Abstract
The Moraxella catarrhalis immunoglobulin D (IgD)-binding protein (MID) is a 200-kDa outer membrane protein displaying a unique and specific affinity for human IgD. MID is found in the majority of M. catarrhalis strains. In the present paper, we show that MID-expressing M. catarrhalis strains agglutinate human erythrocytes and bind to type II alveolar epithelial cells. In contrast, M. catarrhalis isolates with low MID expression levels and two mutants deficient in MID, but with readily detectable UspA1 expression, do not agglutinate erythrocytes and have a 50% lower adhesive capacity. To examine the adhesive part of MID, the protein was dissected into nine fragments covering the entire molecule. The truncated MID proteins were expressed in Escherichia coli, purified, and used for raising polyclonal antibodies in rabbits. Interestingly, by using recombinant fragments, we show that the hemagglutinating and adhesive part of MID is localized within the 150-amino-acid fragment MID764-913. In addition, antibodies against full-length MID, MID764-913, or a 30-amino-acid consensus sequence (MID775-804) inhibited adhesion to alveolar epithelial cells. Antibodies against UspA1, an outer membrane protein expressed in essentially all M. catarrhalis strains, also inhibited adhesion, suggesting that both MID and UspA1 are needed for optimal attachment to epithelial cells. Taken together, in addition to MID-dependent IgD binding, we have demonstrated that the outer membrane protein MID is a novel adhesin that would be a suitable target for a future vaccine against M. catarrhalis.
Moraxella (Branhamella) catarrhalis is often a harmless commensal in the respiratory tract and can be detected in nasopharyngeal cultures from 66% of children during the first year of life and from approximately 4% of adults at any given time. However, the species has increasingly been recognized as an important pathogen in respiratory tract infections in both children and adults (4, 15). After Haemophilus influenzae and Streptococcus pneumoniae, M. catarrhalis is the third most common bacterial agent in acute otitis media in children. In adults and the elderly, M. catarrhalis is a common cause of lower respiratory tract infections, particularly in those with predisposing conditions such as chronic obstructive pulmonary disease. M. catarrhalis is often implicated as a cause of sinusitis in both children and adults. Furthermore, the emergence of antibiotic resistance suggests that the incidence of M. catarrhalis infections may continue to rise. More than 90% of M. catarrhalis clinical isolates are resistant to penicillin, and M. catarrhalis has developed resistance at a rate unprecedented for any bacterial species. The emergence of M. catarrhalis as a significant cause of human disease has generated much interest in the identification of potential vaccine antigens (20). M. catarrhalis vaccine development is at the antigen candidate identification stage, and researchers are searching for potential vaccine antigens that elicit antibodies with capacity to limit the bacterium's pathogenicity.
Two decades ago, M. catarrhalis was shown to display a strong affinity for soluble human immunoglobulin D (IgD) (9). IgD binding at the cellular level explains the strong mitogenic effects of M. catarrhalis on human lymphocytes (3, 10). In addition, it was demonstrated that M. catarrhalis stimulates the proliferation of high-density (mature) B lymphocytes expressing a high density of IgD B-cell receptors (BCR) and that soluble nonmitogenic monoclonal antibodies (MAbs) reactive with human IgD selectively inhibit the B-lymphocyte response. Inhibition by anti-IgD MAbs presumably resulted from covering or capping surface IgD on B lymphocytes, thereby eliminating the bacterium-dependent stimulatory signal delivered through the BCR IgD.
Recently, a novel surface protein of M. catarrhalis that displays a high affinity for IgD, designated MID, was solubilized in Empigen and isolated by ion-exchange chromatography and gel filtration (8). The apparent molecular mass of monomeric MID was estimated to be approximately 200 kDa by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The mid gene was cloned and expressed in Escherichia coli. The complete mid nucleotide gene sequence was determined, and the deduced amino acid sequence consists of 2,123 or 2,139 residues, depending on two alternative translation starts. The sequence of MID has no similarity to other Ig-binding proteins and differs from all previously described outer membrane proteins (OMPs) of M. catarrhalis. MID was found to exhibit unique Ig-binding properties. Thus, in enzyme-linked immunosorbent assays (ELISA), dot blots, and Western blots, MID bound two purified IgD myeloma proteins, four IgD myeloma sera, and finally one IgD standard serum. No binding of MID to IgG, IgM, IgA, or IgE myeloma proteins was detected. MID was also attracted to surface-expressed BCR IgD but not to other membrane molecules on human peripheral blood lymphocytes. MID or MID-Sepharose stimulated human peripheral B cells but not T cells as measured by proliferation (11). IgM secretion was detected in B-cell cultures stimulated with MID or MID-Sepharose and interleukin-2 (IL-2), whereas secretion of IgG and IgA was induced in cultures with the combination of MID or MID-Sepharose and IL-4, IL-10, and soluble CD40 ligand, suggesting that Th2-derived cytokines were required for plasma cell generation.
The mid gene was detected in 98 different strains as revealed by homology of the signal peptide sequence and a conserved area in the 3′ end of the gene (22). When the mid genes from five different strains were compared, identities of 65.3 to 85.0% and homologies of 71.2 to 89.1% were detected. Gene analyses showed several amino acid repeat motifs in the open reading frames. Eighty-four percent of the Moraxella strains expressed high or intermediate levels of MID-dependent IgD binding as revealed by flow cytometry analysis using specific anti-MID polyclonal antibodies and human myeloma IgD, whereas 16% of the strains expressed MID to a low degree. It was shown that bacteria reduced their MID expression by removing a guanosine (G) in their poly(G) tracts. Moraxella strains isolated from the nasopharynx, blood, and sputum expressed MID at approximately the same frequency. In addition, no variation was observed among strains of different geographical origins. MID and the mid gene were found solely in M. catarrhalis, whereas related Neisseria and Moraxella species did not express MID.
To identify the IgD-binding region, MID was digested with proteases (23). In addition, a series of truncated fragments of MID were manufactured and expressed in E. coli, followed by analysis for IgD binding in Western and dot blots. The smallest fragment with essentially preserved IgD binding consisted of 238 amino acid residues (MID962-1200). Ultracentrifugation experiments and gel electrophoresis revealed that native MID962-1200 was a tetramer. Interestingly, tetrameric MID962-1200 attracted IgD more than 20-fold more efficiently than the monomeric form. Thus, a tetrameric structure of MID962-1200 was crucial for optimal IgD-binding capacity.
The present work shows a strict correlation between hemagglutination and MID expression in M. catarrhalis. Isolated MID and a 150-amino-acid recombinant MID-derived protein (MID764-913) bound to erythrocytes and type II alveolar epithelial cells. Antibodies to MID, MID764-913, or the consensus sequence MID775-804 effectively inhibited adherence to the alveolar epithelial cells. Since M. catarrhalis isolates expressing MID at high concentrations bound considerably more effectively to epithelial cells than strains expressing MID at low levels and two MID-deficient mutants, the MID protein, and in particular fragment MID764-913, is suggested to be an attractive vaccine candidate.
MATERIALS AND METHODS
Reagents.
Purification of the M. catarrhalis OMP MID has been described recently (8). The MID protein was conjugated to CNBr-Sepharose according to the manufacturer's instructions. In brief, 4.0 mg of MID or bovine serum albumin (BSA) was diluted in 1 ml of coupling buffer (0.1 M NaHCO3 containing 0.5 M NaCl [pH 8.3]). CNBr-activated Sepharose 4B (1.5 ml) (Amersham Pharmacia Biotech, Uppsala, Sweden) was preswelled and washed in 1 mM HCl. MID and CNBr-Sepharose were mixed and rotated overnight at 4°C. Excess ligand was quantitated by the BCA Protein Assay kit (Pierce, Rockford, Ill.) and afterwards washed away. The remaining active groups were blocked with 0.1 M Tris-HCl (pH 8.0) and 1 M ethanolamine (pH 8.0) for 2 h. Finally, the conjugated protein was washed with three cycles of 0.1 M acetate buffer containing 0.5 M NaCl (pH 4.0) and 0.1 M Tris-HCl containing 0.5 M NaCl (pH 8.0). The amount of MID protein that bound to CNBr-Sepharose was estimated to 2.0 mg. The final product was diluted in 5 ml of 0.1 M NH4CO3 (pH 8.0).
Antisera.
Rabbits were immunized intramuscularly with 200 μg of either purified MID (8), a recombinant MID fragment (one of fragments A to I), or recombinant ubiquitous surface protein A1 (UspA1) emulsified in complete Freund's adjuvant (Difco, Becton Dickinson, Heidelberg, Germany) and were boosted on days 18 and 36 with the same dose of protein in incomplete Freund's adjuvant. Blood was drawn 2 to 3 weeks later. The anti-UspA1 antiserum reacted with both recombinant UspA1 and UspA2 upon examination by Western blotting. A peptide consisting of the KTRASS repeat (MID775-804; sequence, KTRASSIGDVLNAGFNLQGNGEAKDFVSTY) (22) was synthesized and conjugated to the carrier keyhole limpet hemocyanin (Innovagen, Lund, Sweden). The immunization protocol described above was used. Resulting anti-MID775-804 antibodies were isolated by affinity chromatography. The anti-MID775-804 polyclonal antibody (pAb) reacted specifically with MID764-913 (fragment E) and MID902-1200 (fragment F).
Bacteria and culture conditions.
M. catarrhalis strains were clinical isolates from the State Serum Institute (Copenhagen, Denmark) and our department (22). Type strains from the Culture Collection, University of Gothenburg (Department of Clinical Bacteriology, Sahlgrenska Hospital, Gothenburg, Sweden), the National Collection of Type Cultures (Central Public Health Laboratory, London, United Kingdom), and the American Type Culture Collection (Manassas, Va.) were also included. Twenty-one strains expressing high levels of MID and 21 strains expressing low levels as determined by flow cytometry using IgD or MID-specific pAbs were grown overnight in nutrient broth (NB) (Oxoid, Basingstoke, Hampshire, United Kingdom). All strains expressed UspA1 as revealed by Western blotting and an anti-UspA1 pAb. Before agglutination assays, bacteria were washed once in phosphate-buffered saline (PBS) and then resuspended at a concentration of 1 × 109 to 2 × 109/ml in PBS.
Flow cytometry analysis and antibodies.
Binding of IgD to whole bacterial cells was analyzed by using purified human myeloma protein IgD(κ) (The Bindingsite, Birmingham, United Kingdom) and a fluorescein isothiocyanate (FITC)-conjugated rabbit anti-human IgD pAb (Dakopatts, Glostrup, Denmark). Bacteria from liquid cultures were washed twice with PBS containing 1% BSA and incubated with 0.5 μg of IgD(κ)/ml in a final volume of 100 μl of PBS-1% BSA at 4°C for 1 h. After the wash, bacteria were incubated with an anti-IgD pAb for 30 min at 4°C. After two more washes, bacteria were analyzed in an EPICS, XL-MCL flow cytometer (Coulter, Hialeah, Fla.). To exclude nonspecific binding, the FITC-conjugated human anti-IgD pAb was added separately as a negative control for each strain analyzed. To analyze MID, specific rabbit antisera diluted 1/1,000 in PBS-2% BSA were used. After incubation for 1 h at 4°C, bacteria were washed and incubated with an FITC-conjugated goat anti-rabbit pAb for 30 min at 4°C. After additional washes, bacteria were analyzed by flow cytometry.
DNA constructs, recombinant protein expression, and purification of proteins.
The different truncated MID fragments designated A to I, with their specific sizes and primers for generating the proteins, are shown in Fig. 3A and Table 1, respectively. The open reading frame of the mid gene from M. catarrhalis Bc5 (in pET16-MID) (8) was used as a template. All MID constructs, except for MID367-590 (fragment C), were amplified by PCR using specific primers introducing BamHI and HindIII restriction enzyme sites. Due to an internal HindIII restriction enzyme site in fragment C, an XhoI site was used instead of HindIII at the 3′ end. All PCR products, except for MID1616-2139 (fragment I), were cloned into pET26 (Novagen, Madison, Wis.). The PCR product encoding fragment I was cloned into pMAL-c2 (New England Biolabs, Beverly, Mass.). To avoid presumptive toxicity, the resulting plasmids were first transformed into the nonexpressing host E. coli DH5α. Thereafter, plasmids coding for fragments A to D, G, and H were transformed into E. coli BL21(DE3), whereas the host BL21(DE3)-pLysS was used for vectors containing fragments E and F. Both E. coli strains were incubated in the presence of kanamycin, whereas chloramphenicol also was added when BL21(DE3)-pLysS transformants were used. Fragment I was expressed in DH5α. Bacteria were grown to mid-log phase, followed by induction with 1 mM isopropyl-1-thio-β-d-galactoside (IPTG). After 3.5 h, transformants were sonicated and the overexpressed proteins were purified according to the manufacturers' instructions. Resulting recombinant proteins that had a histidine tag or were fused to maltose binding protein were purified on resins containing nickel or amylose, respectively. The concentrations of the eluted proteins were determined by using the BCA Protein Assay kit (Pierce). Thereafter, recombinant proteins were analyzed by SDS-PAGE and Western blotting.
FIG. 3.
MID binds to human erythrocytes, and the active domain is located between amino acid residues 764 and 913 (fragment E). (A) A series of truncated MID proteins (designated A to I) were manufactured and then used to immunize rabbits. The asterisk indicates the IgD-binding part of the MID molecule (23). (B) The resulting rabbit pAbs were equilibrated for antibody content as described in Materials and Methods. (C) The various truncated MID fragments were analyzed for binding to human erythrocytes by using the specific rabbit pAbs. Recombinant truncated proteins were produced in E. coli and purified by affinity chromatography as described in detail in Materials and Methods. On three occasions, rabbits were immunized with recombinant MID fragments. Microtiter plates were coated with a freshly isolated human erythrocyte membrane fraction, and the recombinant MID fragments were then added at the indicated concentrations (4.0, 0.5, or 0.125 μg/ml). Thereafter, specific rabbit anti-MID polyclonal antisera at dilutions equilibrated for specific antibody activity were added. After washes, plates were developed with HRP-conjugated goat anti-rabbit pAbs.
TABLE 1.
Primers used in the present study
| Protein fragment (residues) | 5′ primer | 3′ primer |
|---|---|---|
| A (MID69-186) | CTTATGCTCAACAGGATCCCAGAC | CTACCATCAGGAAGCTTGTATATGTTG |
| B (MID170-377) | CAAAAGTTCCTGGATCCAAAAG | GACCCAAAAGCTTTTTTACGCTATTATTGC |
| C (MID367-590) | GTGGCTAAGCGTCAGGATCCTTTTCAG | TAAAGTCTGCTCGAGTTTTCTCTTAACG |
| D (MID567-774) | CTCTGCCGCCACGGATCCAGACTTATAC | GGCGGCACGAAGCTTGTCGTCGTTAG |
| E (MID764-913) | GGCGTGGATCCTGCACTACAT | GTTCATCAAGCTTAGAGAAGGTTTG |
| F (MID902-1200) | CGCACGGATCCAGGCACAGCAAGCAC | GGTCGGGGAAGCCCAAACCAAGCTTGGC |
| G (MID1011-1466) | CGCAACAACAGGGGATCCCAGTGAAATC | GCCACAAGCTTATGACCTGCATCAAGGGG |
| H (MID1446-1630) | CCCGGATCCCCGATGCGGACAAACTTGCCAATC | CTGTAAGCTTTTTACCAGCATTGGGG |
| I (MID1616-2139) | CAGTGAGGATCCGACTCCAACTGGTCTAAGCC TTGTCAACCC | CCCCCCAAGCTTAAAGTGAAAACCTGCACC AACTGCTGCC |
Construction and characterization of MID-deficient M. catarrhalis.
A kanamycin resistance gene cassette from puC4K was amplified by PCR using specific primers introducing the restriction enzyme site for EcoRV. The resulting PCR product was digested and ligated into the EcoRV site of the mid gene (8). M. catarrhalis strains RH4 and BBH18 were transformed by electroporation using a Genepulser apparatus (Bio-Rad, Sundbyberg, Sweden) set at 2.5 kV, 25 μF, and 200 Ω. After transformation, bacteria were cultured in NB liquid medium (Oxoid) without kanamycin. Resulting mutants were deficient in MID as revealed by Western blotting using pAbs directed against full-length MID and the truncated MID fragments A to I. Furthermore, the two mutants were devoid of IgD binding as revealed by Western blotting and were shown by flow cytometry analysis to have lost >75% of their IgD binding.
SDS-PAGE and detection of proteins on membranes (Western blots).
SDS-PAGE was run at a constant voltage of 150 V by using 10% Bis-Tris gels with running buffer (morpholineethanesulfonic acid [MES]), sample buffer (lithium dodecyl sulfate), and transfer buffer as well as a blotting instrument from Novex (San Diego, Calif.). Gels were stained with Coomassie brilliant blue R-250 (Bio-Rad). Electrophoretic transfer of protein bands from the gel to an Immobilon-P membrane (Millipore, Bedford, Mass.) was carried out at 30 V for 2 to 3 h. After transfer, the Immobilon-P membrane was blocked in PBS with 0.05% Tween 20 (PBS-Tween) containing 5% milk powder. After several washes in PBS-Tween, the membrane was incubated with rabbit anti-MID, truncated MID fragments, or UspA antisera diluted 1/1,000 in PBS-Tween including 2% milk powder for 1 h at room temperature. A horseradish peroxidase (HRP)-conjugated goat anti-rabbit antiserum diluted 1/1,000 (Dakopatts) was added after washes in PBS-Tween. After incubation for 40 min at room temperature and additional washes in PBS-Tween, development was performed with ECL Western blotting detection reagents (Amersham Pharmacia Biotech).
Hemagglutination.
Human erythrocytes were obtained from freshly drawn heparinized human blood. Erythrocytes were washed twice in PBS (pH 7.2) and then suspended in PBS at a final concentration of 1%. Bacteria cultured in NB were harvested by centrifugation, washed, and suspended to 1 × 109 to 2 × 109/ml in PBS. The bacterial and erythrocyte suspensions (50 μl of each) were mixed in round-bottom microtiter plates (Sarstedt, Newton, N.C.). Agglutination was read by the naked eye and semiquantitatively graded as negative (−) to strongly positive (+++). In some experiments, erythrocytes were mixed with MID-Sepharose or BSA-Sepharose in 150 μl of PBS.
ELISA.
To calibrate antibody content in corresponding antisera, purified MID and recombinant truncated MID proteins (1 μg/ml) in 0.1 M Tris-HCl (pH 9.0) were added in 100-μl volumes to microtiter plates (F96 Maxisorb; Nunc, Roskilde, Denmark) and incubated at 4°C overnight. After the plates were washed four times in PBS-Tween, blocking buffer (PBS-Tween containing 1.5% ovalbumin [OVA]) was added. Plates were incubated for 1 h at room temperature and further washed four times with PBS-Tween. Rabbit antisera against MID in twofold dilutions from 1/100 to 1/1,600 in 100 μl of PBS-Tween containing 1.5% OVA were added to each well, and after incubation for 1 h at room temperature, the plates were washed four times with PBS-Tween. After incubation with HRP-conjugated goat anti-rabbit serum (Dakopatts) diluted 1/1,000 and subsequent washes, plates were developed and absorbance at 450 nm was measured. In subsequent experiments, sera were used in dilutions giving similar ELISA values when tested against the respective truncated proteins. Thereafter, an outer membrane fraction of human erythrocytes was prepared. Erythrocytes were lysed in distilled water, followed by centrifugation and washes with 0.1 M Tris-HCl (pH 9.0). The membrane fraction was collected, suspended at a final concentration of 1% in Tris-HCl, and added in 100-μl volumes to microtiter plates. After overnight incubation, four washes in PBS-Tween, incubation with blocking buffer, and another four washes, MID or one of the recombinant truncated MID-derived proteins in twofold dilutions (4 to 0.125 μg) in 100 μl of PBS-Tween containing 1.5% OVA was added to each well. After incubation for 1 h at room temperature and four new washes, antisera in appropriate dilutions from 1/200 to 1/800 giving similar ELISA values (as indicated above) against respective antigens were added. After a new incubation at room temperature and additional washes, an HRP-conjugated goat anti-rabbit antiserum was added, and the plates were incubated, washed, developed, and measured as described above.
Cell lines and adherence assay.
The lung carcinoma cell line A549 (type II alveolar epithelial cells; CCL-185) was obtained from the American Type Culture Collection. A549 cells were cultured in RPMI 1640 medium (Gibco BRL, Life Technologies, Paisley, Scotland) supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 12 μg of gentamicin/ml (referred to below as culture medium). On the day before adherence experiments, cells were harvested, washed twice in gentamicin-free RPMI 1640, and added to 24-well tissue culture plates (Nunc) at a concentration of 105 cells/well in 2.0 ml of gentamicin-free culture medium. Thereafter, cells were incubated overnight at 37°C under 5% CO2. On the day of experiments, M. catarrhalis (≈2 × 108 organisms) in PBS-0.15% gelatin (Sigma) was inoculated onto the monolayers. In neutralization experiments with specific antisera, bacteria were preincubated with a preimmune serum or antisera against MID or MID-derived truncated proteins. All sera were used at a 1/250 dilution, giving approximately the same ELISA values for all antisera included against the corresponding MID-derived truncated protein. Immunosorbent-purified antibodies against the consensus sequence (MID775-804) were used at a concentration of 4 μg/ml. After 1 h at 4°C, bacteria were added to the epithelial cells. In all experiments, tissue culture plates were centrifuged at 3,000 × g for 5 min and incubated at 37°C under 5% CO2. After 30 min, infected monolayers were rinsed twice with PBS-0.15% gelatin with gentle rocking to remove nonadherent bacteria and were then treated with trypsin-EDTA (0.05% trypsin and 0.5 mM EDTA) to release them from the plastic support. Thereafter, the resulting cell-bacterium suspension was seeded onto agar plates containing 1.1% IsoVitaleX, 7.8% human blood, and finally 0.9% proteose peptone. Data were calculated from duplicate cultures.
125I labeling of proteins and binding to eukaryotic cells.
Purified MID or recombinant proteins were radioiodinated (Amersham, Little Chalfont, Buckinghamshire, England) to a high specific activity (0.05 mol of iodine per mol of protein) with Chloramine-T (12). Human erythrocytes or epithelial cells (A549) were incubated with radiolabeled proteins in PBS-2% BSA for 30 min (erythrocytes) or 45 min (epithelial cells) at 37°C. Thereafter, cells were washed three times in the same buffer, followed by measurement in a γ-scintillation counter.
Coiled-coil structure analysis.
The predicted coiled-coil structure of MID was analyzed by using Coils software (version 2.2; http://www.york.ac.uk/depts/biol/units/coils) (19).
Statistics.
Student's t test for paired data was used for statistical calculations. Statistical significance was set at a P value of ≤0.05.
RESULTS
MID agglutinates human erythrocytes.
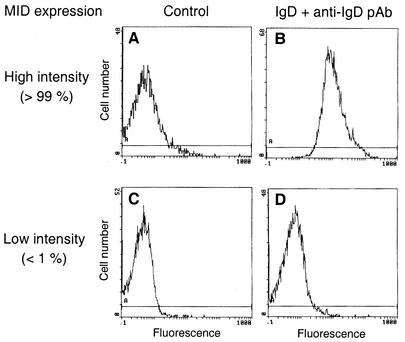
MID expression in several M. catarrhalis strains (n = 98) has previously been characterized by flow cytometry using myeloma IgD and FITC-labeled rabbit anti-human IgD pAbs (22). This assay characteristically shows two peaks, one low-intensity and one high-intensity peak. However, the relative proportions of the two peaks vary and thus reflect phase variation. To investigate a putative involvement of MID in hemagglutination, a series of clinical isolates that expressed MID either at high or at low intensity (22) was selected. Figure 1 shows representative flow cytometry profiles of a high- and a low-intensity strain. Similar profiles were obtained with a specific anti-MID pAb (data not shown). In contrast, Western blots and an anti-UspA1 pAb revealed that UspA1 was approximately equally expressed in all strains and thus was expressed independently of MID. All of the 21 isolates expressing MID at high intensity agglutinated human erythrocytes, whereas most of the low-intensity MID-expressing strains (n = 21) did not agglutinate erythrocytes (Table 2). An almost full correlation between hemagglutinating capacity and MID expression was thus observed. These initial experiments prompted us to examine whether or not purified MID protein from the model strain M. catarrhalis Bc5 (8) agglutinates erythrocytes. To mimic the bacterial surface, MID was conjugated to Sepharose beads and incubated with human erythrocytes. BSA linked to Sepharose was included as a negative control. Interestingly, the human erythrocytes were hemagglutinated in the presence of MID-Sepharose, whereas BSA-Sepharose did not interfere with the erythrocytes.
FIG. 1.
Flow cytometry profiles of M. catarrhalis clinical isolates expressing MID at high or low intensity. IgD binding is shown for the high-intensity MID-expressing strain Bc5 (A and B) and the low-intensity MID-expressing strain BBH17 (C and D). MID expression intensity was defined according to the work of Möllenkvist et al. (22). Bacteria were incubated with human myeloma protein IgD(κ) followed by FITC-conjugated anti-IgD pAbs. Thereafter, fluorescence was analyzed by flow cytometry. Controls incubated with the FITC-conjugated anti-IgD pAb without preincubation with IgD are included (A and C).
TABLE 2.
Characterization of MID-dependent hemagglutination in 42 M. catarrhalis strains
| M. catarrhalis phenotypea | No. of isolates with the following hemagglutination scoreb:
|
|||
|---|---|---|---|---|
| − | + | ++ | +++ | |
| High MID intensity | 0 | 0 | 0 | 21 |
| Low MID intensity | 17 | 2 | 1 | 1 |
M. catarrhalis strains with high or low MID expression were included.
Human erythrocytes were incubated with M. catarrhalis in microtiter plates. Hemagglutination was graded from negative (−) to strongly positive (+++).
High-intensity-MID-expressing M. catarrhalis adheres more efficiently to type II alveolar epithelial cells than low-intensity- MID-expressing strains or two MID-deficient mutants.
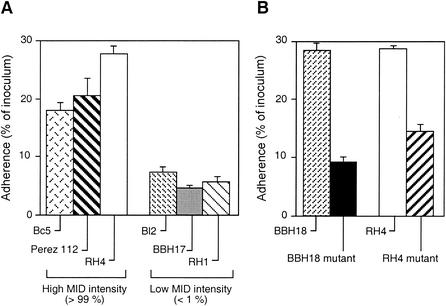
Hemagglutination is often correlated with adhesive capacity. To analyze the influence of MID on M. catarrhalis adherence to type II alveolar epithelial cells, a series of clinical isolates with high or low MID expression was added to the epithelial cell line A549. After centrifugation and incubation at 37°C for 30 min, followed by extensive washes, the epithelial cells were trypsinized and plated at different dilutions. As can be seen in Fig. 2A, low-level MID-expressing Moraxella isolates had an adhesive capacity more than 50% lower than that of bacteria expressing MID to a high degree. For comparison, two MID-deficient mutants showed ≥50% lower adhesion than the corresponding wild-type strains (Fig. 2B). In parallel with the loss of MID expression, the mutants lost their characteristic hemagglutination. Thus, both the experiments with erythrocytes and the results for adhesion of MID-expressing M. catarrhalis to epithelial cells suggested that MID functioned as an adhesin.
FIG. 2.
High-intensity-MID-expressing M. catarrhalis isolates strongly adhere to epithelial cells, whereas low-intensity-MID-expressing strains and MID mutants do not. (A) Adhesion of six different M. catarrhalis strains with high or low expression of the outer membrane protein MID was studied. (B) Two different strains with mutated MID are compared to the corresponding wild-type strains. All clinical isolates expressed UspA1 as revealed by Western blotting. The M. catarrhalis strains were added to the type II alveolar epithelial cell line A549. After centrifugation, cells and bacteria were incubated for 30 min at 37°C, followed by extensive washes. Cells were treated with trypsin-EDTA, and suspensions were plated on blood agar plates and incubated overnight. CFU was counted, and attachment was calculated as a percentage of the inoculum, as follows: (CFU adherent)/(CFU added) × 100. Data are means from four independent experiments with duplicates. Error bars, standard errors of the means. The adhesion of the three high-intensity-MID-expressing strains was statistically different (P ≤ 0.01) from the adhesion of the three low-intensity-MID-expressing isolates.
The hemagglutinating domain of MID is located between amino acid residues alanine764 and serine913.
To dissect the molecule and pinpoint the specific site of the molecule that was responsible for hemagglutination, a series of truncated DNA fragments of the mid gene (Fig. 3A) was cloned and recombinantly expressed in E. coli. Resulting proteins were purified by affinity chromatography, and pAbs against the truncated MID proteins were raised in rabbits and subsequently used in an ELISA.
In preparatory experiments, antibodies to MID and the MID-derived proteins were titrated to give similar values when tested in ELISA against respective antigens. The capacities of the truncated MID proteins to bind to an erythrocyte cell membrane fraction were then measured by an ELISA using the specific antibodies at appropriate concentrations. MID and truncated fragments were added to the erythrocyte membranes at three different concentrations (4.0, 0.5, or 0.125 μg/ml) (Fig. 3C). Interestingly, full-length MID or MID764-913 (fragment E) gave higher ELISA values (more than 4 to 16 times) than the other truncated MID proteins covering amino acids 69 to 774 and 902 to 2139 of MID. Thus, the hemagglutinating capacity of full-length MID1-2139 conjugated to Sepharose was confirmed by using the erythrocyte cell membrane ELISA. Furthermore, the hemagglutinating structure of MID was located within fragment E, which covered amino acid residues 764 to 913.
MID764-913 (fragment E) binds directly both to erythrocytes and to type II alveolar epithelial cells.
To further confirm the importance of MID764-963 as an adhesin, MID and the truncated MID-derived recombinant proteins were radiolabeled and tested for direct binding to human erythrocytes or alveolar epithelial cells (Fig. 4). Both full-length 125I-labeled MID1-2139 and 125I-labeled MID764-913 bound strongly to erythrocytes, whereas the remaining truncated MID fragments covering amino acid residues 69 to 774 or 902 to 2139 did not bind above background levels (Fig. 4A). In parallel, the alveolar epithelial cell line A549 also attracted both 125I-labeled full-length MID and the 125I-labeled truncated MID764-913, whereas the other fragments did not bind to the epithelial cells (Fig. 4B). Taken together, these results demonstrate that MID764-963 was the crucial part of the adhesin MID mediating adherence to mammalian cells.
FIG. 4.
125I-labeled recombinant MID764-913 (fragment E) is specifically bound to erythrocytes and epithelial cells. 125I-labeled MID fragments (fragments A to I) were added to human erythrocytes (A) or to epithelial cells (B). All truncated MID proteins were produced in E. coli and purified by affinity chromatography. The resulting recombinant proteins were labeled with 125I and added to erythrocytes or the epithelial cell line A549. After several washes, bound radioactivity was measured in a γ-counter. Data are means from two experiments with duplicates. Error bars, standard deviations.
Antibodies directed against full-length MID1-2139 or truncated MID764-913 or MID775-804 inhibit adherence of M. catarrhalis to type II alveolar epithelial cells.
To further analyze the influence of full-length MID and MID764-913 on the adherence of M. catarrhalis Bc5 to the A549 cell line, the effects of specific anti-MID pAbs were examined. After preincubation of high- and low-intensity-MID-expressing M. catarrhalis isolates with antibodies at 4°C for 60 min followed by washes, the bacteria were added to alveolar epithelial cells. As demonstrated in Fig. 5, pAbs directed against full-length MID1-2139 or against MID764-913 (fragment E) significantly inhibited attachment (up to 65% inhibition) of the high-level MID-expressing isolate to epithelial cells. We also included rabbit antibodies against a consensus sequence starting with KTRASS (MID775-804), which exists in all mid genes so far examined by us (n = 5) (22). The anti-MID775-804 antibodies were as efficient (72% inhibition) as the anti-MID764-913 pAb. In contrast, a preimmune serum or a pAb directed against MID367-578 (fragment C), which bound efficiently to the bacterial surface as determined by flow cytometry, did not significantly interfere with adhesion.
FIG. 5.
Adhesion of MID-expressing M. catarrhalis to epithelial cells depends on amino acid residues 764 to 913 (fragment E). Decreased adhesion to epithelial cells was observed with MID-expressing bacteria coated with a rabbit anti-MID1-2139, anti-MID764-913 (fragment E), anti-MID775-804, or anti-UspA1 pAb versus a preimmune serum or an anti-MID367-578 (fragment C) pAb. Bacteria were preincubated with the preimmune sera or specific antisera (dilution, 1/250), or with an anti-MID775-804 pAb (final concentration, 4.0 μg/ml), for 1 h at 4°C. Thereafter, bacteria were added to the epithelial cells, followed by centrifugation and incubation for 30 min at 37°C. After washes, cells were treated with trypsin-EDTA, and suspensions were plated onto blood agar plates. CFU were counted after an overnight incubation. The attachment ratio [(CFU adherent/CFU added) × 100] was calculated. All values were related to the adherence ratio obtained with the preimmune control serum (92% ± 4.3% [mean ± standard deviation] of the ratio for the control without any antisera), which was set to 100%. Data are means from four separate experiments with duplicates. Error bars, standard deviations. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05.
It has been demonstrated that UspA1 has a major role in M. catarrhalis attachment (1). In addition, it has recently been shown that the only isolate of 108 Moraxella strains that lacked the uspA1 gene demonstrated reduced adherence to HEp-2 cells (21). Also, when we preincubated the high-level MID-expressing M. catarrhalis isolate Bc5 with the anti-UspA1 pAb, attachment was significantly (70%) inhibited (Fig. 5). Moreover, when the other clinical isolates (Fig. 2A) were preincubated with the anti-MID or anti-UspA1 pAbs, similar patterns were seen. When the two pAbs were combined, no further inhibition was detected. Taken together, our results suggest that the OMPs UspA1 and MID are both needed for optimal attachment to epithelial cells.
DISCUSSION
The pathogenesis of M. catarrhalis infection is not completely understood, but in recent years an increasing number of virulence factors involved in adhesion and colonization have been determined. The M. catarrhalis IgD-binding protein MID is a highly conserved OMP and has been suggested to play an important role in M. catarrhalis pathogenesis (8, 22, 23). Here we show that MID takes part in the agglutination of human erythrocytes and is in part responsible for the adhesion of bacteria to type II alveolar epithelial cells (A549). By using a series of recombinantly expressed truncated fragments of MID extending over the whole 2,139-amino-acid molecule, the sequence MID764-913 (fragment E) was pinpointed as responsible for the hemagglutinating and adhesive activities. pAbs directed against full-length MID1-2139, MID764-913 (fragment E), or the consensus sequence MID775-804 (which starts with KTRASS) significantly inhibited attachment of high-level MID-expressing isolates to alveolar epithelial cells. The identity and similarity of MID764-913 (fragment E) among six different MID sequences (8, 22, 25) were 60 to 96 and 69 to 97%, respectively. Thus, experiments with antibodies confirmed MID764-913 to be responsible for adhesion. In contrast, MID775-804 is most likely not involved in adhesion, because this sequence was also found in MID962-1200 (fragment F), for which neither adhesion nor hemagglutination was detected. Anti-MID775-804 antibodies thus most likely inhibited bacterial adherence via a steric effect independently of binding to the MID adhesive domain per se.
Low-intensity-MID-expressing strains were also included in the present study. These strains showed significantly reduced expression of MID due to an altered poly(G) tract found within the 5′ part of the open reading frame (22). Although these strains showed decreased MID expression, SDS-PAGE, Western blotting, and flow cytometry revealed that many of them expressed MID, most likely due to ongoing phase variation. Hence, they were designated low-intensity-MID-expressing strains. We show here that most of these virtually “MID-deficient” clinical strains, as well as two MID-deficient mutants, neither agglutinated human erythrocytes nor adhered efficiently to epithelial cells.
It has been shown that nonhemagglutinating M. catarrhalis strains do not express a 200-kDa protein as revealed by SDS-PAGE, whereas this high-molecular-weight protein is expressed by hemagglutinating isolates (6). The same authors report from another study that M. catarrhalis isolates obtained from elderly patients with lower respiratory tract infections agglutinated erythrocytes and also adhered to HEp-2 epithelial cells more efficiently than Moraxella strains isolated from healthy carriers. However, a strict correlation between hemagglutination and adhesion was not found (7). The 200-kDa protein described by Fitzgerald et al. (6, 7) is thus probably identical to MID.
During the editorial processing of this paper, Pearson et al. (24) published a report of a study in which the gene encoding the 200-kDa protein of M. catarrhalis was subjected to nucleotide sequence analysis and then was inactivated by insertional mutagenesis. The isogenic mutant lost its abilities to agglutinate human erythrocytes and to bind to human IgD. Most likely the 200-kDa protein (Hag) presented in Pearson's paper and the MID protein studied by us are identical. However, Pearson et al. (24) found that the Hag-deficient mutant still attached at wild-type levels to several human epithelial cell lines, including Chang conjunctival epithelial cells. In contrast, an UspA1-deficient mutant had little or no ability to attach to the same cells. The discrepancy between our results and those of Pearson et al. on the importance of Hag or MID for attachment cannot be explained. Interestingly, in our study, antibodies directed against UspA1 also inhibited adhesion to cells to the same degree as antibodies against MID. Since UspA1 is expressed by essentially all M. catarrhalis isolates, this observation suggested that a synergism existed between UspA1 and MID, i.e., that both proteins were required for optimal adhesion. Thus, our experiments indicate that despite the fact that the hemagglutinating determinant is located on MID, both OMPs MID and UspA1 are likely to play a role in adhesion to epithelial cells.
Several different M. catarrhalis OMPs have been isolated and more or less characterized (20). The most defined group of Moraxella OMPs is the UspA family, consisting of UspA1, UspA2, and UspA2H (1, 5, 16). UspA1 and UspA2H are responsible for adherence to Chang conjunctival epithelial cells in vitro, as revealed in transformation experiments using a nonadhesive H. influenzae strain (17, 18). Interestingly, experiments with uspA1 or uspA2H mutants revealed that the adhesive capacities of mutants decreased as much as 90% from those of their wild-type counterparts. UspA1 has also been suggested to play a role in adherence to HEp-2 epithelial cells, although specific mutants were not included in that study (2). In a recent paper by Meier et al. (21), the only isolate among 108 Moraxella strains which lacked the uspA1 gene demonstrated reduced adherence to HEp-2 cells. Antibodies against UspA1 and UspA2 protect mice from infection in an animal pulmonary clearance model, and it has thus been suggested that the UspA proteins are attractive vaccine candidates (13).
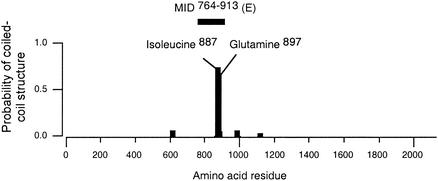
The UspA proteins together with Yersinia YadA belong to a novel class of adhesins consisting of a tripartite organization with an N-terminal oval head domain and a long putative coiled-coil rod terminated by a C-terminal membrane anchor domain (14). In contrast to the UspA proteins, which have a high probability of forming coiled-coils (5), the MID protein harbors only one such stretch of amino acid residues. Interestingly, the coiled-coil is proposed to be located within fragment E (i.e., MID764-913) (Fig. 6), suggesting that the coiled-coil structure is in some way important for the hemagglutinating and adhesive activities. However, our computer analysis has not revealed any “lollipop” structure including a long stalk and a head (23).
FIG. 6.
The MID molecule contains a coiled-coil structure within MID764-913 (fragment E). The full-length Bc5 MID1-2139 protein was analyzed by using Coils, version 2.2, software (19).
It is of the utmost importance to characterize novel bacterial adhesins for the development both of efficient vaccines and of novel therapeutic strategies. In recent years, several interesting and eligible M. catarrhalis OMPs have been identified. In the present communication, we have pinpointed the specific sequence in MID responsible for hemagglutination and adherence to bronchoalveolar epithelial cells. MID will most likely become one of the more interesting adhesins to use as a target for future vaccine development. Before MID can be chosen as a vaccine candidate, however, the intermediate goal is to analyze the protective effect of the novel adhesin in a mouse pulmonary clearance model.
Acknowledgments
This work was supported by grants from the Alfred Österlund Foundation, the Anna and Edwin Berger Foundation, the Greta and Johan Kock Foundation, the Magnus Bergvall Foundation, the Swedish Medical Research Council, the Swedish Society of Medicine, the Åke Wiberg Foundation, and the Cancer Foundation at the Malmö University Hospital.
Editor: D. L. Burns
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect. Immun. 66:3113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bootsma, H. J., H. G. van der Heide, S. van de Pas, L. M. Schouls, and F. R. Mooi.2000. Analysis of Moraxella catarrhalis by DNA typing: evidence for a distinct subpopulation associated with virulence traits. J. Infect. Dis. 181:1376-1387. [DOI] [PubMed] [Google Scholar]
- 3.Calvert, J. E., and A. Calogeres. 1986. Characteristics of human B cells responsive to the T-independent mitogen Branhamella catarrhalis. Immunology 58:37-41. [PMC free article] [PubMed] [Google Scholar]
- 4.Catlin, B. W. 1990. Branhamella catarrhalis: an organism gaining respect as a pathogen. Clin. Microbiol. Rev. 3:293-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cope, L. D., E. R. Lafontaine, C. A. Slaughter, C. A. Hasemann, Jr., C. Aebi, F. W. Henderson, G. H. McCracken, Jr., and E. J. Hansen. 1999. Characterization of the Moraxella catarrhalis uspA1 and uspA2 genes and their encoded products. J. Bacteriol. 181:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald, M., R. Mulcahy, S. Murphy, C. Keane, D. Coakley, and T. Scott. 1997. A 200 kDa protein is associated with haemagglutinating isolates of Moraxella (Branhamella) catarrhalis. FEMS Immunol. Med. Microbiol. 18:209-216. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald, M., S. Murphy, R. Mulcahy, C. Keane, D. Coakley, and T. Scott. 1999. Tissue culture adherence and haemagglutination characteristics of Moraxella (Branhamella) catarrhalis. FEMS Immunol. Med. Microbiol. 24:105-114. [DOI] [PubMed] [Google Scholar]
- 8.Forsgren, A., M. Brant, A. Möllenkvist, A. Muyombwe, H. Janson, N. Woin, and K. Riesbeck. 2001. Isolation and characterization of a novel IgD-binding protein from Moraxella catarrhalis. J. Immunol. 167:2112-2120. [DOI] [PubMed] [Google Scholar]
- 9.Forsgren, A., and A. Grubb. 1979. Many bacterial species bind human IgD. J. Immunol. 122:1468-1472. [PubMed] [Google Scholar]
- 10.Forsgren, A., A. Penta, S. F. Schlossman, and T. F. Tedder. 1988. Branhamella catarrhalis activates human B lymphocytes following interactions with surface IgD and class I major histocompatibility complex antigens. Cell. Immunol. 112:78-88. [DOI] [PubMed] [Google Scholar]
- 11.Gjörloff-Wingren, A., R. Hadzic, A. Forsgren, and K. Riesbeck. 2002. A novel IgD-binding bacterial protein from Moraxella catarrhalis induces human B lymphocyte activation and isotype switching in the presence of Th2 cytokines. J. Immunol. 168:5582-5588. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood, F. C., W. M. Hunter, and J. S. Glover. 1963. The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochem. J. 89:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helminen, M. E., I. Maciver, J. L. Latimer, J. Klesney-Tait, L. D. Cope, M. Paris, G. H. McCracken, Jr., and E. J. Hansen. 1994. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170:867-872. [DOI] [PubMed] [Google Scholar]
- 14.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 19:5989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karalus, R., and A. Campagnari. 2000. Moraxella catarrhalis: a review of an important human mucosal pathogen. Microbes Infect. 2:547-559. [DOI] [PubMed] [Google Scholar]
- 16.Klingman, K. L., and T. F. Murphy. 1994. Purification and characterization of a high-molecular-weight outer membrane protein of Moraxella (Branhamella) catarrhalis. Infect. Immun. 62:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 182:1364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lafontaine, E. R., N. J. Wagner, and E. J. Hansen. 2001. Expression of the Moraxella catarrhalis UspA1 protein undergoes phase variation and is regulated at the transcriptional level. J. Bacteriol. 183:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupas, A. 1996. Prediction and analysis of coiled-coil structures. Methods Enzymol. 266:513-525. [DOI] [PubMed] [Google Scholar]
- 20.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 8(Suppl. 1):S101-S107. [DOI] [PubMed] [Google Scholar]
- 21.Meier, P. S., R. Troller, I. N. Grivea, G. A. Syrogiannopoulos, and C. Aebi. 2002. The outer membrane proteins UspA1 and UspA2 of Moraxella catarrhalis are highly conserved in nasopharyngeal isolates from young children. Vaccine 20:1754-1760. [DOI] [PubMed] [Google Scholar]
- 22.Möllenkvist, A., T. Nordström, C. Halldén, J.-J. Christensen, A. Forsgren, and K. Riesbeck. 2003. The Moraxella catarrhalis IgD-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J. Bacteriol. 185:2285-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordström, T., A. Forsgren, and K. Riesbeck. 2002. The immunoglobulin D-binding part of the outer membrane protein MID from Moraxella catarrhalis comprises 238 amino acids and a tetrameric structure. J. Biol. Chem. 277:34692-34699. [DOI] [PubMed] [Google Scholar]
- 24.Pearson, M. M., E. R. Lafontaine, N. J. Wagner, J. W. St Geme III, and E. J. Hansen. 2002. A hag mutant of Moraxella catarrhalis strain O35E is deficient in hemagglutination, autoagglutination, and immunoglobulin D-binding activities. Infect. Immun. 70:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki, K., R. E. Harkness, and M. H. Klein. September 1998. Nucleic acids encoding high molecular weight major outer membrane protein of Moraxella. U.S. patent 5,808,024.