown), raising the possibility that the inhibition may occur due to sequestration of UDP-glucose.
FIG. 6.
TcdB1-500 inhibition of TcsL CPEs. HeLa cells were treated with TcdB1-500 plus PA for 30 min prior to treatment with TcsL. To enhance TcsL cytopathic activity, cells were treated with the toxin by using an acid pulse where cells were subjected to TcsL in acid medium (pH 4.0) for 10 min followed by replacement with neutral medium (pH 7.4) and TcdB1-500 plus PA. The cells were amended with inhibitor every 30 min for 12 h and then monitored for 18 h to determine CPEs. Samples and corresponding lines are labeled within the figure.
DISCUSSION
Attenuated mutants of TcdB inhibit the wild-type toxin at an intracellular site. To our knowledge this is the first report of the use of attenuated mutants to block wild-type toxin within the cytosol of intoxicated cells. Clearly, while unable to modify the substrate, the mutants carry out functions within the cytosol, which allow inhibition. The exact mode of action within the cytosol is unclear; however, it seems plausible that the inhibitor functions by preventing access to UDP-glucose. This is a feasible possibility since some of the inhibitors do not encompass the region of TcdB reported to interact with Rho, Rac, and Cdc42. Work by Hofmann et al. (17) using chimeric derivatives between the enzymatic domains of TcsL and TcdB suggested that residues 365 to 516 conferred substrate specificity. Our deletion analysis shows that residues 1 to 420 are able to inhibit TcdB intoxication, while the residue 1 to 170 deletion has no inhibitory effect. The residue 1 to 420 fusion apparently lacks a full substrate recognition region, yet contains regions predicted to interact with UDP-glucose. Finally, the mutants also inhibit TcsL, which shares only one substrate, Rac, with TcdB. If inhibition were due to Rho, Rac, and Cdc42 interaction, then the inhibitor should be less effective on TcsL, but it is not. These observations, taken together with our results indicating that Tcnα is not inhibited by TcdB, suggest that inhibition may occur at the cosubstrate level. Previous work by Chaves-Olarte and colleagues found that mutant cell lines with reduced levels of UDP-glucose were resistant to TcdB and TcsL (10). Similar to this, we now present data that suggest that preventing access to UDP-glucose within the cytosol of cells attenuates TcdB's cytotoxic activity. A second reasonable explanation for the protective effects is that LCTs form a higher-ordered complex during the enzymatic modification of small GTPases, and the mutant inhibitors poison this complex. TcdB shares a significantly higher homology with TcsL (80.1% overall) than with Tcnα (31.0% overall). Based on this homology the TcdB inhibitors might be more effective at incorporation into yet undefined complexes of TcsL than at integration into complexes of Tcnα. Both possible scenarios are now under investigation.
The anthrax toxin-derived PA-LFn delivery system is remarkably flexible and has several unique applications. The success of the PA-LFn system is due to the efficiency of cell entry and broad tropism of PA, as well as the amenability of LFn to a variety of heterologous fusions. Since the first description of PA-LFn by Arora et al. (1, 3) and Milne et al. (19), in which enzymatic domains of a variety of toxins were delivered to the cytosol of cells, the system has been used to deliver cytotoxic T-lymphocyte epitopes as well as DNA to cells. The results presented in the present study suggest that PA-LFn may also provide a unique platform for mutant-derived inhibitors.
The results presented here also prompt consideration of a new type of therapeutic against intracellular virulence factors. Recent development of dominant-negative inhibitors (DNIs) against anthrax toxin and Helicobacter pylori VacA provide a new method for treating toxin-based diseases (26, 29). Anthrax toxin-PA and VacA DNIs consist of mutant forms of the toxin, which if incorporated into oligomeric complexes block cytopathic activity. Unfortunately, oligomerization-dependent DNIs may not prove useful for other virulence factors, such as intracellular toxins that do not form higher-order complexes or toxins that preassemble within the bacterium. As an alternative, inhibitors targeting events within the cytosol may prove useful. By generation of mutants attenuated in cosubstrate recognition, transferase activity, or proteolysis, the resulting derivative may be able to protect cells from wild-type toxins. Such an approach may also be applicable to nontoxic virulence factors that function within the cytosol of target cells.
Acknowledgments
This work was supported in part by the Oklahoma Center for the Advancement of Science and Technology.
We thank Rodney Tweten for critical comments during the preparation of the manuscript.
Editor: J. T. Barbieri
REFERENCES
- 1.Arora, N., K. R. Klimpel, Y. Singh, and S. H. Leppla. 1992. Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J. Biol. Chem. 267:15542-15548. [PubMed] [Google Scholar]
- 2.Arora, N., and S. H. Leppla. 1994. Fusions of anthrax toxin lethal factor with Shiga toxin and diphtheria toxin enzymatic domains are toxic to mammalian cells. Infect. Immun. 62:4955-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arora, N., and S. H. Leppla. 1993. Residues 1-254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J. Biol. Chem. 268:3334-3341. [PubMed] [Google Scholar]
- 4.Arora, N., L. C. Williamson, S. H. Leppla, and J. L. Halpern. 1994. Cytotoxic effects of a chimeric protein consisting of tetanus toxin light chain and anthrax toxin lethal factor in non-neuronal cells. J. Biol. Chem. 269:26165-26171. [PubMed] [Google Scholar]
- 5.Ballard, J. D., R. J. Collier, and M. N. Starnbach. 1996. Anthrax toxin-mediated delivery of a cytotoxic T-cell epitope in vivo. Proc. Natl. Acad. Sci. USA 93:12531-12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barth, H., G. Pfeifer, F. Hofmann, E. Maier, R. Benz, and K. Aktories. 2001. Low pH-induced formation of ion channels by Clostridium difficile toxin B in target cells. J. Biol. Chem. 276:10670-10676. [DOI] [PubMed] [Google Scholar]
- 7.Boquet, P. 1999. Bacterial toxins inhibiting or activating small GTP-binding proteins. Ann. N. Y. Acad. Sci. 886:83-90. [DOI] [PubMed] [Google Scholar]
- 8.Busch, C., F. Hofmann, R. Gerhard, and K. Aktories. 2000. Involvement of a conserved tryptophan residue in the UDP-glucose binding of large clostridial cytotoxin glycosyltransferases. J. Biol. Chem. 275:13228-13234. [DOI] [PubMed] [Google Scholar]
- 9.Busch, C., F. Hofmann, J. Selzer, S. Munro, D. Jeckel, and K. Aktories. 1998. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J. Biol. Chem. 273:19566-19572. [DOI] [PubMed] [Google Scholar]
- 10.Chaves-Olarte, E., I. Florin, P. Boquet, M. Popoff, C. von Eichel-Streiber, and M. Thelestam. 1996. UDP-glucose deficiency in a mutant cell line protects against glucosyltransferase toxins from Clostridium difficile and Clostridium sordellii. J. Biol. Chem. 271:6925-6932. [DOI] [PubMed] [Google Scholar]
- 11.Ciesla, W. P., Jr., and D. A. Bobak. 1998. Clostridium difficile toxins A and B are cation-dependent UDP-glucose hydrolases with differing catalytic activities. J. Biol. Chem. 273:16021-16026. [DOI] [PubMed] [Google Scholar]
- 12.Czerucka, D., and P. Rampal. 2002. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect. 4:733-739. [DOI] [PubMed] [Google Scholar]
- 13.Feltis, B. A., S. M. Wiesner, A. S. Kim, S. L. Erlandsen, D. L. Lyerly, T. D. Wilkins, and C. L. Wells. 2000. Clostridium difficile toxins A and B can alter epithelial permeability and promote bacterial paracellular migration through HT-29 enterocytes. Shock 14:629-634. [DOI] [PubMed] [Google Scholar]
- 14.Garrett, W. S., L. M. Chen, R. Kroschewski, M. Ebersold, S. Turley, S. Trombetta, J. E. Galan, and I. Mellman. 2000. Developmental control of endocytosis in dendritic cells by Cdc42. Cell 102:325-334. [DOI] [PubMed] [Google Scholar]
- 15.Giry, M., M. R. Popoff, C. von Eichel-Streiber, and P. Boquet. 1995. Transient expression of RhoA, -B, and -C GTPases in HeLa cells potentiates resistance to Clostridium difficile toxins A and B but not to Clostridium sordellii lethal toxin. Infect. Immun. 63:4063-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez, J., C. Martinez, M. Giry, A. Garcia, and A. Rebollo. 1997. Rho prevents apoptosis through Bcl-2 expression: implications for interleukin-2 receptor signal transduction. Eur. J. Immunol. 27:2793-2799. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann, F., C. Busch, and K. Aktories. 1998. Chimeric clostridial cytotoxins: identification of the N-terminal region involved in protein substrate recognition. Infect. Immun. 66:1076-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Just, I., J. Selzer, M. Wilm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 19.Milne, J. C., S. R. Blanke, P. C. Hanna, and R. J. Collier. 1995. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 15:661-666. [DOI] [PubMed] [Google Scholar]
- 20.Mylonakis, E., E. T. Ryan, and S. B. Calderwood. 2001. Clostridium difficile-associated diarrhea: a review. Arch. Intern. Med. 161:525-533. [DOI] [PubMed] [Google Scholar]
- 21.Nusrat, A., C. von Eichel-Streiber, J. R. Turner, P. Verkade, J. L. Madara, and C. A. Parkos. 2001. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69:1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pothoulakis, C., and J. T. Lamont. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. II. The integrated response of the intestine to Clostridium difficile toxins. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G178-G183. [DOI] [PubMed] [Google Scholar]
- 23.Qa'Dan, M., M. Ramsey, J. Daniel, L. M. Spyres, B. Safiejko-Mroczka, W. Ortiz-Leduc, and J. D. Ballard. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell. Microbiol. 4:425-434. [DOI] [PubMed] [Google Scholar]
- 24.Qa'Dan, M., L. M. Spyres, and J. D. Ballard. 2001. pH-enhanced cytopathic effects of Clostridium sordellii lethal toxin. Infect. Immun. 69:5487-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qa'Dan, M., L. M. Spyres, and J. D. Ballard. 2000. pH-induced conformational changes in Clostridium difficile toxin B. Infect. Immun. 68:2470-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sellman, B. R., M. Mourez, and R. J. Collier. 2001. Dominant-negative mutants of a toxin subunit: an approach to therapy of anthrax. Science 292:695-697. [DOI] [PubMed] [Google Scholar]
- 27.Spyres, L. M., M. Qa'Dan, A. Meader, J. J. Tomasek, E. W. Howard, and J. D. Ballard. 2001. Cytosolic delivery and characterization of the TcdB glucosylating domain by using a heterologous protein fusion. Infect. Immun. 69:599-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Starnbach, M. N., and M. J. Bevan. 1994. Cells infected with Yersinia present an epitope to class I MHC-restricted CTL. J. Immunol. 153:1603-1612. [PMC free article] [PubMed] [Google Scholar]
- 29.Vinion-Dubiel, A. D., M. S. McClain, D. M. Czajkowsky, H. Iwamoto, D. Ye, P. Cao, W. Schraw, G. Szabo, S. R. Blanke, Z. Shao, and T. L. Cover. 1999. A dominant negative mutant of Helicobacter pylori vacuolating toxin (VacA) inhibits VacA-induced cell vacuolation. J. Biol. Chem. 274:37736-37742. [DOI] [PubMed] [Google Scholar]
- 30.Wagenknecht-Wiesner, A., M. Weidmann, V. Braun, P. Leukel, M. Moos, and C. von Eichel-Streiber. 1997. Delineation of the catalytic domain of Clostridium difficile toxin B-10463 to an enzymatically active N-terminal 467 amino acid fragment. FEMS Microbiol. Lett. 152:109-116. [DOI] [PubMed] [Google Scholar]
- 31.Whilhite, D. C., and S. R. Blanke. 1998. Soluble expression and one-step purification of recombinant Bacillus anthracis protective antigen. Protein Pept. Lett. 5:273-278. [Google Scholar]