Abstract
Some common childhood infections appear to prevent the development of atopy and asthma. In some Mycobacterium bovis BCG-vaccinated populations, strong delayed-type hypersensitivity responses to mycobacterial antigens are associated with a reduced risk of atopy. Although BCG exposure decreases allergen-induced lung eosinophilia in animal models, little attention has been given to the effect of immunity to BCG on responses against live pathogens. We used the murine Cryptococcus neoformans infection model to investigate whether prior BCG infection can alter such responses. The present study shows that persistent pulmonary BCG infection of C57BL/6 mice induced an increase in gamma interferon, a reduction in interleukin-5, and a decrease in lung eosinophilia during subsequent Cryptococcus infection. This effect was long lasting, depended on the presence of live bacteria, and required persistence of mycobacterial infection in the lung. Reduction of eosinophilia was less prominent after infection with a mutant BCG strain (ΔhspR), which was rapidly cleared from the lungs. These observations have important implications for the development of vaccines designed to prevent Th2-mediated disease and indicate that prior lung BCG vaccination can alter the pattern of subsequent host inflammation.
The ability of infections to modulate unrelated immune responses is becoming increasingly recognized. It has previously been shown that influenza A virus infection in mice prevents subsequent T helper 2 (Th2)-mediated illnesses, including pulmonary eosinophilia, in mice exposed to respiratory syncytial virus proteins (27). Clinical studies have suggested that infections in childhood may prevent the development of atopy (14, 15) (26), and strong immune responses to mycobacterial proteins such as purified protein derivative (PPD) seem to be linked to a lower prevalence of atopy (23).
The regulation of T-cell responses against antigens after mucosal delivery is of major interest. It has been postulated that a default type 2 cytokine response occurs in infancy, which is replaced in later life by a type 1 polarized response dominated by gamma interferon (IFN-γ) production (7). Infections in early life (14, 6) and the presence of normal gastrointestinal flora (25) may play an important role in this maturation process. The rising incidence in atopy worldwide has been linked to decreased exposure to infections in early life, leading to the persistence of inappropriate Th2 immune responses.
The ability of Mycobacterium bovis bacillus Calmette-Guérin (BCG), which induces a Th1 immune phenotype, to down-regulate Th2 immune responses to subsequent unrelated antigens has been demonstrated with ovalbumin (OVA)-induced airway eosinophilia (4). BCG or related substances may therefore hold promise as vaccines against Th2-mediated illness. However, the extent and mechanisms of interactions between BCG-induced and unrelated immune reactions require further clarification. One aspect requiring further investigation is the effect of BCG exposure on subsequent infection-induced lung illness.
We utilize the murine model of Cryptococcus neoformans infection to study the effect of prior BCG exposure on the Th2-mediated immunopathology of infection with an unrelated live pathogen. C. neoformans is a fungus, and recent interest in this organism results from an increasing incidence of human cryptococcal infections, mostly associated with the worldwide AIDS epidemic. The organism is, however, also associated with pulmonary eosinophilia in immunocompetent hosts (16) and with summer-type hypersensitivity pneumonitis in Japan (1). Generally, resistant mouse strains produce higher concentrations of type 1 cytokines, with IFN-γ and interleukin-12 (IL-12) being important components of cell-mediated immunity in the lungs. Both CD4+ and CD8+ T cells are required for protection (10). Susceptible mice, such as those of the C57BL/6 strain, fail to clear Cryptococcus infection and develop a response marked by high levels of IL-5, chronic lung eosinophilia, and low levels of IFN-γ and IL-12 (10).
In the following report, we demonstrate for the first time that intranasal BCG administration reduces infection-induced lung eosinophilia for prolonged periods. This effect is accompanied by a change in type 1 and type 2 cytokines and may depend on the persistence of BCG in the lung. The findings have important implications for future approaches to designing BCG-related vaccines against Th2-mediated illness.
MATERIALS AND METHODS
All chemicals were purchased from Sigma, Poole, Dorset, United Kingdom, unless stated otherwise.
C. neoformans.
C. neoformans strain 52 (ATCC 24067) was obtained from the American Type Culture Collection (Rockville, Md.). For infection, yeast was grown to stationary phase (48 to 72 h) at room temperature on a shaker in Sabouraud dextrose broth (1% neopeptone and 2% dextrose; Difco, Detroit, Mich.). The cultures were washed in saline, counted on a hemocytometer, and diluted in sterile nonpyrogenic saline to the required infective dose (2 × 104 CFU/mouse). The inocula were plated on Sabouraud agar plates (Difco), and colonies were counted after 48 h of culture at room temperature to assess viability.
BCG.
M. bovis BCG Pasteur (Pasteur Institute, Paris, France) was grown in Middlebrook 7H9 medium (Difco) supplemented with 10% albumin-dextrose-catalase (Difco), 0.2% glycerol, and 0.05% Tween 80 or on Middlebrook 7H11 agar plates supplemented with 10% oleic acid-albumin-dextrose-catalase (Difco), 0.2% glycerol, and 10 μg of amphotericin B/ml. A mutant strain of BCG Pasteur, ΔhspR, overexpressing heat shock protein 70, has been described previously (24). This strain was generously supplied by G. Stewart. Bacteria for infection were obtained from late-logarithmic-growth-phase cultures and quantified by measurement of the optical density at a wavelength of 600 nm, with an optical density of 1 representing 108 CFU. Heat killing of bacteria was performed at 56°C for 20 min. Plating of serial dilutions on 7H11 medium was done to assess the number of viable bacteria in infective inocula and in aliquots of organ homogenates.
Mice and infections.
Eight- to twelve-week-old female C57BL/6 mice and IFN-γ gene-disrupted (IFN-γ−/−) C57BL/6 mice (Harlan Olac Ltd., Bicester, United Kingdom) were kept in pathogen-free conditions, according to institutional and Home Office guidelines. For infections, mice were anaesthetized with vaporized isoflurane (Rhône Mérieux, Harlow, Essex, United Kingdom) before administration of BCG (inoculum of 2 × 105 CFU unless stated otherwise) or phosphate-buffered saline (PBS). BCG was administered intranasally unless otherwise stated. Six weeks later, mice were again anaesthetized and infected intranasally with 2 × 104 CFU of Cryptococcus per mouse. Animals were sacrificed by intraperitoneal injection of a lethal dose of pentobarbital 13 days later, followed by exsanguination via the femoral arteries.
Cell recovery.
Bronchoalveolar lavage (BAL) fluids, lung tissues, and serum samples were harvested by using methods described previously (11). Briefly, the lungs of each mouse were inflated six times with 1 ml of 12 mM lidocaine in Eagle's minimal essential medium and BAL fluid kept on ice. BAL fluid (100 μl) was cytocentrifuged onto glass slides and stained with hematoxylin and eosin (H&E). The remainder of the BAL fluid was centrifuged, the supernatant was retained at −80°C, and the pellet was resuspended at a concentration of 106 cells/ml. Lungs and spleens were homogenized by passage through 100-μm-pore-size cell strainers (Falcon), red blood cells were lysed in ammonium chloride buffer, and the remaining cells were washed and resuspended in RPMI medium with 10% fetal calf serum. Cell numbers were determined by counting cells on hemocytometer slides by using a microscope and trypan blue exclusion to identify viable cells.
Flow cytometry analysis.
Cells were stained with Cychrome-conjugated anti-CD4, fluorescein isothiocyanate (FITC)-conjugated CD45RB, and phycoerythrin (PE)-conjugated anti-CD8 or Cychrome-conjugated anti-B220 and PE-conjugated anti-DX5 (all from Becton Dickinson-Pharmingen, Heidelberg, Germany) for 30 min on ice. Samples were washed with PBS containing 1% bovine serum albumin (BSA) and 0.1% sodium azide. Cells were then fixed for 20 min at room temperature with 2% formaldehyde and analyzed on a FACSCalibur flow cytometer (Becton Dickinson-Pharmingen) collecting data on at least 40,000 lymphocytes. To detect intracellular cytokines, 106 cells per ml were incubated with 50 ng of phorbol myristate acetate/ml, 500 ng of ionomycin (Calbiochem)/ml, and 10 mg of brefeldin A/ml for 4 h at 37°C. Cells were then stained for CD8-Cychrome- and allophycocyanin-conjugated anti-CD4 as described above and fixed. After permeabilization with 0.5% saponin in PBS containing 1% BSA and 0.1% azide for 10 min, FITC-conjugated anti-mouse IFN-γ (XMG1.2; Pharmingen) and PE-conjugated anti-mouse IL-5 (TRFK-5; Pharmingen) or FITC-conjugated anti-tumor necrosis factor (TNF; JES 2A5; Pharmingen) diluted 1/40 in saponin buffer was added. After 30 min, all samples were washed with PBS containing 1% BSA and 0.1% sodium azide and analyzed by collecting data on at least 40,000 lymphocytes. Directly conjugated isotype-matched control antibodies were used to set limits of background fluorescence.
Enumeration of eosinophils.
Eosinophils were enumerated as granulocytes by using flow cytometry to identify them by their distinctive forward- and side-scatter properties. Identification was confirmed by counting eosinophils in H&E-stained cytocentrifuge preparations by making use of the characteristic nuclear morphology of eosinophils and the acidophilic granules in the cytoplasm.
Cytokine ELISA.
IL-4, IL-5, IFN-γ, transforming growth factor β (TGF-β), and TNF were assessed in lung lavage supernatants and serum samples by using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Becton Dickinson-Pharmingen). Total (acid-activated) and bioactive (not acid-activated) TGF-β levels were determined. Briefly, Immunosorb ELISA plates (Nunc) were coated with capture antibody and left overnight at 4°C. Wells were then washed five times with PBS-0.05% Tween 20 and blocked with PBS-10% fetal bovine serum for 1 h at room temperature. One hundred microliters of sample (undiluted) or standard was added to blocked wells for 2 h at room temperature. Bound cytokine was detected by using biotinylated anti-cytokine antibody, Avidin horseradish peroxidase, and tetramethylbenzidine. Color development was blocked with 2 N H2SO4, and optical densities were read at 450 nm. The concentration of cytokine in each sample was determined from the standard curve.
EliSpot assays.
Sterile filter plates (Millipore, Bedford, Mass.) were coated with anti-IL-4, anti-IL-5, and anti-IFN-γ antibodies (Pharmingen International, Becton Dickinson) in 0.1 M bicarbonate buffer, pH 9.6, overnight before being washed and blocked with RPMI medium containing 10% fetal bovine serum. Single-cell lung homogenate was obtained by homogenizing lungs through 100-μm-pore-size cell strainers (Falcon, Becton Dickinson), and 5 × 105 cells were added to the wells with four doubling dilutions. Cells were cultured for 48 h with medium, PPD (PPD-RT46; Statens Seruminstitut, Copenhagen, Denmark), or heat-killed C. neoformans (avirulent, unencapsulated strain J305, ATCC 52816; American Type Culture Collection). The rationale for using the unencapsulated strain is that it ensures that any responses are specific for C. neoformans and not for surface antigens that may potentially cross-react with other organisms. It has previously been reported that strain J305 stimulates antigen-specific proliferation of splenocytes from mice infected with strain 52D but not that of splenocytes from uninfected mice (10). The cells were removed after 48 h by washing, and cytokine production was detected by biotin-labeled rat anti-murine IL-4, IL-5, or IFN-γ monoclonal antibody by using 5-bromo-4-chloro-3-indolylphosphate-Nitro Blue Tetrazolium (BCIP-NBT) as an alkaline phosphatase substrate. Spots were counted by an investigator who was unaware of the experimental group to which the animals belonged. The number of background spots was deducted from the number of those obtained by antigen stimulation.
Statistics.
Statistics were obtained with the use of the Minitab software program for Windows. The method used was that of two-tailed analysis of variance, assuming unequal variance, and results were expressed as mean values with standard deviations of the mean. P values of less than 0.05 were judged to indicate statistical significance. If more than one test was performed in a particular study, the alpha level was adjusted downward by using Bonferroni's correction (described at http://home.clara.net/sisa/bonhlp.htm) to consider chance capitalization.
RESULTS
Prior BCG infection decreases total cell recruitment to the lung and alters differential cell composition of BAL fluid during subsequent Cryptococcus infection.
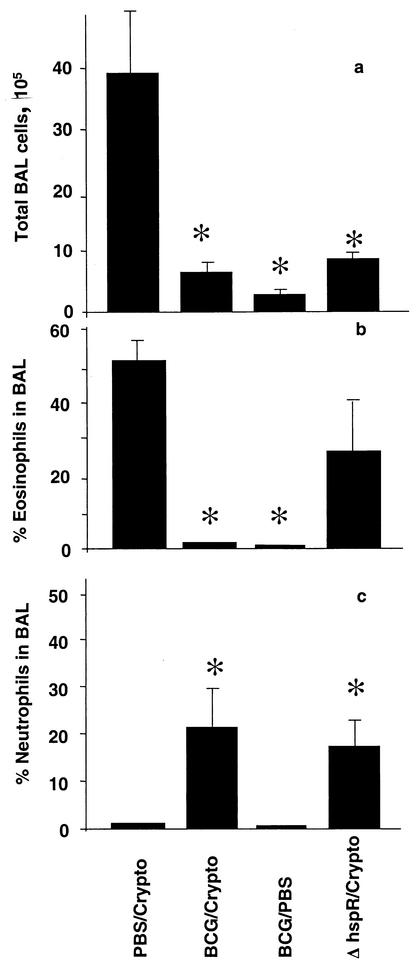
It has previously been shown that one viral lung infection significantly reduces lung inflammation during a second, unrelated viral infection (27). To determine whether the immune modulatory phenomenon is restricted to viruses, mice were infected with 2 × 105 CFU of BCG Pasteur or given PBS intranasally and then infected with 2 × 104 CFU of C. neoformans or given PBS via the same route 6 weeks later. Animals were sacrificed 13 days after the fungal infection (at the time of peak BAL fluid eosinophilia), and BAL fluids were obtained. Prior BCG infection decreased the total number of inflammatory cells recruited to the BAL fluid in response to Cryptococcus infection (Fig. 1a). The very low number of cells in the BAL fluids of mice infected with BCG alone (BCG followed by PBS [BCG/PBS]) suggests only low-grade inflammation 6 weeks after BCG infection. Cryptococcus infection alone (PBS followed by Cryptococcus [PBS/Cryptococcus]) resulted in extensive eosinophilia (Fig. 1b), which was dramatically reduced by prior BCG infection. BCG alone (BCG/PBS) did not lead to lung eosinophilia. Prominent neutrophil recruitment into the BAL fluid was seen in the dual-infected group but not in those infected with Cryptococcus or BCG alone (Fig. 1c). The absolute numbers of eosinophils in the respective groups followed the same pattern as the differential counts (data not shown).
FIG. 1.
Wild-type BCG alters Cryptococcus-induced eosinophilic inflammation, but ΔhspR BCG is less effective. Six weeks prior to infection with 2 × 104 CFU of C. neoformans (Crypto), 2 × 105 CFU of wild-type BCG Pasteur or ΔhspR BCG (ΔhspR) was given intranasally. Control groups were given PBS instead of either Cryptococcus or BCG. Thirteen days after fungal infection, BAL was performed. (a) The total cell recruitment to BAL fluid was assessed by hemocytometer count, and the percentages of eosinophils (b) and neutrophils (c) in BAL fluids were determined by counting cells on H&E-stained cytocentrifugation slides. Each column represents the mean ± the standard deviation of results from one of at least two representative experiments with groups of four to five mice. P values smaller than 0.05 for comparison with the PBS/Cryptococcus-infected group are denoted by asterisks.
As immature precursors of eosinophils and neutrophils might migrate into the airways under chronic conditions and are often difficult to distinguish from each other, the total percentage and the absolute number of granulocytes were determined by differential cell count by using cytocentrifugation slides. PBS/Cryptococcus infection was characterized by a 59% ± 4.1% proportion of granulocytes and by 5.7 × 105 ± 1.6 × 105 total granulocytes in the BAL fluid, whereas with BCG infection followed by Cryptococcus infection (BCG/Cryptococcus infection), levels of 21% ± 9.6% and 0.7 × 105 were found (P < 0.01). The percentage of macrophages was higher in the BCG-exposed animals (59 ± 10 versus 26 ± 7.6; P < 0.01), while the proportions of lymphocytes were not different in the groups.
ΔhspR BCG is less effective than wild-type BCG in reducing lung eosinophilia.
Previously, mycobacterial strains with a deleted heat shock protein (hsp) repressor gene which overexpress Hsp70 and induce a stronger IFN-γ response were described (24). These mycobacteria are cleared from infected animals more quickly than wild-type mycobacteria. We used the ΔhspR BCG strain to determine whether reduced eosinophilia in response to Cryptococcus infection required enhanced type 1 cytokines or the persistence of mycobacteria. Increased clearance of ΔhspR BCG compared to that of wild-type BCG was reconfirmed in the present study (titers were 1.2 × 104 ± 0.2 × 104 CFU and 3.8 × 103 ± 1.4 × 103 CFU, respectively, in spleen homogenates 10 weeks after intravenous [i.v.] infection with 2 × 105 CFU; P < 0.01). Similar to that with wild-type BCG, intranasal infection with ΔhspR BCG decreased total cell recruitment to the lung in Cryptococcus-challenged mice (Fig. 1a) but the effect on BAL fluid eosinophilia was less pronounced (P of 0.07 for comparison with the eosinophilia induced by PBS/Cryptococcus infection) (Fig. 1b). This BCG strain also resulted in a prominent BAL fluid neutrophil content during Cryptococcus infection (Fig. 1c).
Live BCG is required to reduce Cryptococcus-induced eosinophilia.
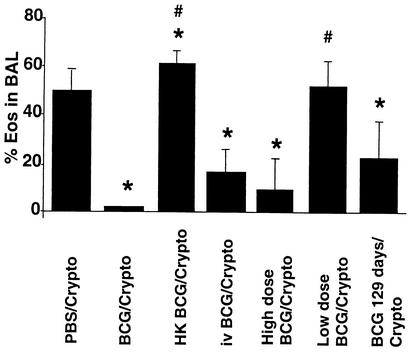
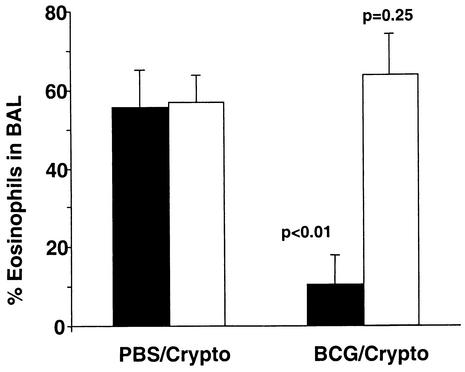
To determine if live BCG was required for a reduction in eosinophilia, 2 × 105 CFU of BCG was heat-killed (HK-BCG) and administered to mice 6 weeks prior to infection with 2 × 104 CFU of Cryptococcus. HK-BCG alone led to transient lymphocytosis in the BAL fluid which was resolved after 7 days. A mild eosinophilic infiltrate was observed (2.1% ± 0.8% in five mice). HK-BCG actually increased the percentage of eosinophils (Fig. 2) in the BAL fluid but not the total number of cells recovered (data not shown) compared to those in the BAL fluids of naïve Cryptococcus-infected mice. This may be related to the cytokine production pattern. The frequency of PPD-specific IFN-γ-producing cells in lung homogenate after live BCG infection was 1,092 ± 372 cells per million, compared to only 120 ± 110 cells with dead BCG. Frequencies of IL-4-producing cells (150 to 200 cells per million) and IL-5-producing cells (320 to 440 cells per million) were not different for mice infected with live BCG and those exposed to dead BCG. The ratio of IFN-γ-producing cells versus IL-5-producing cells is therefore skewed towards a type 2 pattern after exposure to dead BCG.
FIG. 2.
Reduced Cryptococcus-induced BAL fluid eosinophilia requires live BCG infection and is influenced by the route, dose, and timing of BCG infection. BCG was administered intranasally or i.v. prior to intranasal infection with 2 × 104 CFU of Cryptococcus (Crypto). The time interval between BCG and Cryptococcus infection was 42 days, except in one group for which the interval was stretched to 129 days. The BCG inoculum was 2 × 105 CFU per animal in all but two groups, with which the effects of a high dose of BCG (2 × 106 CFU) and a low dose of BCG (103 CFU) were investigated. The effect of intranasal administration of 2 × 105 CFU of HK-BCG on Cryptococcus-induced BAL fluid eosinophilia is also shown. A control group was given PBS instead of BCG. Thirteen days after the fungal infection, BAL was performed and the percentages of eosinophils (Eos) in the lavage fluids were determined by counting cells on H&E-stained cytocentrifugation slides. Each column represents the mean with standard deviations of results from one experimental group of five mice. The data shown is from one of two representative experiments with five mice per group. The asterisks designate statistically significant differences from the PBS/Cryptococcus-infected group (P < 0.05), and the number signs designate significant differences from the BCG/Cryptococcus-infected group.
The route of BCG infection, the BCG inoculum dose, and the extended time interval between the two infections affect the reduction of eosinophils.
The effect of the BCG administration route was evaluated by i.v. infection with 2 × 105 CFU of BCG 6 weeks prior to Cryptococcus infection. Although total cell recruitment (data not shown) and BAL fluid eosinophilia were reduced after i.v. infection with wild-type BCG, the effects were less pronounced than those seen after intranasal BCG infection (Fig. 2). Not shown here is that i.v. administration of ΔhspR BCG is also not as effective as intranasal administration in altering the pattern of host inflammation in response to C. neoformans.
A report by Power et al. (19) suggests that mycobacterial dose determines the Th1 or Th2 nature of the immune response independently of the route of infection. High doses of BCG skew the response towards Th2 predominance. The ability of a higher dose of BCG to down-regulate Cryptococcus-induced lung eosinophilia was therefore investigated. We observed that 2 × 106 CFU of BCG (a 10-fold increase over the dose used in the previous experiments) still decreased total cell recruitment (from 9.7 × 105 ± 2.5 × 105 to 3.4 × 105 ± 1.8 × 105; P < 0.01) and BAL fluid eosinophilia (P < 0.01; Fig. 2). To evaluate the effect of low doses of BCG, 103 CFU was given intranasally and no effect on cell recruitment (data not shown) or BAL fluid eosinophilia was found after fungal infection 6 weeks later (Fig. 2).
Remarkably, the effect of prior BCG infection (2 × 105 CFU) on Cryptococcus-induced BAL fluid eosinophilia was still apparent when C. neoformans was introduced 129 days after BCG inoculation (P < 0.01; Fig. 2), although it was not as pronounced as it was after 42 days. BCG titers of 3 × 104 ± 2.7 × 104 CFU were recovered from the lungs of coinfected mice even after this interval.
Flow cytometry analysis of BAL fluid cell phenotypes.
It has previously been shown that the protective effect of one viral infection against another heterologous virus is mediated by the immune response to the first pathogen. We therefore investigated the lymphocytic component of BAL fluid cells by flow cytometry. Lymphocyte and granulocyte percentages were compatible with the differential cell counts obtained by using cytospin preparations. BCG-immune mice infected with Cryptococcus had significantly more activated CD4+ and CD8+ T cells in the BAL fluids than PBS/Cryptococcus-infected animals (Table 1). Activation was assessed as the percentages of CD4+ and CD8+ cells expressing a CD45RBlo phenotype. B cells were reduced in BCG-immune mice compared to those in mice infected with Cryptococcus alone, whereas NK cell numbers were unaffected. The results from flow cytometry analysis of intracellular cytokine production by lung homogenate cells are shown in Table 2. Coinfection increased CD4+ T-cell and CD8+ T-cell IFN-γ and TNF production. IL-5 production was decreased by dual infection in five of six coinfected mice but did not reach statistical significance due to one outlier. As expected, only low IL-5 production was observed in mice infected with BCG alone. Due to the low level of inflammation in the BCG/PBS-infected group, only limited data were obtained from these animals and TNF production could not be assessed.
TABLE 1.
Increased T-cell activation in BCG-immune mice infected with C. neoformansa
| Inoculum (a) | Granulocytesb | CD4+ CD45RBlo cellsc | CD8+ CD45RBlo cellsc | B cellsc | NK cellsc |
|---|---|---|---|---|---|
| Cryptococcus only | 54 +/− 5.9 | 24 +/− 9.0 | 4.4 +/− 1.8 | 14 +/− 1.1 | 2.8 +/− 0.9 |
| BCG only | 5.7 +/− 0.8d | 21 +/− 4.4 | 15 +/− 4.6d | 11 +/− 2.0d | 2.5 +/− 1.1 |
| BCG/Cryptococcus | 16 +/− 3.0d | 43 +/− 6.9d,e | 15 +/− 2.9d | 6.0 +/− 2.1d,e | 3.2 +/− 0.2 |
Values are mean percentages and standard deviations (five mice per group).
Granulocytes were identified by their forward- and side-scatter properties by using flow cytometry.
Percentages are for those within the lymphocyte population.
Significantly different (P < 0.05) from values for PBS/Cryptococcus-infected group.
Significantly different (P < 0.05) from values for BCG/PBS-infected group.
TABLE 2.
Prior exposure to BCG increases IFN-γ and TNF levels in C. neoformans-infected micea
| Inocula | CD4+ IFN-γ | CD8+ IFN-γ | CD4+ IL-5 | CD8+ IL-5 | CD4+ TNF | CD8+ TNF |
|---|---|---|---|---|---|---|
| PBS/Cryptococcus | 2.7 +/− 0.8 | 7.4 +/− 1.6 | 1.8 +/− 1.1 | 2.5 +/− 1.5 | 7.7 +/− 2.4 | 8.6 +/− 2.4 |
| BCG/Cryptococcus | 15 +/− 4.6b | 15 +/− 3.9b | 0.7 +/− 0.9 | 1.9 +/− 2.7 | 17 +/− 4.6b | 17 +/− 5.0b |
| BCG/PBS | 3.8 +/− 1.3c | 2.0 +/− 0.7b,c | 0.2 +/− 0.2b | 0.1 +/− 0.2b | NDd | NDd |
The values represent the mean percentages and standard deviations of CD4 or CD8 T cells expressing the cytokine indicated (five mice per group).
Significantly different (P < 0.05) from values for PBS/Cryptococcus-infected group.
Significantly different (P < 0.05) from values for BCG/PBS-infected group.
ND, not done due to inadequate number of cells.
BCG infection alters levels of cytokines, including antigen-specific IL-5, in serum and BAL fluid during coinfection.
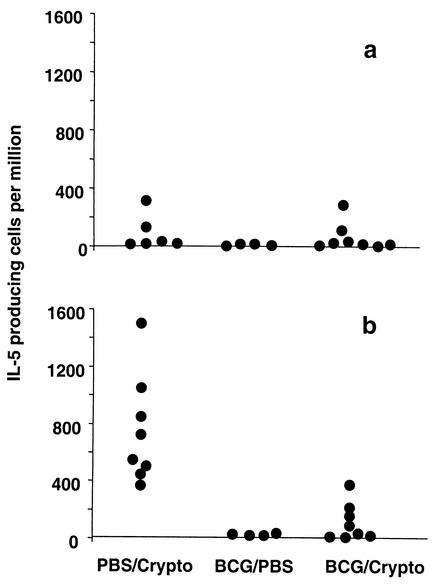
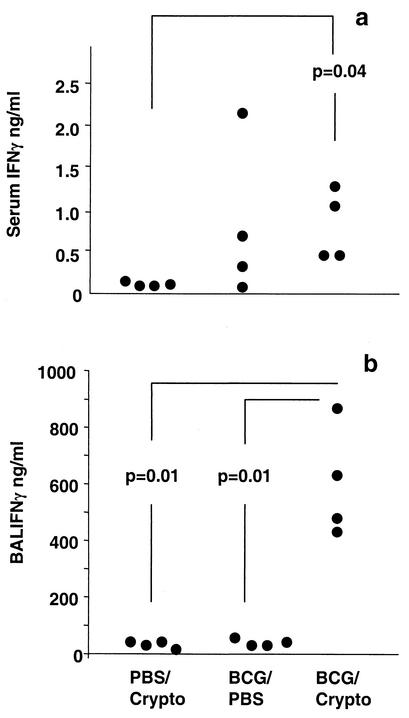
Since Cryptococcus-induced eosinophilia relies on IL-5, the frequency of cytokine-producing cells in lung homogenate was determined by EliSpot assay. The frequencies of BCG-specific and Cryptococcus-specific IL-4- and IFN-γ-producing cells were not significantly altered (data not shown). BCG-specific IL-5 production was consistently low in all groups (Fig. 3a). Cryptococcus-specific IL-5 production was decreased, however, by prior exposure to BCG (Fig. 3b). The IL-5 and TNF levels in serum and BAL fluid and the IL-4 levels in serum as measured by ELISA were similar for the different groups. However, the coinfected group displayed higher levels of IFN-γ in serum and BAL fluid than the PBS/Cryptococcus- and BCG/PBS-exposed animals (Fig. 4a and b). Additionally, IL-4 was absent in BAL fluids from all BCG/PBS-infected mice whereas low levels of this cytokine were detected in the BCG/Cryptococcus-infected animals (37 ± 30 pg/ml). The IL-4 levels in BAL fluids from PBS/Cryptococcus-infected mice were not significantly different from those in BAL fluids from the coinfected group (data not shown). Interestingly, the BCG/PBS-infected group had the highest average serum IFN-γ levels of all groups (Fig. 4a), although variability rendered the difference not statistically significant. This suggests either an ongoing inflammatory response due to persistent BCG infection or prolonged elevation of this type 1 cytokine after successfully controlled BCG infection.
FIG. 3.
Prior BCG exposure decreases antigen-specific IL-5 production during Cryptococcus infection. Mice were infected intranasally with 2 × 105 CFU of BCG or given PBS. After 6 weeks, Cryptococcus (Crypto) or PBS was administered intranasally, and animals were sacrificed 13 days later. EliSpot assays to detect IL-5-producing cells were performed with lung homogenate cells. HK-BCG (a) and heat-killed Cryptococcus (b) were used as stimulants, and results from medium-stimulated wells were subtracted from those from antigen-stimulated samples. Each dot represents one animal, and the results from two representative experiments are pooled.
FIG. 4.
Systemic and lung IFN-γ levels are increased after BCG/Cryptococcus coinfection. Mice were infected intranasally with 2 × 105 CFU of BCG or given PBS. After 6 weeks, Cryptococcus (Crypto) or PBS was administered intranasally and animals were sacrificed 13 days later. Serum (a) and BAL fluid (b) IFN-γ levels were determined by ELISA. The significant differences between groups are indicated in the figure. Each dot represents one animal, and the results are from one of two representative experiments.
Levels of the immune modulatory cytokine, TGF-β, were determined by ELISA. No significant differences in levels of total or bioactive TGF-β were found between singly and dually infected groups (data not shown), but both Cryptococcus and BCG infection increased levels above those found in uninfected mice.
Splenocyte transfer from BCG-immune mice does not protect from Cryptococcus-induced lung eosinophilia.
It has previously been shown that transfer of influenza-immune splenocytes to naïve mice reduces the eosinophilic response to the heterologous virus respiratory syncytial virus (27). To determine if the effect was also valid in this model, donor mice were infected intranasally with BCG (2 × 105 CFU) or given PBS. The animals were sacrificed 6 weeks later, and splenocytes were adoptively transferred i.v. to syngeneic mice. These mice were infected the following day with C. neoformans, and BAL fluids were obtained 13 days later. No effect of BCG-immune splenocytes on BAL fluid eosinophilia was found when the BAL fluids were compared to those of mice naïve for splenocyte transfer (57% ± 13% versus 50% ± 17%; P = 0.23).
BCG/Cryptococcus coinfection in the lung increases replication of BCG but does not affect Cryptococcus growth.
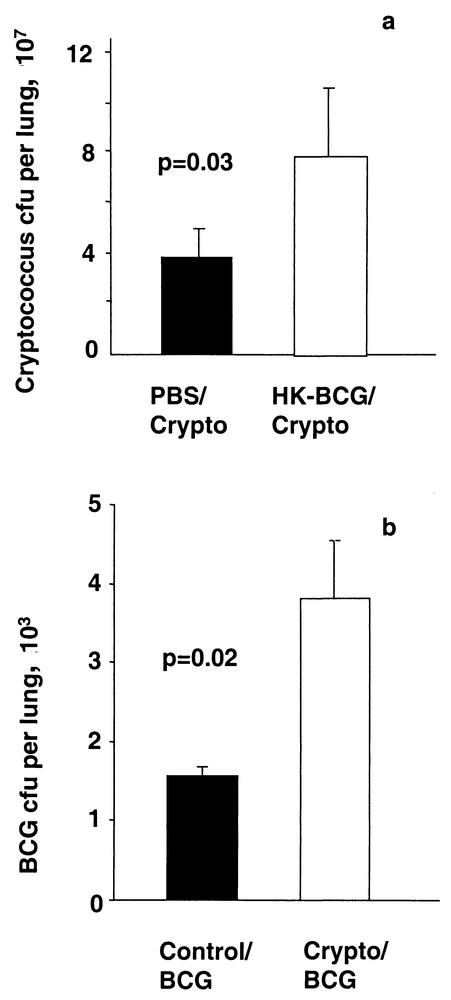
Both mycobacteria and cryptococci are cleared by type 1 immune responses in resistant animals, while Cryptococcus induces nonprotective type 2 responses in susceptible C57BL/6 mice. As type 1 and 2 immune phenotypes can reciprocally inhibit each other, the effect on replication of the respective pathogens was evaluated. Six weeks prior to infection with C. neoformans, live wild-type BCG Pasteur or HK-BCG Pasteur was given intranasally. Control groups were given PBS instead of Cryptococcus or BCG. Thirteen days after the fungal infection, lungs were homogenized and aliquots were plated out on Middlebrook and Sabouraud agar. No significant change in Cryptococcus growth was induced by prior live BCG infection, in spite of the alteration of the cytokine phenotype induced by coinfection (data not shown). Increased fungal recovery from lungs was seen, however, after intranasal HK-BCG administration (Fig. 5a). Compared to that in the BCG/PBS-infected group, significantly increased mycobacterial growth was seen in BCG/Cryptococcus-infected mice (Fig. 5b).
FIG. 5.
Coinfection increases BCG replication in the lung, and prior exposure to dead BCG increases the Cryptococcus lung burden. An inoculum of 2 × 105 CFU of HK-BCG or of live BCG Pasteur was given intranasally 6 weeks prior to infection with 2 × 104 CFU of Cryptococcus (Crypto). Lungs were homogenized 13 days later and plated out on Sabouraud dextrose medium and Middlebrook agar to assess pathogen burden. (a) Prior HK-BCG exposure increased Cryptococcus recovery from lungs. (b) A significant increase in BCG titers was found in Cryptococcus coinfected mice. Each column represents the mean CFU count ± the standard deviation from five mice in one of at least two representative experiments.
BCG-induced reduction of eosinophilia depends on IFN-γ.
A previous study has demonstrated the IFN-γ dependence of BCG-induced alteration of allergic inflammation (4). To assess the role of IFN-γ in protection from live-pathogen-induced illness, we infected C57BL/6 IFN-γ knockout mice and their littermate controls with 2 × 105 CFU of BCG or gave them PBS intranasally before the introduction of the fungal pathogen. Both wild-type and IFN-γ knockout mice developed lung eosinophilia after C. neoformans infection. However, only wild-type mice showed a reduction in eosinophilia as a result of prior BCG infection (Fig. 6). This suggests that IFN-γ plays a central role in BCG-induced alteration of the Th2 immune response.
FIG. 6.
IFN-γ is required for the protective effect of BCG on Cryptococcus-induced lung eosinophilia. Six weeks prior to infection with 2 × 104 CFU of C. neoformans (Crypto), 2 × 105 CFU of BCG Pasteur was given intranasally to IFN-γ gene knockout (IFN-γ−/−) mice and their wild-type C57BL/6 littermates. Thirteen days after fungal infection, BAL was performed. Eosinophil recruitment to BAL fluid was assessed by hemocytometer count of H&E-stained cytocentrifugation slides. Open columns represent the mean values ± standard deviations for a group of five IFN-γ−/− mice, and filled columns represent the corresponding values for wild-type littermates. BCG infection of wild-type animals reduced Cryptococcus-induced BAL fluid eosinophilia (P < 0.01), whereas no reduction was found in IFN-γ−/− animals (P = 0.3).
DISCUSSION
Polarization of immune responses into type 1 or type 2 phenotypes has a major effect on the outcome of certain infections, host responses against foreign antigens, and autoimmune conditions. The determinants of the development of these diverse responses remain controversial, but the nature and concentration of antigens, the availability of costimulatory signals, the local microenvironment of secreted substances, host genetic factors, and the maturation and activation state of antigen-presenting cells, especially dendritic cells, seem to play a pivotal role (12). In the present study, we show that the immunopathology of infection due to a live pathogen is altered in a microenvironment shaped by prior BCG infection. BCG infection suppresses Cryptococcus-induced airway eosinophilia, an effect that is long lasting, requires persisting mycobacteria in the lung, and depends on IFN-γ production. It should be stressed that the distinction of eosinophils both by flow cytometry and in H&E-stained cytospin preparations is problematic. While the flow cytometer does not distinguish between neutrophils and eosinophils, cytospin analysis does not account for degranulated eosinophils. However, in the group infected with Cryptococcus only, all of the granulocytes observed contained acidophilic granules. These were significantly reduced in mice additionally infected with BCG. We therefore believe that our comparisons are valid. The effect on eosinophilia does not extend to decreased fungal proliferation, and unlike the protective effect of BCG against OVA-induced inflammation, the reduction in Cryptococcus-induced eosinophilia requires live organisms. Remarkably, Cryptococcus infection increases the number of mycobacteria recovered from the lungs. The lack of an effect of BCG on the number of C. neoformans CFU does not preclude a potential survival benefit. Due to Home Office restrictions, such studies cannot be performed.
Prior intranasal or systemic BCG infection inhibits OVA-induced airway eosinophilia in mice (4, 30), but only little data exist for interactions between BCG and live pathogens. Erb et al. (4) showed that lung eosinophilia induced by Nippostrongylus brasiliensis infection is inhibited by BCG infection 1 week prior to helminth infection. If BCG is administered 4 and 8 weeks prior to worm infection, however, eosinophilia is reduced by only 50 and 30%, respectively. In contrast, Cryptococcus-induced lung eosinophilia is virtually abolished by prior BCG infection at 6 weeks and is reduced by 50% at 17 weeks, demonstrating that the effect of BCG on different live pathogens varies but can be pronounced and long lasting.
Early cytokine production during pulmonary Cryptococcus infection determines susceptibility (5). Th1-type cell-mediated immunity with IL-12, IFN-γ, (5) and TNF production (9) is critical for clearance of the organism. IL-5, on the other hand, is required for eosinophil and mononuclear cell recruitment during infection in susceptible C57BL/6 mice (8) that produce less of the type 1 cytokines. The increase in intracellular IFN-γ production by lung cells and in IFN-γ secretion in serum and BAL fluid seen in BCG-immune animals presumably reciprocally inhibits C. neoformans-induced Th2 cells. This idea is confirmed by our EliSpot data, which show a decrease in the frequency of IL-5-producing lung cells. This reduction in IL-5 correlates with reduced eosinophil recruitment to the BAL fluid in BCG-immune mice.
Though BCG exposure decreased Cryptococcus-specific IL-5 production, the frequency of cells secreting IL-4 and IFN-γ was unaltered. This is in contrast to results of earlier work employing the OVA model, in which BCG altered OVA-specific IFN-γ secretion (30), although OVA-specific IL-4 production was also not altered in that study. The differences probably relate to the methods of cytokine analysis and the models of eosinophilia used. Despite the altered cytokine profile in BCG-immune mice, C. neoformans clearance was not affected. The inability of the BCG-induced immune phenotype to decrease fungal growth may indicate (i) that the magnitude of the alteration in responses is not adequate in these susceptible mice, (ii) that IFN-γ alone is not sufficient for effective cryptococcal killing, or (iii) that BCG interferes with other anticryptococcal defenses, such as functions of antigen-presenting cells. Furthermore, both pathogens increased TGF-β levels, which could inhibit Cryptococcus clearance and override protection offered by increased IFN-γ levels.
The failure of splenocyte transfer from BCG-immune mice to protect against lung eosinophilia also supports the concept that it is the cytokine environment provided by low-grade BCG infection in the lung rather than a cellular component of the immune response which is required for altering Cryptococcus-induced eosinophilia. It is therefore unlikely that bystander activation or cross-reactive T- or B-cell activity plays a major role in this model.
The presence of neutrophils in BCG/Cryptococcus-infected mice with lowered eosinophil counts is striking. This suggests altered chemokine secretion and may be a factor in the skewing of the immune phenotype towards a type 1 pattern. Neutrophils contribute to effective antifungal responses (17), and although neutrophil numbers in BAL fluids were prominent in experimental groups with decreased BAL fluid eosinophilia, no decrease in fungal proliferation accompanied these changes.
Protective immunity to mycobacterial infection is not completely understood, but CD4+ T cells (22), CD8+ T cells (3), γδ T cells (21), and NK cells (18) all play a role. The elevated percentage of activated CD4+ and CD8+ T cells in BAL fluids in our studies suggested an appropriate immune response against mycobacterial infection in BCG/PBS-infected mice. These cells might be instrumental in the diversion of the lung cytokine microenvironment from a type 2 to a type 1 phenotype. However, a type 2 component of the immune response against mycobacteria is detrimental for successful control of infection (20). Some type 2 cytokines, i.e., IL-4, were still found in the BAL fluids of coinfected mice, and this might explain the increased mycobacterial recovery from the lungs. This observation also lends support to the hypothesis that unrelated Th2-inducing infections might be instrumental in reactivating tuberculosis in patients (2).
Even though ΔhspR BCG induces more CD8+ T-cell production of IFN-γ (24), Cryptococcus-induced eosinophilia was less dramatically altered than it was after wild-type BCG infection. Our previous studies show that ΔhspR mycobacteria are cleared more rapidly from mice than are wild-type bacteria. We therefore believe that the reduction of Cryptococcus-induced lung eosinophilia depends on ongoing low-grade mycobacterial replication in the lung. This hypothesis is further supported by the observations that a low dose of BCG has no effect and that i.v. administration of either strain of BCG was less effective at reducing lung eosinophilia, as this route of infection is associated with lower BCG titers in the lung.
The increased Cryptococcus-induced BAL fluid eosinophilia and fungal growth after heat-inactivated-BCG administration may be due to different pathways of antigen presentation. The increased activation of CD8+ T cells in mice with BCG/Cryptococcus coinfection compared to that in mice with Cryptococcus infection alone was not observed in mice exposed to killed BCG. Furthermore, HK-BCG was not able to induce the same magnitude of IFN-γ response in lung cells as was live BCG. This has important implications for vaccine design, as dead vaccines can exacerbate immunopathology of unrelated infections (as seen in this study) and related infections (e.g., after formaldehyde-inactivated respiratory syncytial virus vaccination [29]). The effect depends on the model used, as killed Mycobacterium vaccae (28) and killed BCG (13) used in other studies reduced allergen-induced Th2 responses successfully. Soluble antigen was used in these studies, whereas we used a live pathogen.
In summary, prior BCG exposure decreases lung eosinophilia due to an unrelated infection, C. neoformans. We believe that this effect is related to ongoing, low-grade BCG infection resulting in an altered cytokine environment and especially increased IFN-γ production, whereas antigen-specific responses are not affected. The study suggests that BCG-based vaccinations against Th2-mediated conditions may need to consist of live organisms, which induce low-grade, chronic inflammatory changes at the target site.
Acknowledgments
We thank Brigitte Askonas and Douglas Young for invaluable discussion and Graham Stewart for supplying the ΔhspR70 BCG strain. G.W. was supported by a National Asthma Campaign grant (00/007), and T.H. was supported by the Royal Society (RSRG21219), the Medical Research Council, United Kingdom (B00/0077), and the National Asthma Campaign (00/007). B.G.M. was funded by the British Medical Association and the Wellcome Trust-supported PJMO (program grant 054797/Z/98).
Editor: T. R. Kozel
REFERENCES
- 1.Ando, M., M. Suga, Y. Nishiura, and M. Miyajima. 1995. Summer-type hypersensitivity pneumonitis. Intern. Med. 34:707-712. [DOI] [PubMed] [Google Scholar]
- 2.Bentwich, Z., A. Kalinkovich, Z. Weisman, G. Borkow, N. Beyers, and A. D. Beyers. 1999. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol. Today 20:485-487. [DOI] [PubMed] [Google Scholar]
- 3.Cho, S., V. Mehra, S. Thoma-Uszynski, S. Stenger, N. Serbina, R. J. Mazzaccaro, J. L. Flynn, P. F. Barnes, S. Southwood, E. Celis, B. R. Bloom, R. L. Modlin, and A. Sette. 2000. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. USA 97:12210-12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erb, K. J., J. W. Holloway, A. Sobeck, H. Moll, and G. Le Gros. 1998. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 187:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoag, K. A., M. F. Lipscomb, A. A. Izzo, and N. E. Street. 1997. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am. J. Respir. Cell Mol. Biol. 17:733-739. [DOI] [PubMed] [Google Scholar]
- 6.Holt, P. G. 1995. Environmental factors and primary T-cell sensitisation to inhalant allergens in infancy: reappraisal of the role of infections and air pollution. Pediatr. Allergy Immunol. 6:1-10. [DOI] [PubMed] [Google Scholar]
- 7.Holt, P. G., and C. Macaubas. 1997. Development of long-term tolerance versus sensitisation to environmental allergens during the perinatal period. Curr. Opin. Immunol. 9:782-787. [DOI] [PubMed] [Google Scholar]
- 8.Huffnagle, G. B., M. B. Boyd, N. E. Street, and M. F. Lipscomb. 1998. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 160:2393-2400. [PubMed] [Google Scholar]
- 9.Huffnagle, G. B., G. B. Toews, M. D. Burdick, M. B. Boyd, K. S. McAllister, R. A. McDonald, S. L. Kunkel, and R. M. Strieter. 1996. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J. Immunol. 157:4529-4536. [PubMed] [Google Scholar]
- 10.Huffnagle, G. B., J. L. Yates, and M. F. Lipscomb. 1991. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect. Immun. 59:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussell, T., L. C. Spender, A. Georgiou, A. O'Garra, and P. J. M. Openshaw. 1996. Th1 and Th2 cytokine induction in pulmonary T-cells during infection with respiratory syncytial virus. J. Gen. Virol. 77:2447-2455. [DOI] [PubMed] [Google Scholar]
- 12.Jankovic, D., Z. Liu, and W. C. Gause. 2001. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22:450-457. [DOI] [PubMed] [Google Scholar]
- 13.Major, T., G. Wohlleben, B. Reibetanz, and K. J. Erb. 2002. Application of heat killed Mycobacterium bovis-BCG into the lung inhibits the development of allergen-induced Th2 responses. Vaccine 20:1532-1540. [DOI] [PubMed] [Google Scholar]
- 14.Martinez, F. D. 1994. Role of viral infections in the inception of asthma and allergies during childhood: could they be protective? Thorax 49:1189-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez, F. D., D. A. Stern, A. L. Wright, L. M. Taussig, M. Halonen, et al. 1995. Association of non-wheezing lower respiratory tract illnesses in early life with persistently diminished serum IgE levels. Thorax 50:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marwaha, R. K., A. Trehan, K. Jayashree, and R. K. Vasishta. 1995. Hypereosinophilia in disseminated cryptococcal disease. Pediatr. Infect. Dis. J. 14:1102-1103. [PubMed] [Google Scholar]
- 17.Miller, M. F., and T. G. Mitchell. 1991. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect. Immun. 59:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orme, I. M. 2001. Immunology and vaccinology of tuberculosis: can lessons from the mouse be applied to the cow? Tuberculosis 81:109-113. [DOI] [PubMed] [Google Scholar]
- 19.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rook, G. A., and A. Zumla. 2001. Advances in the immunopathogenesis of pulmonary tuberculosis. Curr. Opin. Pulm. Med. 7:116-123. [DOI] [PubMed] [Google Scholar]
- 21.Saunders, B. M., A. A. Frank, A. M. Cooper, and I. M. Orme. 1998. Role of γδ T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect. Immun. 66:5508-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirakawa, T., T. Enomoto, S. Shimazu, and J. M. Hopkin. 1997. The inverse association between tuberculin responses and atopic disorder. Science 275:77-79. [DOI] [PubMed] [Google Scholar]
- 24.Stewart, G. R., V. A. Snewin, G. Walzl, T. Hussell, P. Tormay, P. O'Gaora, M. Goyal, J. Betts, I. N. Brown, and D. B. Young. 2001. Overexpression of heat-shock proteins reduces survival of Mycobacterium tuberculosis in the chronic phase of infection. Nat. Med. 7:732-737. [DOI] [PubMed] [Google Scholar]
- 25.Sudo, N., S. Sawamura, K. Tanaka, Y. Aiba, C. Kubo, and Y. Koga. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J. Immunol. 159:1739-1745. [PubMed] [Google Scholar]
- 26.von Mutius, E., F. D. Martinez, C. Fritzsch, T. Nicolai, G. Roell, and H. H. Thiemann. 1994. Prevalence of asthma and atopy in two areas of West and East Germany. Am. J. Respir. Crit. Care Med. 149:358-364. [DOI] [PubMed] [Google Scholar]
- 27.Walzl, G., S. Tafuro, P. Moss, P. J. Openshaw, and T. Hussell. 2000. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J. Exp. Med. 192:1317-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, C. C., and G. A. Rook. 1998. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology 93:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 70:2852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, X., S. Wang, Y. Fan, and L. Zhu. 1999. Systemic mycobacterial infection inhibits antigen-specific immunoglobulin E production, bronchial mucus production and eosinophilic inflammation induced by allergen. Immunology 98:329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]