Abstract
Th1 immune response is essential in the protection against mycobacterial intracellular pathogens. Lipoproteins trigger both humoral and cellular immune responses and may be candidate protective antigens. We studied in BALB/c mice the immunogenicity and the protection offered by the recombinant 27-kDa Mycobacterium tuberculosis lipoprotein and the corresponding DNA vaccine. Immunization with the 27-kDa antigen resulted in high titers of immunoglobulin G1 (IgG1) and IgG2a with a typical Th1 profile and a strong delayed hypersensitivity response. A strong proliferation response was observed in splenocytes, and significant nitric oxide production and gamma interferon secretion but not interleukin 10 secretion were measured. Based on these criteria, the 27-kDa antigen induced a typical Th1-type immune response thought to be necessary for protection. Surprisingly, in 27-kDa-vaccinated mice (protein or DNA vaccines) challenged by M. tuberculosis H37Rv or BCG strains, there was a significant increase in the numbers of CFU in the spleen compared to that for control groups. Furthermore, the protection provided by BCG or other mycobacterial antigens was completely abolished once the 27-kDa antigen was added to the vaccine preparations. This study indicates that the 27-kDa antigen has an adverse effect on the protection afforded by recognized vaccines. We are currently studying how the 27-kDa antigen modulates the mouse immune response.
The Th1-type immune response is believed to be necessary for protection against mycobacterial pathogens, such as Mycobacterium tuberculosis and Mycobacterium bovis. These facultative intracellular pathogens reside in mononuclear cells, which allow them to escape the immune response of the host. Therefore, there is a crucial requirement for a coordinated cellular immune response to control the infection (3, 17, 24). It has been shown that innate and cell-mediated immune responses can act as a double-edged sword. On one hand, an appropriate immune response can eradicate and control the pathogens; on the other hand, the same effectors might enhance the pathogens' survival, damage the host tissues, and cause death (37, 38). Thus, it is essential to define distinct mycobacterial components that might influence this immunological balance and choose the protective elements for vaccination while avoiding the harmful ones.
Lipoproteins are reported to affect both innate and adaptive immunity (27, 34) and have been identified as major antigens in M. tuberculosis, Treponema pallidum, and Mycoplasma hyorhinis (9, 36, 43). Several mycobacterial lipoproteins have been shown to be an important trigger in the activation of humoral and cellular immune responses to mycobacteria (43). One such protein, the 19-kDa antigen, is able to access the major histocompatibility complex class I pathway in macrophages, which is important in order to induce a protective Th1-type immune response against M. tuberculosis (33). The 19-kDa lipoprotein was also shown to induce antimicrobial activity and host defense mechanisms triggered through toll-like receptors (10, 39). Furthermore, lipoproteins were successfully used as potential protective antigens against different pathogens (11, 18). These observations indicate that lipoproteins might be appropriate candidates for mycobacterial vaccines.
Recently, the M. bovis 27-kDa lipoprotein was identified and shown to be recognized by the sera of cattle naturally infected with M. bovis (7). This lipoprotein is exclusively present in the M. tuberculosis species complex and forms an operon with a 55-kDa multidrug-resistant pump. Subcellular fractionation of M. bovis exclusively located the native 27-kDa lipoprotein in the membrane fraction (6, 7). In the present study we cloned the gene encoding the 27-kDa lipoprotein from M. tuberculosis and expressed its recombinant form in Escherichia coli. The immunogenicity and the potential protective efficacy of this antigen, delivered as subunit or as DNA vaccines, were analyzed in the M. tuberculosis and BCG challenge models in BALB/c mice.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female BALB/c mice aged 5 to 6 weeks old were purchased from Harlan (Jerusalem, Israel). Animals were maintained under specific-pathogen-free conditions during the experiments.
Plasmids.
M. tuberculosis H37Rv DNA was prepared as described previously (5). pQE70 vector (Qiagen, Hilden, Germany) was used to clone the LprG gene and express the recombinant 27-kDa protein in E. coli. In this system, a histidine tail is added to the C terminus of the expressed protein. The primers that were used to amplify the LprG gene encoding the 27-kDa protein from H37Rv DNA contained SphI and BglII restriction sites, and the sequences were 5-CCTGGCATGCGGACCCCCAGACGCCACTG and 3-CCAGATCTGCTCACCGGGGCTTCGTG. The plasmid encoding the LprG gene was termed pRHB27. For DNA vaccination, the LprG gene from the pRHB27 plasmid was cloned into the pcDNA3.1 vector (Invitrogen, San Diego, Calif.) under the control of the CMV promoter to generate the pcIHB27 vector. The pcIHB27 vector was then transfected to the E. coli XLI strain and purified using the Endo-free Giga prep kit (Qiagen). In both cases, the LprG gene was cloned with its signal peptide sequence. Sequence of the 27-kDa clone was confirmed to be correct in an automated sequencer (ABI; Perkin-Elmer, Applied Biosystems). Expression of the pcIHB27 vector in HeLa cells was tested following transfection with FuGENE 6 (Boehringer Mannheim Corp.) according to the manufacturer's instructions. The vector pRHB27 was used as a negative control. Isolation of mRNA was performed with the SV total RNA isolation system (Promega), and reverse transcription (RT)-PCR was carried out with the Access RT-PC system (Promega).
Purification of the 27-kDa recombinant protein expressed in E. coli.
The E. coli SG13009 strain harboring the pRHB27 plasmid was grown in Luria-Bertani medium containing kanamycin (10 μg/ml) (Sigma Chemical Co.) and ampicillin (100 μg/ml) (Biochemie GmbH, Kundl, Austria). At an optical density at 600 nm (OD600) of 0.7, the synthesis of the 27-kDa protein was induced by adding a 1 mM concentration of isopropyl-β-d-thiogalactopyranoside (IPTG) (Ornat, Rehovot, Israel). The incubation continued for 3 h at 37°C. Then cells were harvested and lysed, and the recombinant 27-kDa protein was purified using a nickel-nitrilotriacetic acid column (Qiagen, Hilden, Germany). The purified protein was dialyzed against saline and then transferred to a column containing immobilized polymyxin B (Sigma Chemical Co.) to remove endotoxin. The amount of lipopolysaccharide (LPS) in the protein fraction was measured quantitatively with the Limulus amebocyte lysate assay (Biowhittaker, Walkersville, Md.) and found to be <0.11 endotoxin unit (EU) per μg of antigen. The purified protein was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue.
Metabolic labeling with [3H]palmitate.
E. coli strain SG13009 harboring the pRHB27 vector was grown in 10 ml of Luria-Bertani medium containing ampicillin (100 μg/ml) and 5 μCi of n-[9,10-H3]palmitic acid (51 Ci mmol−1; Amersham). At an OD600 of 0.7, 1 mM IPTG was added, and the culture was incubated for an additional hour. The bacteria were harvested, and the recombinant 27-kDa protein was purified as described previously, submitted to SDS-PAGE, and stained with Coomassie blue. The gel was then dried, treated with N-salicylic acid enhancing solution, and exposed to X-ray film (SuperRX; Fujifilm) for 4 weeks at room temperature.
Mass spectrometry analysis of the 27-kDa antigen.
The recombinant M. tuberculosis 27-kDa antigen was separated by SDS-PAGE, and the bands corresponding to the recombinant protein were excised. The mass spectrometry was carried out with Qtof2 (Micromass, Manchester, England) using nanospray attachment. Data analysis was done using the biolynx package (Micromass), and database searches were performed with the Mascot package (Matrix Science, London, England).
Identification of the 27-kDa antigen in BCG and M. tuberculosis.
BCG Pasteur and M. tuberculosis H37Rv strains (107 cells/lane) were boiled in loading buffer and separated on an SDS-PAGE gel. The gel was transferred to a polyvinylidene difluoride membrane and incubated with rabbit anti-27-kDa serum for 2 h. Then, mouse anti-rabbit-horseradish peroxidase (Promega) (diluted 1:20,000) was added and incubated for 1 h. After 6 washes in phosphate-buffered saline (PBS), the membrane was dried and visualized by enhanced chemiluminescence. Rabbit anti-27-kDa serum was obtained by immunizing rabbits twice at 21-day intervals with the recombinant 27-kDa antigen emulsified in incomplete Freund adjuvant.
Immunization.
Mice were injected subcutaneously three or two times at 2-week intervals with either 10 or 50 μg of the recombinant 27-kDa antigen alone, emulsified in monophosphoryl lipid A-trehalose dicorynomycolate) Ribi adjuvant (Sigma Chemical Co.) or in dimethyldioctadecylammonium chloride (DDA) adjuvant (Fluka, Switzerland). Control mice were injected with saline, Ribi, or DDA. In other experiments mice were immunized twice at 2-week intervals with 85B, Esat6, and L7/L12 antigens (10 μg/antigen) alone or emulsified in Ribi, with or without the 27-kDa antigen (10 μg). For DNA vaccination, mice were injected intramuscularly three times at 2-week intervals in both quadricepses with pcIHB27 plasmid in PBS. Mice received 100 μg of DNA at each injection. Control mice were injected with the same amount of pcDNA3.1 vector. The amount of LPS in the DNA vaccine was measured and found to be <0.15 EU per 100 μg of DNA.
For BCG vaccination, groups of mice were vaccinated subcutaneously with 2 × 105 CFU of BCG Pasteur 1173 P2 (a gift from G. Marchal, Pasteur Institute, Paris, France) with or without 10 μg of the 27-kDa antigen. Parts of the groups were injected again with 10 μg of the 27-kDa antigen 14 days after the BCG vaccination.
Proliferation assay.
Three weeks after the last immunization, mice were sacrificed and splenocytes were aseptically harvested for the proliferation assay. Splenocytes from each group of mice were pooled and grown in 96-well plates (Nunc, Denmark) at a concentration of 5 × 105 cells per well. RPMI 1640 medium supplemented with 10% fetal calf serum, 1 mM glutamine, 25 mM HEPES, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 12.5 U of nystatin/ml (all purchased from Biological industries, Israel), and 5 × 10−5 M 2-mercaptoethanol (Merck, Germany) was used for in vitro cultures. Splenocytes were restimulated with 5 or 20 μg of 27-kDa antigen/ml or with 5 μg of concanavalin A (Sigma Chemical Co.)/ml. In some experiments, the antigen was preincubated with polymyxin B (10 μg/ml; Sigma Chemical Co.) for 1 h at 37°C before being added to the splenocytes. After 96 h of incubation, splenocytes were pulsed with 0.5 μCi of methyl-[3H]thymidine (25 Ci/mmol−1; Amersham Pharmacia Biotec)/well, harvested 16 h later with a cell harvester, and lysed, and the amount of incorporated methyl-[3H]thymidine was determined. We defined the stimulation index (SI) as the count-per-minute values obtained from the cells stimulated by the antigen divided by the count-per-minute values obtained from the cells without antigen stimulation.
Cytokine assays.
Secretion of interleukin 10 (IL-10) and gamma interferon by splenocytes of immunized mice was monitored as described above in supernatants collected at different intervals after in vitro stimulation of these splenocytes. Cytokine levels were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions, using commercial pairs of antibodies and recombinant cytokines (Pharmingen International). Ninety-six-well plates (Nunc, Roskilde, Denmark) were coated overnight with 2 μg of primary anti-cytokine capture monoclonal antibody/ml. The plates were then washed twice with PBS-0.05% Tween 80, and blocking was performed for 2 h with PBS-10% fetal calf serum. Subsequently, supernatant samples of splenocytes, stimulated by the antigens, or standards of recombinant cytokines were added to the coated microplates and incubated overnight at 4°C. Then, the plates were washed four times in PBS-0.05% Tween 80 followed by the addition of 1 μg of biotinylated rat anti-mouse cytokine detection monoclonal antibody/ml. After incubation for 1 h at room temperature, plates were washed six times before addition of peroxidase-conjugated streptavidin (Jackson Immunoresearch Laboratories). Plates were washed eight times, and 1 mg of O-phenylenediamine dihydrochloride/ml was added as a substrate (Sigma Chemical Co.) during 4 min at room temperature. Absorption was read at 450 nm using an ELISA reader (ELX-800UV; Bio-Tec instruments). The amount of cytokine in samples was extrapolated from a standard curve established with the relevant cytokine.
Nitric Oxide (NO) determination.
Nitric oxide levels were measured by the Griess assay (25). One hundred microliters of the supernatant of the splenocyte culture was added to 96-well plates, followed by 100 μl of Griess reagent (Sigma Chemical Co.). After incubation at room temperature for 4 min, absorption was read at 550 nm using an ELISA reader (ELX-800UV). Units of NO were determined by comparison with a standard curve using sodium nitrate (Sigma Chemical Co.).
Serum analysis.
Three weeks after the last immunization, blood was drawn from the mice; the sera were kept at −70°C until used. Anti-27-kDa-specific antibodies (immunoglobulin G1 [IgG1] and IgG2a) were measured by ELISA for each mouse. Ninety-six-well plates (Nunc) were coated overnight at 4°C with 1 μg of the recombinant 27-kDa antigen/well in PBS (pH 7.4) solution. The plates were washed twice with PBS-0.02% Tween/0.08% gelatin and blocked with PBS-0.4% gelatin (2 h at room temperature). Subsequently, mouse serum samples diluted serially in PBS-0.4% gelatin were added to the wells for overnight incubation at 4°C. This was followed by four washes in PBS-0.4% gelatin and the addition of anti-mouse IgG1 alkaline phosphatase conjugate (Southern Biotechnology) or anti-mouse IgG2a alkaline phosphatase conjugate (Southern Biotechnology). After incubation for 2 h at room temperature, plates were washed six times and 1 mg of P-nitrophenylphosphate solution/ml was added for 4 min (Kirkegaard & Perry Laboratories). Absorption was read at 405 nm using an ELISA reader (ELX-800UV).
DTH evaluation.
Three weeks after the last immunization, delayed-type hypersensitivity (DTH) was determined by injecting 10 μg of the recombinant 27-kDa protein in 20 μl of normal saline into the right footpad. The left footpad was injected with 20 μl of saline. To measure the DTH response to purified protein derivative (PPD), 1 week later the same mice were injected with 20 μl of saline containing 2.5 μg of PPD (Statens Serum Institute, Copenhagen, Denmark) into the left footpad and 20 μl of saline into the right footpad. DTH was also evaluated in mice previously vaccinated subcutaneously with 2 × 105 CFU of BCG Pasteur in the base of the tail. Two months after the BCG vaccination, mice were injected in the right footpad with the 27-kDa antigen (10 μg in 20 μl of saline) and with an equal volume of saline in the left footpad. Local swelling was measured with an engineer micrometer (Mitutoyo, Aurora, Japan).
Challenge.
Four weeks after the last immunization, mice were challenged intravenously in the tail vein with 5 × 105 CFU of M. tuberculosis strain H37Rv (a gift from G. Marchal, Pasteur Institute) or with 5 × 106 CFU of BCG Pasteur, both in 200 μl of saline. Four weeks (unless otherwise indicated) after the challenge, spleens were removed, homogenized in PBS, and plated on Middlebrook 7H9 agar (Difco) supplemented with 10% oleic acid-albumin-dextrose-catalase (Becton Dickinson). Numbers of CFU were determined after 4 weeks' incubation at 37°C.
Ethical considerations.
All experiments were performed in accordance with the regulations of the animal experimentation ethic committee of the Hebrew University-Hadassah Medical School.
Statistics analysis.
Data were analyzed by Student's t test, and P values of <0.05 were considered significant. The results shown are means. Analyses of proliferation, NO, cytokines, and serum antibody were done with pooled samples of three to five mice. The immunization experiments were repeated two to four times. DTH studies of mice were performed with five to seven mice for each group in three different experiments.
RESULTS
Expression of the recombinant 27-kDa antigen in E. coli.
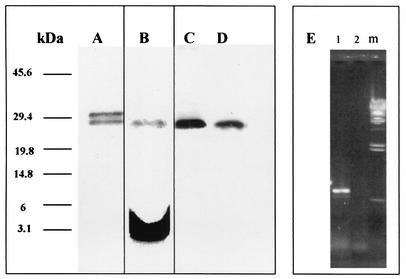
The sequence of the cloned M. tuberculosis LprG gene in pRHB27 was identical to that of the previously sequenced M. tuberculosis LprG gene (EMBL accession number Z80108, the Rv1411c gene) (13) and to the M. bovis gene (EMBL accession number AJ000500) (7). The cloned 27-kDa antigen was overexpressed with a histidine tag at the C-terminal portion. SDS-PAGE identified two distinct bands of 30 and 27 kDa (Fig. 1A). Mass spectrometry analysis identified both bands as the 27-kDa antigen, corresponding to the premature form and the mature form. The 27-kDa band and not the 30-kDa band was acylated (Fig. 1B) as shown by a [3H]palmitic acid incorporation experiment. N-terminal sequence analysis by the Edmann degradation method of the acylated 27-kDa band failed repeatedly. This was probably due to the posttranslational acylation of the first cysteine as has been shown for other lipoproteins (19, 35). The 27-kDa antigen preparations used in this work for mice immunization contained a mixture of both bands. The presence of the 27-kDa antigen in the mycobacterial strains used in this study was also evaluated. We found that both BCG Pasteur and M. tuberculosis H37Rv expressed the 27-kDa antigen (Fig. 1C and D, respectively) and therefore were relevant for the immunological study of this antigen.
FIG. 1.
Purification and metabolic radiolabeling of the recombinant 27-kDa antigen in E. coli and expression of the native form in BCG and M. tuberculosis. E. coli SG13009 cells harboring the plasmid pRHB27 were grown to an OD600 of 0.7 with or without [3H]palmitic acid. Protein production was induced by IPTG. The induced protein was purified using a nickel-nitrilotriacetic acid column, and samples were analyzed by SDS-PAGE and stained with Coomassie blue. The radioactively labeled samples were exposed to an X-ray film for 1 month. Numbers at the left indicate molecular masses in kilodaltons. Lane A, SDS-PAGE of the purified recombinant 27-kDa lipoprotein; lane B, an autoradiogram of the purified radiolabeled 27-kDa lipoprotein after SDS-PAGE analysis; lanes C and D, Western blot of BCG and M. tuberculosis H37Rv lysates, respectively, exposed to rabbit-anti-27-kDa serum; lane E, RT-PCR of the LprG gene in HeLa cells transfected with pIHB27 (1), pRHB27 as a control vector (2), and λ HindIII DNA marker (m).
Antibody responses to the 27-kDa antigen.
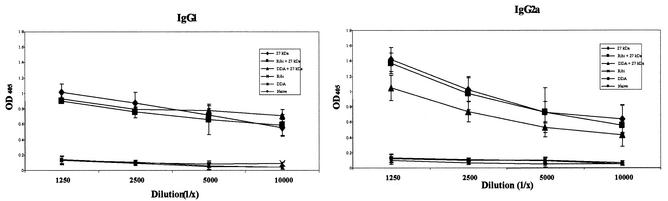
Mice were immunized with the 27-kDa antigen alone or emulsified in Ribi or DDA adjuvants. Mice were also immunized with the DNA of the pcIHB27 plasmid. Sera were collected from mice in the different groups 21 days after the last immunization, and titers of antibody were tested individually. Figure 2 illustrates the IgG1 and IgG2a antibody titers in the different groups of mice. High titers of antibody were detected in sera of mice immunized with the 27-kDa antigen alone as well as emulsified in Ribi or in DDA. IgG2a titers were higher than the IgG1 titers in the sera of 27-kDa- or Ribi-27-kDa-immunized mice (P < 0.05), whereas for the DDA-27-kDa group the titers of IgG1 and of IgG2a were similar. Mice that were immunized with the pcIHB27 DNA showed low titers of IgG2a (OD, 0.204 ± 0.07 at a 1:100 dilution) and no detectable titer of IgG1 antibody. No antibodies against the 27-kDa antigen were found in the control groups. Analysis of total IgG in sera from BCG-vaccinated mice indicated that the 27-kDa antigen was indeed recognized by the BCG-vaccinated mice (OD, 0.230 ± 0.04 at a 1:100 dilution; P < 0.0001).
FIG. 2.
Antibody response to the 27-kDa antigen in vaccinated mice. BALB/c mice were immunized subcutaneously with the 27-kDa antigen, Ribi-27-kDa, DDA-27-kDa, Ribi, DDA, and saline three times 2 weeks apart. Sera were collected and analyzed by ELISA for the presence of anti-27-kDa IgG1 and IgG2a antibodies. Each point represents the average and standard deviation of three independent experiments; in each experiment five individual mice were tested.
Proliferation and cytokine secretion analyses.
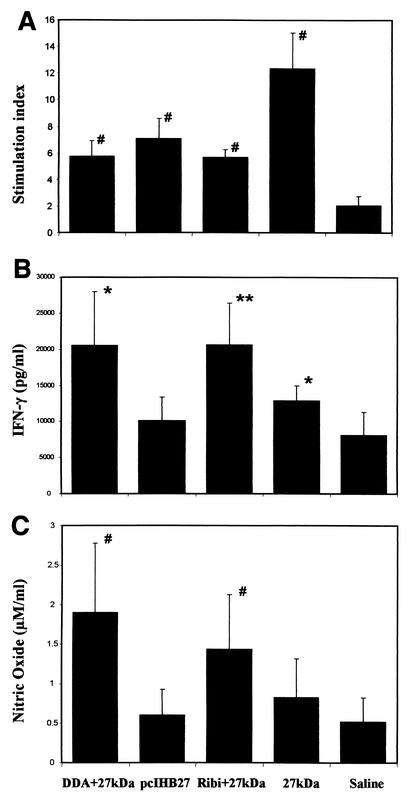
The Th1-type immune response is characterized by T-cell proliferation and a typical cytokines secretion pattern in response to antigen stimulation (31). We measured splenocyte proliferation, cytokine secretion profiles (IFN-γ and IL-10), and NO production to determine the type of immune response induced by the various immunization protocols. Figure 3A shows representative results of the proliferation resulting from stimulation with the 27-kDa antigen (20 μg/ml). A high SI was shown in the 27-kDa-immunized group (SI = 12), and weaker but still significant proliferation responses were shown in the other groups (SI = 6 to 7). Splenocytes of control groups immunized with adjuvants or the empty DNA vector alone and stimulated by the recombinant 27-kDA antigen showed an SI similar to that for the naive group (SI = 1.6 to 2). The Th1-related IFN-γ production was measured on supernatants of splenocyte cultures after 96 h of incubation with the recombinant 27-kDa antigen (Fig. 3B). The highest level of IFN-γ secretion was found in the Ribi-27-kDa-immunized (20,614 pg/ml; P < 0.01) and DDA-27-kDa-immunized (20,466 pg/ml; P < 0.05) groups, whereas the group immunized with the 27-kDa protein alone showed a low but still significant IFN-γ secretion compared to the naive group (12,814 pg/ml; P < 0.05). No significant IFN-γ secretion was found in the pcIHB27-immunized groups. IFN-γ levels of the naive group were 8,131 pg/ml. Control groups inoculated with Ribi or DDA alone showed IFN-γ production similar to that for the naive group (Ribi, 8,157 ± 800 pg/ml; DDA, 8,693 ± 276 pg/ml). Addition of polymyxin B to the recombinant antigen did not change the IFN-γ secretion levels. The results for NO production showed a pattern similar to that described for IFN-γ secretion. The emulsified 27-kDa antigen induced significant NO production (Ribi-27 kDa, 1.43 ± 0.68 μM/ml and P < 0.005; DDA-27 kDa, 1.89 ± 0.8 μM/ml and P < 0.001), whereas the 27-kDa protein alone and pcIHB27 induced only weak or nonsignificant NO production. Splenocytes from the control groups produced a smaller amount of NO production than the naive mice (Ribi, 0.25 ± 0.01 μM/ml; DDA, 0.45 ± 0.03 μM/ml; naive, 0.51 ± 0.3 μM/ml). We also examined the secretion of the Th2-associated cytokine IL-10. Our results (naive, 1,323 ± 788 pg/ml; 27 kDa, 2,400 ± 1,831 pg/ml; Ribi-27 kDa, 1,831 ± 1,183 pg/ml; pcIHB27, 2,022 ± 1,808 pg/ml; DDA-27 kDa, 1,860 ± 469 pg/ml) demonstrated that there was no significant secretion of this cytokine in the vaccinated groups compared to the control mice. The overall cytokines secretion profile supports the antibody isotype analysis, indicating that the immune response against the 27-kDa antigen was a Th1-type response (31).
FIG. 3.
Proliferation response and IFN-γ and NO secretion in 27-kDa-immunized mice after in vitro stimulation of the mouse splenocytes with the 27-kDa antigen. (A) Splenocyte proliferation for immunized mice exposed to 27-kDa antigen (20 μg/ml). The proliferation results presented are from one representative experiment (out of three experiments) with five mice in each group. IFN-γ secretion (B) and nitric oxide production (C) from splenocytes of immunized mice after 96 h of in vitro stimulation with the 27-kDa antigen (20 μg/ml) are shown. The IFN-γ and NO secretion results shown are the mean and standard deviation for seven experiments with three to five mice in each group. Data were analyzed by Student's t test. ∗, P < 0.05; ∗∗, P < 0.01; #, P < 0.005 (compared to the naive group).
DTH evaluation.
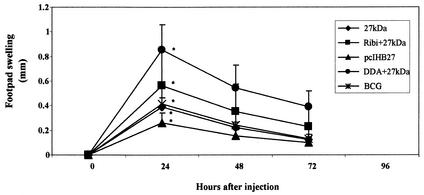
To investigate the ability of the 27-kDa antigen to prime a DTH response, we injected the 27-kDa antigen in the footpads of immunized mice 4 weeks after the last immunization. There was a very strong DTH response after 24 h (Fig. 4) in the DDA-27-kDa-immunized group (0.85 ± 0.19 mm) and a weaker but still significant DTH response in the other groups: Ribi-27 kDa (0.56 ± 0.3 mm), 27 kDa (0.39 ± 0.2 mm), and pcIHB27 (0.26 ± 0.07 mm). All these results were significantly different from the swelling measured in the contralateral footpad injected with saline (P < 0.001) and in the footpad of the control mice (P < 0.001). Injection of PPD (2.5 μg per footpad) into the footpads of the mice immunized with the different 27-kDa preparations did not result in footpad swelling, ruling out the presence of native 27-kDa in the PPD preparation. We also tested if the 27-kDa antigen induced a DTH response in BCG-vaccinated mice. Figure 4 shows that the DTH response in BCG-vaccinated mice was very similar to that of the 27-kDa-immunized mice (0.39 ± 0.2 mm in 27-kDa-immunized mice and 0.41 ± 0.04 mm in BCG-immunized mice after 24 h). The footpad swelling was of the same amplitude when the DTH was measured 1 or 3 months after the BCG vaccination (Fig. 4). To rule out the possibility that the DTH response in the BCG-vaccinated mice was due to lipids or other cell derivative contamination, we injected the footpads of BCG-vaccinated mice with a nonrelevant protein. We used for this purpose the E. coli protein IIBbgl, a component of the E. coli sugar transport system, expressed in E. coli strain SG13009 and purified the same way as the 27-kDa antigen. The results showed no footpad swelling in the BCG-vaccinated mice after 24 h (data not shown). Control groups also showed no significant footpad swelling in response to the 27-kDa injection (data not shown).
FIG. 4.
DTH evaluation in 27-kDa- or BCG-vaccinated mice. Mice were injected in the footpads with the 27-kDa antigen (10 μg) 4 weeks after the last vaccination or 1 and 3 months after BCG vaccination. Footpad swelling was measured, and the results shown are the means and standard deviations for three to five experiments with three to seven mice in each group. Data were analyzed by Student's t test. ∗, P < 0.001 (compared to naive control mice after 24 h).
Protection against M. tuberculosis H37Rv and a BCG challenge.
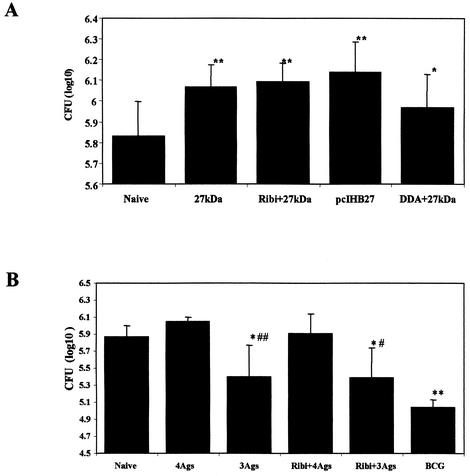
The DTH results, the high IgG2a/IgG1 ratio, and the significant level of IFN-γ and NO production in vaccinated mice demonstrated that the 27-kDa antigen was able to elicit a Th1-type immune response in mice. Such a response is believed to be necessary for bacterial clearance in mycobacterium-infected mice. Therefore, we challenged mice with 5 × 105 CFU of the M. tuberculosis H37Rv strain or with 5 × 106 CFU of the BCG Pasteur strain. Figure 5A and B and Fig. 6A present the CFU values for the different experiments with M. tuberculosis- and BCG-infected mice, respectively. Unexpectedly, CFU counts from the spleens of the 27-kDa-immunized group were consistently and significantly higher than those found for the control mice. The differences were remarkably greater with M. tuberculosis H37Rv-infected mice (Fig. 5A and B) compared to the BCG-infected mice (Fig. 6A). We then verified whether or not the 27-kDa antigen was able to influence the level of protection obtained by immunization with three other mycobacterial antigens. As shown in Fig. 6B, vaccination with the 85B antigen, the ribosomal L7/L12 protein, and the ESAT-6 antigen emulsified or not in Ribi resulted in a statistically significant reduction of the number of BCG CFU in the spleen (0.49 log; P < 0.05). However, adding the 27-kDa antigen to the antigen mixture eliminated the protection afforded by the three antigens alone (Fig. 6B). Furthermore, when mice were vaccinated with BCG and the 27-kDa antigen together (Fig. 5C), the relative protection offered by BCG against a challenge with the virulent M. tuberculosis H37Rv strain was completely abolished. The protection was also decreased even when the 27-kDa antigen was injected 2 weeks after the BCG immunization (Fig. 5C).
FIG. 5.
Intravenous M. tuberculosis H37Rv challenge. Mice were immunized twice with different formulations of the 27-kDa antigen; 4 weeks after the last injection, mice were challenged (intravenously) with 5 × 105 CFU of M. tuberculosis strain H37Rv. Four weeks (A) or seven weeks (B) after infection, numbers of CFU in mouse spleens were determined. (C) Mice were immunized subcutaneously with 2 × 105 CFU of BCG Pasteur with or without the 27-kDa antigen. After 2 weeks, parts of the groups (BCG/27 and BCG + 27/27) were injected with the 27-kDa antigen alone, and 6 weeks later mice were challenged (intravenously) with 5 × 105 CFU of M. tuberculosis H37Rv. (D) Timelines of the vaccination protocols used in Fig. 5C. Four weeks after the challenge, CFU at the spleens were counted. Data were analyzed by Student's t test. ∗, P < 0.05; ∗∗, P < 0.01; #, P < 0.005 (compared to the naive group).
FIG. 6.
Intravenous BCG Pasteur challenge. (A) Mice were immunized three times with the different 27-kDa antigen preparations. Four weeks after the last injection, mice were challenged intravenously with 5 × 106 CFU of BCG Pasteur. Four weeks after the infection, CFU in mouse spleens were measured. (B) Mice were injected twice with 85B, Esat6, and L7/L12 antigens in the absence (3Ags) or the presence of the 27-kDa antigen (4Ags). Another group was vaccinated subcutaneously with 2 × 105 CFU of BCG Pasteur. Four weeks after the last injection, mice were challenged intravenously with 5 × 106 CFU of BCG Pasteur, and after 4 weeks, CFU values in the spleen were determined. Data were analyzed by Student's t test. ∗, P < 0.05; ∗∗, P < 0.0001 (compared to the naive group). #, P < 0.01; ##, P < 0.005 (compared to the relevant 4Ags group).
DISCUSSION
In this study we analyzed the immunogenicity and the protective efficacy of the recombinant 27-kDa mycobacterial antigen. This antigen exists only in the M. tuberculosis species complex and was characterized by sequence analysis as a lipoprotein with a known signal peptidase type II motif. Subcellular fractionation of M. bovis exclusively located the native 27-kDa lipoprotein in the membrane fraction (7). The DNA sequences of M. tuberculosis and M. bovis LprG genes are identical. The LprG gene from M. tuberculosis was cloned for this study. Expression of the 27-kDa antigen in E. coli showed two distinct bands in SDS-PAGE of 30 and 27 kDa. The 30-kDa band was definitively identified by mass spectrometry as the 27-kDa prelipoprotein. Metabolic radiolabeling with palmitic acid indicated that only the recombinant 27-kDa band is acylated. The fact that the 30-kDa band was not radiolabeled indicates that the labeling is not due to nonspecific lipid absorption. Furthermore, it has been shown that the 27-kDa antigen has a signal peptidase type II site (7). For E. coli, the signal peptidase II enzyme required diacylglyceryl modification prior to cleavage (20, 26); this means that diacylglyceryl modification precedes cleavage of the signal peptide. Therefore, we assume that the mature 27-kDa antigen is actually acylated on the cysteine, which allowed its cleavage. Finally, the failure to sequence the mature lipoprotein by N-terminal sequence analysis can be explained by the presence of a lipid on the first cysteine that prevents sequencing as has been shown for other lipoproteins (19, 35). It is likely that posttranslation acylation in E. coli is different than that for mycobacteria. Therefore, the immune response against the recombinant acylated 27-kDa antigen might be different from the response against the native 27-kDa lipoprotein. Both forms of the recombinant 27-kDa antigen were used to immunize mice, since signal peptides were also found to take part in the immunogenicity (21, 28).
As mentioned before, serum from cattle naturally infected with M. bovis recognized the 27-kDa antigen (7), indicating that this antigen is a strong immunogen that induces specific antibody production in a natural infection. We also found specific anti-27 kDa antibodies in mice after immunization with BCG, which points out that the 27-kDa lipoprotein is actively recognized by the host during the course of infection. Indeed, preliminary results demonstrate that like the 19- and 38-kDa mycobacterial lipoproteins, the 27-kDa antigen is recognized by serum from BCG-vaccinated patients and tuberculosis patients (A. Acosta and H. Bercovier, personal communication).
Immunization with the 27-kDa antigen alone or with adjuvants resulted in a strong antibody response of both IgG1 and IgG2a isotypes. Furthermore, immunization with the 27-kDa antigen, alone or emulsified with Ribi, resulted in higher titers of IgG2a than of IgG1 (at a serum dilution of 1/1,250, the ratio was 1.4:1; P < 0.05). This indicates that the 27-kDa antigen induces preferentially a Th1-type response (22) even in BALB/c mice, where IgG1 (a Th2 isotype marker) is usually the predominant isotype (32). However, the DDA adjuvant induced a lesser titer of IgG2a that equaled the level of IgG1 (at a dilution of 1/1,250, the ratio was 1:1). This correlates with previous observations showing that DDA induces significant titers of IgG1 in mice although it predominantly stimulates Th1 cells (29). As expected, in contrast to the subunit vaccination, DNA vaccination induced low titers of antibody, mainly of the IgG2a isotype as reported for other DNA vaccination (14). The ability of the 27-kDa protein to induce strong antibody production, even without an adjuvant, might be due to the lipid moiety. Lipid moieties of lipoproteins have been found to enhance antibody production, since the elimination of the lipid reduced the immune response of the host against the antigen (2).
Lipoproteins may be important also in inducing a cell-mediated immune response. It has been shown that acylation can enhance a DTH response (4, 15). The 27-kDa-immunized mice developed a significant DTH response with all the protocols tested in this study. The DTH response for BCG-vaccinated mice was also similar to that for the 27-kDa-vaccinated mice. This indicates that the recombinant 27-kDa antigen is well recognized by the cellular immune response in mice during an experimental BGC infection. It might be of interest to test in the future whether this antigen can be used as a substitute of PPD to detect TB or contact patients or animals, as has been proposed for the 38-kDa and DPPD antigens (12, 40).
Cytokine secretion followed a pattern similar to that of the DTH response. Secretion of IFN-γ and NO in mice immunized with the emulsified 27-kDa antigen was higher than the secretion in mice immunized with the 27-kDa antigen alone or as a DNA vaccine (Fig. 3B and C). The relatively high basal level of IFN-γ secreted in naive splenocytes (8,131 pg/ml) was not due to LPS contamination, since the addition of polymyxin B did not abolish this secretion (data not shown). This finding may indicate that the 27-kDa antigen is able to induce IFN-γ secretion by itself, through a mechanism that might not involve antigen processing. It also has been found that there were no significant differences in the IL-10 secretion level between the vaccinated mice and the control groups. Thus, cytokine secretion profiles demonstrated that 27-kDa-immunized mice preferentially induced a Th1 response that has been found necessary for protection (16, 23).
Considering all the immunological test results, we tested the ability of the 27-kDa antigen to provide protection against M. tuberculosis or BCG challenges. However, immunization with the 27-kDa antigen did not provide protection when mice were challenged with M. tuberculosis or BCG. On the contrary, we noticed that the CFU numbers for the 27-kDa-immunized groups were higher than for the control groups after either M. tuberculosis (Fig. 5A and B) or BCG (Fig. 6A) infection. These results were similar using a lower antigen dose that could have been critical for obtaining an optimal immunization (8, 41). The differences in the CFU numbers between the immunized and the naive groups were statistically significant and varied between 0.5 and 0.8 logs after M. tuberculosis challenge. We also found that even 7 weeks after the M. tuberculosis challenge, the differences were still significant, although with lower values. In another experiments, we checked the influence of the 27-kDa antigen on the protection afforded by BCG vaccination against M. tuberculosis challenge (Fig. 5C). Surprisingly, the 27-kDa antigen completely abolished protection when it was injected simultaneously with the BCG vaccine. Protection was also reduced even if the 27-kDa antigen was injected 2 weeks after the BCG vaccination (Fig. 5C). The results for the BCG-challenged mice (Fig. 6A) were relatively lower than the M. tuberculosis results (up to 0.3 log) but nevertheless were reproducible and highly significant, as shown in the 27-kDa-immunized groups (P < 0.0001) (Fig. 6). Thus, we believe that the increase in CFU numbers in the BCG-challenged mice actually has an immunological meaning. We could not clarify yet why the deleterious effect of the 27-kDa protein was stronger in M. tuberculosis-infected mice than in BCG-infected mice, but we assume that it might be related to the virulence of the strains. We also found that the addition of the 27-kDa antigen to three other mycobacterial antigens abolished the protection provided by these three antigens against a BCG challenge (Fig. 6B). Our findings demonstrated the harmful influence of the 27-kDa antigen on the immune system submitted to a bacterial challenge. Since the deleterious effect of the 27-kDa antigen was measured only in the spleen, other organs, such as the lungs, should be investigated in the future to assess fully the effect of the 27-kDa protein on the immune response.
An apparently similar phenomenon was reported for another mycobacterial antigen, the 19-kDa lipoprotein. This antigen has a deleterious effect on protection when expressed in Mycobacterium vaccae or Mycobacterium smegmatis, although it induces a strong Th1-type immune response (1, 42). However, in contrast to results with the 27-kDa antigen, immunization with the 19-kDa antigen as a protein or as a DNA vaccine showed no such negative effect on protection. Therefore, we can assume that the 27-kDa antigen acts in a different manner than the 19-kDa antigen.
Recently it has been suggested that mycobacterial antigens exacerbate disease manifestation in M. tuberculosis-infected mice (30). Antigens like the 27-kDa antigen and the 19-kDa antigen, which are immunologically recognized by the host but which diminish the protective immune response, might take part in such a phenomenon. In the quest for a better vaccine against mycobacterial pathogens, individual mycobacterial proteins have been screened for their ability to induce a Th1 response. The data presented in this work point out the fact that this strategy does not always result in the finding of a protective antigen. The study of antigens like the 19-kDa and the 27-kDa lipoproteins, which are strong elicitors of the Th1-type response but have a negative effect on protection, underlines the complexity in mycobacterial vaccine design.
Acknowledgments
This work was supported in part by a grant from The Israel Science Foundation (Center of Excellence Program No. 8004/98) and by a grant from the CSED.
We thank Roni Itai and J. M. Gershoni for their helpful comments. We also thank Ariel Gaathon for his contribution in protein analysis by mass spectrometry and Edmann degradation.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abou-Zeid, C., M. P. Gares, J. Inwald, R. Janssen, Y. Zhang, D. B. Young, C. Hetzel, J. R. Lamb, S. L. Baldwin, I. M. Orme, V. Yeremeev, B. V. Nikonenko, and A. S. Apt. 1997. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect. Immun. 65:1856-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkoyunlu, M., A. Melhus, C. Capiau, O. van Opstal, and A. Forsgren. 1997. The acylated form of protein D of Haemophilus influenzae is more immunogenic than the nonacylated form and elicits an adjuvant effect when it is used as a carrier conjugated to polyribosyl ribitol phosphate. Infect. Immun. 65:5010-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, P. 1997. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand. J. Immunol. 45:115-131. [DOI] [PubMed] [Google Scholar]
- 4.Bachrach, G., M. Banai, S. Bardenstein, G. Hoida, A. Genizi, and H. Bercovier. 1994. Brucella ribosomal protein L7/L12 is a major component in the antigenicity of brucellin INRA for delayed-type hypersensitivity in brucella-sensitized guinea pigs. Infect. Immun. 62:5361-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bercovier, H., O. Kafri, and S. Sela. 1986. Mycobacteria possess a surprisingly small number of ribosomal RNA genes in relation to the size of their genome. Biochem. Biophys. Res. Commun. 136:1136-1141. [DOI] [PubMed] [Google Scholar]
- 6.Bigi, F., A. Alito, M. I. Romano, M. Zumarraga, K. Caimi, and A. Cataldi. 2000. The gene encoding P27 lipoprotein and a putative antibiotic-resistance gene form an operon in Mycobacterium tuberculosis and Mycobacterium bovis. Microbiology 146:1011-1018. [DOI] [PubMed] [Google Scholar]
- 7.Bigi, F., C. Espitia, A. Alito, M. Zumarraga, M. I. Romano, S. Cravero, and A. Cataldi. 1997. A novel 27 kDa lipoprotein antigen from Mycobacterium bovis. Microbiology 143:3599-3605. [DOI] [PubMed] [Google Scholar]
- 8.Bretscher, P. A. 1992. A strategy to improve the efficacy of vaccination against tuberculosis and leprosy. Immunol. Today 13:342-345. [DOI] [PubMed] [Google Scholar]
- 9.Bricker, T. M., M. J. Boyer, J. Keith, R. Watson-McKown, and K. S. Wise. 1988. Association of lipids with integral membrane surface proteins of Mycoplasma hyorhinis. Infect. Immun. 56:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 11.Brown, J. S., A. D. Ogunniyi, M. C. Woodrow, D. W. Holden, and J. C. Paton. 2001. Immunization with components of two iron uptake ABC transporters protects mice against systemic Streptococcus pneumoniae infection. Infect. Immun. 69:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos-Neto, A., V. Rodrigues-Junior, D. B. Pedral-Sampaio, E. M. Netto, P. J. Ovendale, R. N. Coler, Y. A. Skeiky, R. Badaro, and S. G. Reed. 2001. Evaluation of DPPD, a single recombinant Mycobacterium tuberculosis protein as an alternative antigen for the Mantoux test. Tuberculosis (Edinburgh) 81:353-358. [DOI] [PubMed] [Google Scholar]
- 13.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 14.Coler, R. N., A. Campos-Neto, P. Ovendale, F. H. Day, S. P. Fling, L. Zhu, N. Serbina, J. L. Flynn, S. G. Reed, and M. R. Alderson. 2001. Vaccination with the T cell antigen Mtb 8.4 protects against challenge with Mycobacterium tuberculosis. J. Immunol. 166:6227-6235. [DOI] [PubMed] [Google Scholar]
- 15.Coon, J., and R. Hunter. 1975. Properties of conjugated protein immunogens which selectively stimulate delayed-type hypersensitivity. J. Immunol. 114:1518-1522. [PubMed] [Google Scholar]
- 16.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper, A. M., and J. L. Flynn. 1995. The protective immune response to Mycobacterium tuberculosis. Curr. Opin. Immunol. 7:512-516. [DOI] [PubMed] [Google Scholar]
- 18.Cote-Sierra, J., A. Bredan, C. M. Toldos, B. Stijlemans, L. Brys, P. Cornelis, M. Segovia, P. de Baetselier, and H. Revets. 2002. Bacterial lipoprotein-based vaccines induce tumor necrosis factor-dependent type 1 protective immunity against Leishmania major. Infect. Immun. 70:240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De, B. K., J. S. Sampson, E. W. Ades, R. C. Huebner, D. L. Jue, S. E. Johnson, M. Espina, A. R. Stinson, D. E. Briles, and G. M. Carlone. 2000. Purification and characterization of Streptococcus pneumoniae palmitoylated pneumococcal surface adhesin A expressed in Escherichia coli. Vaccine 18:1811-1821. [DOI] [PubMed] [Google Scholar]
- 20.Dev, I. K., and P. H. Ray. 1984. Rapid assay and purification of a unique signal peptidase that processes the prolipoprotein from Escherichia coli B. J. Biol. Chem. 259:11114-11120. [PubMed] [Google Scholar]
- 21.Faith, A., C. Moreno, R. Lathigra, E. Roman, M. Fernandez, S. Brett, D. M. Mitchell, J. Ivanyi, and A. D. Rees. 1991. Analysis of human T-cell epitopes in the 19,000 MW antigen of Mycobacterium tuberculosis: influence of HLA-DR. Immunology 74:1-7. [PMC free article] [PubMed] [Google Scholar]
- 22.Finkelman, F. D., J. Holmes, I. M. Katona, J. F. Urban, Jr., M. P. Beckmann, L. S. Park, K. A. Schooley, R. L. Coffman, T. R. Mosmann, and W. E. Paul. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8:303-333. [DOI] [PubMed] [Google Scholar]
- 23.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 26.Hussain, M., S. Ichihara, and S. Mizushima. 1980. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J. Biol. Chem. 255:3707-3712. [PubMed] [Google Scholar]
- 27.Infante-Duarte, C., and T. Kamradt. 1997. Lipopeptides of Borrelia burgdorferi outer surface proteins induce Th1 phenotype development in alphabeta T-cell receptor transgenic mice. Infect. Immun. 65:4094-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamb, J. R., A. D. Rees, V. Bal, H. Ikeda, D. Wilkinson, R. R. De Vries, and J. B. Rothbard. 1988. Prediction and identification of an HLA-DR-restricted T cell determinant in the 19-kDa protein of Mycobacterium tuberculosis. Eur. J. Immunol. 18:973-976. [DOI] [PubMed] [Google Scholar]
- 29.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreira, A. L., L. Tsenova, M. Haile Aman, L. G. Bekker, S. Freeman, B. Mangaliso, U. Schroder, J. Jagirdar, W. N. Rom, M. G. Tovey, V. H. Freedman, and G. Kaplan. 2002. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect. Immun. 70:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 32.Natsuume-Sakai, S., K. Motonishi, and S. Migita. 1977. Quantitative estimations of five classes of immunoglobulin in inbred mouse strains. Immunology 32:861-866. [PMC free article] [PubMed] [Google Scholar]
- 33.Neyrolles, O., K. Gould, M. P. Gares, S. Brett, R. Janssen, P. O'Gaora, J. L. Herrmann, M. C. Prevost, E. Perret, J. E. Thole, and D. Young. 2001. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166:447-457. [DOI] [PubMed] [Google Scholar]
- 34.Oftung, F., H. G. Wiker, A. Deggerdal, and A. S. Mustafa. 1997. A novel mycobacterial antigen relevant to cellular immunity belongs to a family of secreted lipoproteins. Scand. J. Immunol. 46:445-451. [DOI] [PubMed] [Google Scholar]
- 35.Prestidge, R. L., P. M. Grandison, D. W. Chuk, R. J. Booth, and J. D. Watson. 1995. Production of the 19-kDa antigen of Mycobacterium tuberculosis in Escherichia coli and its purification. Gene 164:129-132. [DOI] [PubMed] [Google Scholar]
- 36.Radolf, J. D., E. J. Robinson, K. W. Bourell, D. R. Akins, S. F. Porcella, L. M. Weigel, J. D. Jones, and M. V. Norgard. 1995. Characterization of outer membranes isolated from Treponema pallidum, the syphilis spirochete. Infect. Immun. 63:4244-4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reina-San-Martin, B., A. Cosson, and P. Minoprio. 2000. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol. Today 16:62-67. [DOI] [PubMed] [Google Scholar]
- 38.Rook, G. A., and R. Hernandez-Pando. 1996. The pathogenesis of tuberculosis. Annu. Rev. Microbiol. 50:259-284. [DOI] [PubMed] [Google Scholar]
- 39.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 40.Vordermeier, H. M., D. P. Harris, P. K. Mehrotra, E. Roman, A. Elsaghier, C. Moreno, and J. Ivanyi. 1992. M. tuberculosis-complex specific T-cell stimulation and DTH reactions induced with a peptide from the 38-kDa protein. Scand. J. Immunol. 35:711-718. [DOI] [PubMed] [Google Scholar]
- 41.Weinrich Olsen, A., L. A. van Pinxteren, L. Meng Okkels, P. Birk Rasmussen, and P. Andersen. 2001. Protection of mice with a tuberculosis subunit vaccine based on a fusion protein of antigen 85b and esat-6. Infect. Immun. 69:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeremeev, V. V., I. V. Lyadova, B. V. Nikonenko, A. S. Apt, C. Abou-Zeid, J. Inwald, and D. B. Young. 2000. The 19-kD antigen and protective immunity in a murine model of tuberculosis. Clin. Exp. Immunol. 120:274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young, D. B., and T. R. Garbe. 1991. Lipoprotein antigens of Mycobacterium tuberculosis. Res. Microbiol. 142:55-65. [DOI] [PubMed] [Google Scholar]