Abstract
Purified Actinobacillus actinomycetemcomitans serotype b lipopolysaccharide (LPS) was found to be able to bind Fusobacterium nucleatum cells and to inhibit binding of F. nucleatum to A. actinomycetemcomitans serotype b. Sugar binding studies showed that the requirements for binding of A. actinomycetemcomitans serotype b LPS to the F. nucleatum lectin are the presence of a metal divalent ion, an axial free hydroxyl group at position 4, and free equatorial hydroxyl groups at positions 3 and 6 of d-galactose, indicating that the β-N-acetyl-d-galactosamine in the serotype b LPS trisaccharide repeating unit is the monosaccharide residue recognized by the F. nucleatum lectin. These data strongly suggest that A. actinomycetemcomitans serotype b LPS is one of the receptors responsible for the lactose-inhibitable coaggregation of A. actinomycetemcomitans to fusobacteria.
Lactose-inhibitable coaggregation is a common interaction among oral bacteria, including periodontal microorganisms such as Actinobacillus actinomycetemcomitans and Fusobacterium nucleatum. Binding of these two bacteria is mediated by a galactoside moiety on the A. actinomycetemcomitans surface and a lectin on F. nucleatum. Protease treatment or heating the F. nucleatum at 85°C completely prevents coaggregation with A. actinomycetemcomitans. On the other hand, when these treatments were applied to the A. actinomycetemcomitans partner, coaggregation was not affected (9).
A. actinomycetemcomitans is a nonmotile, gram-negative capnophilic, fermentative coccobacillus that has been implicated in the etiology and pathogenesis of juvenile (30) and adult (24) periodontitis as well as systemic infections (20). A. actinomycetemcomitans strains isolated from the human oral cavity are divided into six serotypes, a to f (3, 7, 19, 31). The serotype-specific antigens are major targets of the humoral response in periodontitis patients colonized by these species (1, 22). These antigens are located in the O-polysaccharide (O-PS) region of the lipopolysaccharide (LPS) (16, 18, 27). The chemical structures of the A. actinomycetemcomitans serotype a to f antigenic O-PSs were determined (7, 17, 18), and the DNA sequences of the genes involved in their synthesis have been described previously (7, 14, 15, 25, 28, 29). The structural differences between these antigens are the basis for the absence of cross-reactivity among the different A. actinomycetemcomitans serotypes (1, 22), with the exception of serotypes b and f, which show serological cross-reactivity, probably due to a common β-N-acetyl-galactosamine epitope (7).
Of these strains, serotype b is most frequently isolated from subjects with localized juvenile periodontitis (30, 31), who exhibit elevated serum antibody levels to serotype b-specific antigen (1, 22). The serotype b O-PS region of the LPS (18) consists of a polymer of repeating trisaccharide units with the structure →3)α-d-Fucp-(1→2)-3-O-(β-d-GalpNAc)-α l-Rhap(1→.
F. nucleatum strains are the most numerous gram-negative bacteria isolated from healthy periodontal sites and are the most common predominant pathogen in subsequent periodontal destruction (4, 13). F. nucleatum strains were shown to be able to coaggregate all species of oral bacteria tested (9, 10) and thus play an important part in the development of dental plaque.
Two different galactose-binding adhesins of F. nucleatum were proposed to be responsible for the lactose-inhibitable coaggregation with Porphyromonas gingivalis (9) and to its attachment to mammalian cells (26): a major 42-kDa membrane protein (8) and a surface 30-kDa polypeptide extracted from the surface of the bacteria (21). While these preliminary studies were focused on the characterization of the F. nucleatum adhesins, there have been no reports on the identification and characterization of the complementary receptors on the gram-negative anaerobic partners. The aim of the present study was to examine the role of LPS from A. actinomycetemcomitans serotype b as a possible receptor for the lactose-inhibitable coaggregation with F. nucleatum. The minimal carbohydrate structural requirements for recognition by the F. nucleatum galactose-binding lectin are also reported.
(Work by I.N. was performed as part of the M.Sc. degree at Hebrew University, Jerusalem, Israel.)
A. actinomycetemcomitans strains Y4, JP2 (serotype b), and ATCC 29523 (serotype a) were grown as previously described (23) at 37°C in 5% CO2. F. nucleatum PK 1594 was grown in Wilkins-Chalgren anaerobe broth (Oxoid) at 37°C under anaerobic conditions. For the coaggregation or binding assays, bacterial cells were harvested, washed with coaggregation buffer (CB; 10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.02% NaN3), and stored at 4°C until used.
LPS was prepared as previously described (18). After ultracentrifugation, the LPS was further purified by gel filtration on a Sephacryl S-400 HR (900 by 16 mm; Pharmacia Fine Chemicals in an AKTA explorer system; Amersham Biosciences) at room temperature with disaggregation buffer (0.05 M Tris-HCl [pH 9.0], 0.001 M EDTA, 0.3% deoxycholate) as eluent. Fractions containing LPS were identified by silver staining and precipitated by addition of 0.15 M NaCl and 4 volumes of 95% ethanol. The precipitates were isolated by centrifugation at 12,000 × g for 20 min at 4°C, pooled, dissolved in water, dialyzed against water, and lyophilized. The O-PS was prepared and purified as described by Perry et al. (18). The high-molecular-weight fraction obtained from the Sephadex G-50 column chromatography contained the O-PS (Kav, 0.04).
Coaggregation was routinely assayed by the visual coaggregation assay as described by Kolenbrander et al. (9). Coaggregation scores from 0 to 4+ were monitored visually according to the scale described by Cisar et al. (2).
Coaggregate formation by accretion onto a partner cell-coated microtiter well surface was adapted from the assay described by Jenkinson et al. (6). Unlabeled A. actinomycetemcomitans cells, adjusted to a density of 108 cells per ml in CB, and 50-μl samples were applied to the wells of 96-well microtiter plates (Maxisorp, Nunc, Denmark). The plates were centrifuged at 800 × g at 20°C for 5 min and further incubated at 4°C for 16 h. The plates were blocked for 2 h at room temperature by adding 200 μl of 0.4% Tween 20 in CB.
Radioactively labeled samples of 50 μl of [3H]N-acetyl-glucosamine-labeled F. nucleatum cells (1.7 × 107 cells; specific radioactivity about 103 cells per cpm) were added to the wells, and the plates were incubated for 2 h on a rotary shaker. The wells were washed four times with 0.05% Tween 20 in CB. Accreted cells were removed from the plastic surface by adding 100 μl of a solution containing 1% sodium dodecyl sulfate and 0.4 M NaOH for 2 h and transferring the liquid contents for determination of radioactivity. The assays were performed in quadruplicate, and wells without the unlabeled partner were used as control wells.
Binding of 3H-labeled F. nucleatum cells to either A. actinomycetemcomitans LPS or O-PS was tested by the same assay, but the plates were coated with either 100 μl of LPS or O-PS (100 μg/ml of CB). When the inhibitory effect of sugars, EDTA, or LPS was tested, the 3H-labeled F. nucleatum cells were preincubated for 30 min at room temperature at the indicated concentrations before being added to the plates. All sugars were obtained from Sigma and are of the d-configuration and in pyranose form, unless otherwise indicated.
The percentage of inhibition was calculated as [(binding in the absence of inhibitor − binding in the presence of inhibitor)/binding in the absence of inhibitor] × 100.
For each of the measures (before and after inhibition), the mean, standard deviation, and coefficient of variation were calculated. Statistical analysis consisted of a two-tailed nonpaired t test for comparing the mean inhibition with A. actinomycetemcomitans ATCC 29523 LPS versus that with Y4 or JP2 LPS.
A. actinomycetemcomitans strains Y4 and JP2 (serotype b) coaggregated with F. nucleatum PK 1594, while A. actinomycetemcomitans ATCC 29523 (serotype a) showed no visible coaggregation (Table 1). The coaggregation between the two serotype b A. actinomycetemcomitans strains and F. nucleatum was completely inhibited by 10 mM galactose (Gal), while 20 mM glucose (Glc) was without effect. EDTA (2 mM) also completely inhibited coaggregation.
TABLE 1.
Coaggregation of F. nucleatum PK 1594 and different A. actinomycetemcomitans strains in the presence of monosaccharides or EDTA
| A. actinomycetemcomitans strain | Inhibitor additiona | Coaggregation scoreb |
|---|---|---|
| Y4 | None | 3 |
| Gal or GalNAc (10 mM) | 0 | |
| Glc (20 mM) | 3 | |
| JP2 | None | 2 |
| Gal or GalNAc (10 mM) | 0 | |
| Glc (20 mM) | 2 | |
| ATCC 29523 | None | 0 |
| Y4 or JP2 | EDTA (2 mM) | 0 |
Final inhibitor concentration.
The coaggregation score was determined by the visual coaggregation assay (9).
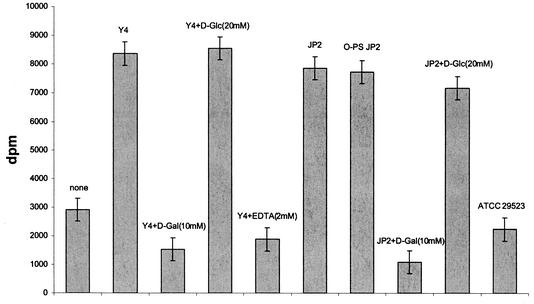
The purified LPSs from the two serotype b A. actinomycetemcomitans strains (Y4 and JP2) and the serotype a strain (ATCC 29523) as well as O-PS from strain JP2 were tested for their capacity to bind to F. nucleatum. LPSs from A. actinomycetemcomitans serotype b strains Y4 and JP2 were found to bind to F. nucleatum cells as compared to controls (without LPS) and to LPS from A. actinomycetemcomitans serotype a, which did not bind to F. nucleatum (Fig. 1). Furthermore, O-PS from A. actinomycetemcomitans JP2 bound F. nucleatum to the same extent as LPS from A. actinomycetemcomitans JP2 (Fig. 1). Binding of F. nucleatum to LPS from serotype b strains Y4 and JP2 was completely inhibited by 10 mM Gal or 2 mM EDTA (not shown for LPS from strain JP2), while no inhibition by 20 mM Glc could be observed (Fig. 1).
FIG. 1.
Binding of 3H-labeled F. nucleatum cells to LPS of A. actinomycetemcomitans strains Y4, JP2, and ATCC 29523 and to O-PS from strain JP2 in the absence or presence of monosaccharides and EDTA. Binding is expressed as radioactivity accreted to the wells.
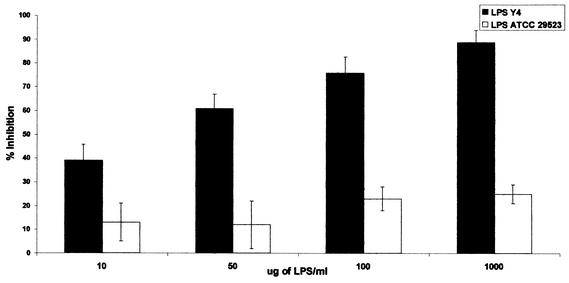
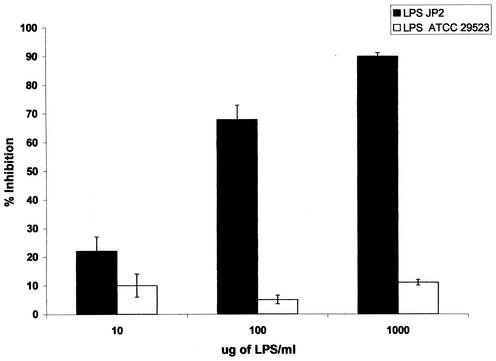
The LPSs from A. actinomycetemcomitans serotype b strains Y4 and JP2 were tested for their capacity to interfere with the binding of F. nucleatum to A. actinomycetemcomitans cells. A. actinomycetemcomitans serotype a LPS was used as a control. As shown in Fig. 2, Y4 LPS inhibited binding of F. nucleatum to Y4 A. actinomycetemcomitans cells in a dose-dependent manner. Inhibition by serotype b LPS Y4 was significantly greater than the inhibition observed with serotype a LPS (75% inhibition for the Y4 LPS versus 25% inhibition for the ATCC 29523 LPS at 100 μg/ml). A similar dose-dependent inhibition was observed when JP2 LPS was used to inhibit binding of F. nucleatum to A. actinomycetemcomitans JP2 cells (Fig. 3): 70% inhibition for JP2 LPS versus 5% inhibition for the serotype a LPS at 100 μg/ml.
FIG. 2.
Inhibition of binding of 3H-labeled F. nucleatum cells to Y4 A. actinomycetemcomitans cells at the indicated Y4 LPS concentrations. P < 0.01 for inhibition by A. actinomycetemcomitans Y4 LPS versus ATCC 29523 LPS at 10, 50, and 100 μg/ml; P < 0.001 for inhibition by LPS at 1,000 μg/ml.
FIG. 3.
Inhibition of binding of 3H-labeled F. nucleatum cells to JP2 A. actinomycetemcomitans cells at the indicated JP2 LPS concentrations. P < 0.05 for inhibition by A. actinomycetemcomitans JP2 LPS versus ATCC 29523 LPS at 10 μg/ml; P < 0.001 for inhibition by LPS at 100 and 1,000 μg/ml.
To study the interaction between the F. nucleatum lectin and the serotype b A. actinomycetemcomitans LPS receptor, the effect of a range of concentrations of different saccharides on binding of F. nucleatum to Y4 LPS was tested. For each compound examined, inhibition curves were constructed. Based on these curves, we estimated the concentration of each saccharide that inhibited binding of F. nucleatum to Y4 LPS by 50% (I50). Table 2 shows the I50s of the different carbohydrates. The best inhibitors of the F. nucleatum lectin were Gal, lactose, and related compounds with a free and axial hydroxyl group at position 4 and equatorial free hydroxyl groups at positions 3 and 6: N-acetyl-galactosamine (GalNAc), 2-deoxy-Gal, methyl-α-galactoside (α-MeGal), raffinose, and mellibiose. Glc and mannose (Man) at 100 mM and l-rhamnose (l-Rha) at 50 mM with an equatorial hydroxyl at position 4 could not inhibit bacterial binding. Furthermore, cellobiose was at least 60 times less active than lactose. Taking into account that cellobiose is identical to lactose, except for the orientation of the 4-hydroxyl group in the nonreducing sugar (Gal in lactose and Glc in cellobiose), it is reasonable that the lectin interacts with the 4-hydroxyl group of Gal. This is also indicated by the loss of inhibitory activity of the galactose derivative substituted at position 4, Gal-4 sulfate. d-Gulose, the 3-epimer of Gal, had also no inhibitory effect, indicating that the interaction probably involves the equatorial orientation of the 3-hydroxyl group of Gal. In contrast, Gal derivatives with substituents at position 2 were substantially as inhibitory as Gal: GalNAc and 2-deoxy-Gal showed I50s comparable to those of Gal. Position 6 of Gal also appears to be important for binding, since fucose (Fuc; 6-deoxy-galactose) was 22 times less active than Gal, and the derivative with a negatively charged group at this position (Gal-6 sulfate) was virtually inactive. Structural analysis of LPS from the serotype a A. actinomycetemcomitans strain (ATCC 29523) indicated that its O-PS contains 6-deoxy-d-talose (6dTalp) and O-acetyl (2:1) and is a polymer of disaccharide repeating units with the structure:→3)-α-d-6dTalp(1→2)-α-d-6dTalp-(1→ (17). F. nucleatum cells neither coaggregate with A. actinomycetemcomitans strain ATCC 29523 nor bind to its LPS. Although talose is the 2-epimer of Gal, a position that is not necessary for binding, the substitution of the C-6 hydroxyl group probably renders the A. actinomycetemcomitans serotype a LPS incapable of binding to F. nucleatum under our experimental conditions. α-MeGal, raffinose, mellibiose, and lactose also had the same I50s as Gal, indicating that substitution at the anomeric carbon atom (α or β) does not influence the binding activity. The comparable I50s of lactose and Gal also indicate that the hydroxyl groups of Glc do not participate in the binding, as in the case of mammalian galectins (5).
TABLE 2.
Inhibition of binding of F. nucleatum to Y4 LPS by a series of saccharides
| Inhibitor | Formula | I50 (mM)a |
|---|---|---|
| Gal | 0.78 | |
| GalNAC | 0.78 | |
| 2-Deoxy-Gal | 0.85 | |
| Gal-4-sulphate | NI (20) | |
| Gal-6-sulphate | NI (50) | |
| Gulose | NI (50) | |
| Fuc | 17 | |
| l-Rha | NI (50) | |
| Glc | NI (100) | |
| Man | NI (100) | |
| α-MeGal | 0.78 | |
| Lactose | Gal β1-4Glc | 0.78 |
| Raffinose | Gal α1-6Glc β1-2Frucf | 0.78 |
| Mellibiose | Gal α1-6Glc | 0.78 |
| Cellobiose | Glc β1-4Glc | NI (50) |
I50 is the inhibitor concentration that caused 50% reduction in binding. NI, not inhibitory at the millimolar concentration shown in parentheses.
The present results demonstrate that serotype b A. actinomycetemcomitans LPS acts as a receptor for coaggregation with F. nucleatum. This conclusion is supported by the ability of serotype b LPS to bind to cells of F. nucleatum and its inhibitory effect on the binding of F. nucleatum to A. actinomycetemcomitans cells.
Different serotypes of A. actinomycetemcomitans differ in their ability to coaggregate with F. nucleatum and in the chemical structure of the O-PS moieties of their LPSs (17, 18). The lipid A and core polysaccharide structures of the LPS were found to be identical among the different serotypes (12, 18). The serotype b A. actinomycetemcomitans LPS is capable of binding F. nucleatum, probably through its galactose-binding lectin. Our inhibition studies suggest that the most important characteristics of the binding site of this lectin are as follows. (i) It is dependent on a divalent metal ion for its carbohydrate binding activity, since EDTA completely inhibited binding, thus resembling the ion requirements of the C-type animal lectins (11). (ii) A free axial hydroxyl group at position 4 and free equatorial hydroxyl groups at positions 3 and 6 of Gal are necessary for binding.
To our knowledge, this is the first report identifying polysaccharide receptors for coaggregation on the surface of gram-negative late colonizers of the dental plaque.
In summary, the results of the present study indicate that LPS from A. actinomycetemcomitans cells plays a role in their attachment to other microorganisms in dental plaque, thus creating a reservoir of bacteria that are involved in the pathogenesis of periodontal as well as systemic diseases. Furthermore, knowledge of the structural requirements of the galactose-binding lectin may lead to the development of derived saccharides that may be used as inhibitors of coaggregation and therein point to a mechanism for inhibiting subgingival plaque formation.
Acknowledgments
We thank Mario Lebendiker, Protein Purification Laboratory, Hebrew University, for the purification of LPS and O-PS.
This study was supported by the Chief Scientist of the Ministry of Health (grants to G.R. and M.N.S.).
Editor: J. T. Barbieri
REFERENCES
- 1.Califano, J. V., H. A. Schenkein, and J. G. Tew. 1989. Immunodominant antigen of Actinobacillus actinomycetemcomitans Y4 in high-responder patients. Infect. Immun. 57:1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cisar, J. O., P. E. Kolenbrander, and F. C. McIntire. 1979. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect. Immun. 24:742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gmur, R., H. McNabb, T. J. van Steenberg, P. Baehni, A. Mombelli, A. J. van Winkehoff, and B. Guggenheim. 1993. Seroclassification of hitherto nontypeable Actinobacillus actinomycetemcomitans strains: evidence for a new serotype e. Oral Microbiol. Immunol. 8:116-120. [DOI] [PubMed] [Google Scholar]
- 4.Haffajee, A. D., and S. S. Socransky. 1994. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 5:78-111. [DOI] [PubMed] [Google Scholar]
- 5.Hirabayashi, J., T. Hashidate., Y. Arata, N. Nishi, T. Nakamura, M. Hirashima., T. Urashima, T. Oka, M. Futai, W. E. G. Muller, F. Yagi, and K. Kasai. 2002. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572:232-254. [DOI] [PubMed] [Google Scholar]
- 6.Jenkinson, H. F., S. D. Terry, R. McNab, and G. W. Tannock. 1993. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect. Immun. 61:3199-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan, J. B., M. B. Perry, L. L. MacLean, D. Furgang, M. E. Wilson, and D. H. Fine. 2001. Structural and genetic analyses of O polysaccharide from Actinobacillus actinomycetemcomitans serotype f. Infect. Immun. 69:5375-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinder, S. A., and S. C. Holt. 1993. Localization of the Fusobacterium nucleatum T18 adhesin activity mediating coaggregation with Porphyromonas gingivalis T22. J. Bacteriol. 175:840-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolenbrander, P. E., R. N. Andersen, and L. V. H. Moore. 1989. Coaggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infect. Immun. 57:3194-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolenbrander, P. E., and J. London. 1993. Adhere today, here tomorrow: oral bacterial adherence. J. Bacteriol. 175:3247-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, R. T., Y. Ichikawa, M. Fay, K. Drickamer, M. Shao, and Y. Lee. 1991. Ligand-binding characteristics of rat serum-type mannose-binding protein (MBP-A). Homology of binding site architecture and chicken hepatic lectins. J. Biol. Chem. 266:4810-4815. [PubMed] [Google Scholar]
- 12.Masoud, H., S. T. Weintraub, R. Wang, R. Cotter, and S. C. Holt. 1991. Investigation of the structure of lipid A from Actinobacillus actinomycetemcomitans strain Y4 and human clinical isolate PO 1021-7. Eur. J. Biochem. 200:775-779. [DOI] [PubMed] [Google Scholar]
- 13.Moore, W. E. C., and L. V. H. Moore. 1994. The bacteria of periodontal diseases. Periodontol. 2000 5:66-77. [DOI] [PubMed] [Google Scholar]
- 14.Nakano, Y., Y. Yoshida, N. Suzuki, Y. Yamashita, and T. Koga. 2000. A gene cluster for the synthesis of serotype d-specific polysaccharide antigen in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1493:259-263. [DOI] [PubMed] [Google Scholar]
- 15.Nakano, Y., Y. Yoshida, Y. Yamashita, and T. Koga. 1998. A gene cluster for 6-deoxy-l-talan synthesis in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1442:409-414. [DOI] [PubMed] [Google Scholar]
- 16.Page, R. C., T. J. Sims, L. D. Engel, B. J. Moncla, B. Bainbridge, J. Stray, and R. P. Darveau. 1991. The immunodominant outer membrane antigen of Actinobacillus actinomycetemcomitans is located in the serotype-specific high-molecular-mass carbohydrate moiety of lipopolysaccharide. Infect. Immun. 59:3451-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry, M. B., L. M. Maclean, J.-R. Brisson, and M. E. Wilson. 1996. Structures of the antigenic O-polysaccharides of lipopolysaccharides produced by Actinobacillus actinomycetemcomitans serotypes a, c, d and e. Eur. J. Biochem. 242:682-688. [DOI] [PubMed] [Google Scholar]
- 18.Perry, M. B., L. L. MacLean, R. Gmür, and M. E. Wilson. 1996. Characterization of the O-polysaccharide structure of lipopolysaccharide from Actinobacillus actinomycetemcomitans serotype b. Infect. Immun. 64:1215-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saarela, M., S. Asikainen, S. Alaluusua, L. Phyala, C.-H. Lai, and H. Jousimies-Somer. 1992. Frequency and stability of mono- and poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d and e. Oral Microbiol. Immunol. 7:277-279. [DOI] [PubMed] [Google Scholar]
- 20.Sailler, L., B. Marchou, J. Lemozy, E. Bonnet, Z. Elias, L. Cuzin, and P. Massip. 2000. Successful treatment of Actinobacillus actinomycetemcomitans endocarditis with ofloxacin. Clin. Microbiol. Infect. 6:55-56. [DOI] [PubMed] [Google Scholar]
- 21.Shaniztki, B., D. Hurwitz, N. Smorodinsky, N. Ganeshkumar, and E. I. Weiss. 1997. Identification of a Fusobacterium nucleatum PK1594 galactose-binding adhesin which mediates coaggregation with periopathogenic bacteria and hemagglutination. Infect. Immun. 65:5231-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sims, T. J., B. J. Moncla, R. P. Darveau, and R. C. Page. 1991. Antigens of Actinobacillus actinomycetemcomitans recognized by patients with juvenile periodontitis and periodontally normal subjects. Infect. Immun. 59:913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slots, J. 1982. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J. Clin. Microbiol. 15:606-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slots, J., L. Bragd, M. Wikstrom, and G. Dahlen. 1986. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J. Clin. Periodontol. 13:570-577. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki, N., Y. Nakano, Y. Yoshida, H. Nakao, Y. Yamashita, and T. Koga. 2000. Genetic analysis of the gene cluster for the synthesis of serotype a-specific polysaccharide antigen in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1517:135-138. [DOI] [PubMed] [Google Scholar]
- 26.Weiss, E. I., B. Shaniztki, M. Dotan, N. Ganeshkumar, P. E. Kolenbrander, and Z. Metzger. 2000. Attachment of Fusobacterium nucleatum PK1594 to mammalian cells and its coaggregation with periopathogenic bacteria are mediated by the same galactose-binding adhesin. Oral Microbiol. Immunol. 15:371-377. [DOI] [PubMed] [Google Scholar]
- 27.Wilson, M. E., and R. E. Schifferle. 1991. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect. Immun. 59:1544-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida, Y., Y. Nakano, N. Suzuki, H. Nakao, Y. Yamashita, and T. Koga. 1999. Genetic analysis of the gene cluster responsible for synthesis of a serotype e-specific polysaccharide antigen in Actinobacillus actinomycetemcomitans. Biochim. Biophys. Acta 1489:457-461. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida, Y., Y. Nakano, Y. Yamashita, and T. Koga. 1998. Identification of a genetic locus essential for serotype b-specific antigen synthesis in Actinobacillus actinomycetemcomitans. Infect. Immun. 66:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]
- 31.Zambon, J. J., J. Slots, and R. J. Genco. 1983. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 41:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]