Abstract
Helicobacter pylori activates the transcription factor NF-κB, leading to proinflammatory cytokine production by gastric epithelial cells. However, the receptors for the initial bacterial interaction with host cells which activate downstream signaling events have not been completely defined. Recently, it has been shown that microbial components activate Toll-like receptors (TLRs), thereby leading to AP-1- and NF-κB-dependent transcription and resulting in the production of proinflammatory cytokines. The aim of this study was to determine whether H. pylori activates TLR4. Reverse transcription-PCR showed that both type I and type II H. pylori clinical isolates induced TLR4 mRNA expression in AGS cells compared with that by uninfected controls. H. pylori upregulated TLR4 protein expression in two gastric epithelial cell lines (AGS and MKN45) and one intestinal epithelial cell line (T84). Monoclonal TLR4 antibody inhibited lipopolysaccharide-induced interleukin-8 secretion from THP-1 macrophages but not from gastric epithelial cells infected with H. pylori. H. pylori demonstrated increased adherence to CHO TLR4-transfected cells compared with that to both CHO TLR2-transfected and nontransfected CHO cells (P < 0.01). These results indicate that H. pylori activates TLR4 expression in epithelial cells and that TLR4 can serve as a receptor for H. pylori binding.
Helicobacter pylori is a gram-negative bacterium that plays an etiologic role in the development of gastritis, peptic ulceration, and gastric adenocarcinoma (2). Several bacterial factors are proposed to play a role in disease pathogenesis. Type I H. pylori strains contain a pathogenicity island, which carries a number of virulence factors, including cagA and cagE (7), and is associated with more severe gastroduodenal disease (2). Studies using isogenic mutants demonstrate that certain genes carried on the cag pathogenicity island, including cagE but not cagA, are responsible for nuclear factor-κB (NF-κB) activation resulting in the transcription of a number of proinflammatory genes such as interleukin-8 (IL-8), IL-1β, gamma interferon, and tumor necrosis factor alpha (20, 29). However, the eukaryotic receptors involved in H. pylori activation of the innate immune response have not been clearly defined.
Toll-like receptors (TLRs) play a crucial role in host innate and adaptive immune responses to microbial pathogens and their products (1). TLRs have leucine-rich motifs in their extracellular domains similar to those of other pattern-recognition proteins that promote ligand binding (1). TLR proteins also contain a cytoplasmic tail that is homologous to the IL-1 and IL-18 receptor and hence can trigger intracellular signaling pathways (23). To date, 10 TLRs have been described (31), with TLR2 and TLR4 the two best characterized. TLR2 responds to peptidoglycan, lipoteichoic acid (24), and bacterial lipoproteins (19). TLR4 is activated by the lipopolysaccharide (LPS) of gram-negative bacteria (3). Recently, it has been demonstrated that TLR2 and TLR4 are expressed on human intestinal epithelial cell lines (4, 5) and that Escherichia coli (O26:B6)-derived LPS induces TLR4 trafficking in epithelial cells (3). Maeda et al. (20) showed that TLR4 mRNA is also expressed on gastric epithelial MKN45 cells. In contrast to macrophages, TLR4 is not involved in H. pylori-induced NF-κB activation in gastric epithelia (21). Therefore, the precise function(s) of TLR4 in gastric epithelial cells is still not known.
MATERIALS AND METHODS
Reagents.
H. pylori-derived LPS was kindly provided by Mario Monteiro (Institute for Biological Sciences, National Research Council, Ottawa, Ontario, Canada). Polyclonal anti-TLR4 and anti-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.), and monoclonal anti-TLR4 (HTA125) antibody was purchased from eBioscience (San Diego, Calif.). Polyclonal H. pylori immune serum was purchased from DAKO (Copenhagen, Denmark). Mouse immunoglobulin G2a (IgG2a) and IgG2b were purchased from R&D Systems (Minneapolis, Minn.). R-phycoerythrin (R-PE)-conjugated mouse anti-human CD14 monoclonal antibody and R-PE-conjugate mouse IgG2a (as a negative control for monoclonal anti-CD14 antibody) were purchased from PharMingen (Franklin Lakes, N.J.). Fluorescein isothiocyanate-conjugated anti-mouse IgG and horseradish peroxidase-labeled anti-rabbit IgG were purchased from Santa Cruz Biotechnology. Ciprofloxacin was kindly provided by Bayer Pharmaceuticals (West Haven, Conn.). G418 and hygromycin were purchased from Gibco Life Technologies, Inc. (Grand Island, N.Y.).
Bacteria and growth conditions.
The H. pylori strains employed in this study included type I strain LC11 (cagA+, cagE+, VacA+) and type II strain LC20 (lacking cagA, lacking cagE, VacA−) originally isolated from children with peptic ulceration and with gastritis alone, respectively (9). Bacterial cultures were grown for 72 h on agar plates containing 5% sheep blood under microaerophilic conditions (5% O2, 85% N2, 10% CO2). Bacteria were inoculated into brucella broth with 10% fetal bovine serum (FBS), grown at 37°C under microaerophilic conditions with shaking overnight, and washed once with sterile phosphate-buffered saline (PBS) (pH 7.4). Bacteria were resuspended in antibiotic-free medium (Gibco) 1-2.5% FBS for the period of bacterial infection.
Tissue culture cells.
Human gastric epithelial AGS CRL-1739 cells (American Tissue Culture Collection, Manassas, Va.) and Chinese hamster ovary (CHO) fibroblast cells (CHO TLR2- and CHO TLR4-transfected cells), kindly provided by Douglas T. Golenbock (Boston University, Boston, Mass.) (22), were grown in Ham's F-12 medium (Gibco) with 10% FBS. T84 cells (ATCC CCL-248) were grown in a 1:1 mixture of Dulbecco's minimum essential medium (Gibco) and Ham's F-12 medium.
MKN45 cells (kindly provided by Sheila Crowe, University of Virginia, Charlottesville) were grown in RPMI 1640 supplemented with 10% FBS plus antibiotics. At 20 h prior to infection, cells were grown in the absence of antibiotics in tissue culture medium plus 1% FBS. THP-1 cells (ATCC TIB-202) were grown in RPMI 1640 medium and stimulated with the phorbol ester phorbol-12-myristate-13-acetate (0.1 to 10 ng/ml) for 3 to 4 days to become macrophage-like cells (8). AGS, MKN45, T84, and THP-1 cells were grown in medium containing 2 mM l-glutamine, 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). G418 (500 μg/ml), ciprofloxacin (10 μg/ml), and hygromycin (250 μg/ml) were added to TLR2- and TLR4-transfected CHO cells. All cells were grown in a humidified 5% CO2 atmosphere at 37°C.
Bacterial infection of tissue culture cells.
Tissue culture cells were seeded onto 4-well slide chambers or 6-cm-diameter petri dishes (Nunc, Naperville, Ill.). Coverslips were placed into 24- and 6-well plates for immunostaining and scanning electron microscopy, respectively. Cells were directly seeded into 6-cm-diameter dishes for whole-cell protein and RNA isolation experiments. Cells were grown in medium with 2.5% FBS and without antibiotics for 20 h at 37°C prior to bacterial infection. Cells were washed once with sterile PBS, and then H. pylori bacteria were added at a multiplicity of infection (MOI) of 100:1 for differing time points, followed by washing with PBS six times to remove nonadherent bacteria.
RNA isolation and RT-PCR.
Using TRIZol reagent (Gibco) according to the manufacturer's instructions, total RNA was extracted from cells. Using oligo-dT random primers, reverse transcription-PCR (RT-PCR) analysis was carried out with 5 μg of total RNA. cDNA products were amplified using specific primer pairs (Table 1) for human TLR4 (hTLR4) primers and human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as described previously (5, 26). PCR products were resolved by agarose (1.5%) gel electrophoresis and visualized by staining with ethidium bromide. The relative amounts of the PCR products were calculated as the ratio of the hTLR mRNA to GAPDH mRNA, as analyzed by Fluorchem software (Alpha Innotech Corporation, San Leandro, Calif.).
TABLE 1.
Primer sequences employed in this study
Western blotting for TLR4 expression.
Tissue culture cells grown in 6-cm-diameter petri dishes were infected with bacteria for 2 to 6 h and then washed with ice-cold sterile PBS. The cells were lysed with radioimmunoprecipitation buffer (10 mM Tris HCl [pH 8.0], 150 mM NaCl, 0.5% Triton X-100) containing a mixture of proteinase inhibitors (Roche Molecular Biochemicals, Mannheim, Germany) for 40 min on ice. Lysates were then centrifuged at 10,000 × g for 15 min at 4°C, snap frozen, and stored at −70°C. Lysates were subjected to electrophoresis in sodium dodecyl sulfate-7.5 or 12% polyacrylamide gels and then transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). A THP-1 whole-cell lysate (Santa Cruz Biotechnology) served as the positive control for TLR4 expression (5). Membranes were probed with polyclonal antiserum against TLR4 (Santa Cruz H80) at a dilution of 1 in 200 at 4°C overnight, washed three times with TBST, probed with secondary antibody conjugated with horseradish peroxidase (1:1,000), and detected by enhanced chemiluminescence (Amersham Pharmacia Biotechnology Inc., Piscataway, N.J.). The blots were stripped using stripping buffer (1 mM glycine, pH 3.5) for 1 h and then reprobed using actin immune serum to monitor protein loading. TLR4 was quantified relative to actin expression with Fluorchem software.
Immunofluorescence.
Cells were grown in 2.5% FBS in antibiotic-free culture medium on coverslips or 4-well-chamber slides for 16 to 20 h. A bacterial suspension was then added to the cell monolayer for 2 h at 37°C. Cells were washed six times with PBS and then fixed in 4% paraformaldehyde. Cells were washed in PBS and permeabilized with 0.1% Triton X-100 in PBS. Nonspecific binding was blocked with 1% bovine serum albumin-0.1% Triton X-100 in PBS or normal goat serum (Santa Cruz Biotechnology) for 1 h at room temperature. Cells were rinsed four times with PBS and incubated with anti-TLR4 (1:100) at 4°C overnight. Cells were then rinsed three times with PBS. Expression of TLR2 and TLR4 was visualized by adding rhodamine-conjugated goat anti-rabbit antibody at a dilution of 1 in 100 and donkey anti-goat IgG at a dilution of 1 in 100 for 30 min at room temperature. Normal goat serum, preimmune rabbit serum, and secondary antibody alone were employed as negative controls. Cells were then examined using fluorescence microscopy (Leitz Dialux 22; Leica Canada Inc., Willowdale, Ontario, Canada).
Scanning electron microscopy.
CHO cells were seeded onto 6-well plates with 25-mm-diameter glass coverslips at an approximate density of 2 × 105 cells/well. Cells were allowed to adhere for 16 to 20 h and were then washed twice with PBS, and 2.5% FBS antibiotic-free medium was added. Cells were infected with H. pylori strain LC11 (MOI, 100:1) for 2 h at 37°C in 5% CO2. Wells were then washed six times with PBS to remove nonadherent bacteria and fixed in Universal fixative (Sigma) (4% paraformaldehyde and 1% glutaraldehyde [pH 7.0]; 1:1 ratio). Coverslips were then removed from the multiwell plates and incubated in osmium tetroxide for 1 h at room temperature. Cells were then dehydrated in a graded series of ethanol washes (50 to 100%), dried through a critical point dryer, and sputter coated with gold. Samples were viewed with a JSM 820 scanning electron microscope (JEOL Ltd., Boston, Mass.).
Flow cytometric analysis of membrane-bound CD14 (mCD14).
AGS and THP-1 cells were plated in 6-well plates at 3 × 105 cells. After 1 day of growth, cells were detached (using 0.25% trypsin-EDTA [Gibco] for 5 min at 37°C) from plastic plates. Cells were then washed three times with PBS and blocked followed by incubation with either isotype control mouse IgG2a conjugated with PE or mouse anti-human mCD14 conjugated with PE for 30 min at room temperature. Cells were then washed three times with PBS and fixed with 2% paraformaldehyde for 1 h before being subjected to flow cytometry using a FACScan microfluorometer (Becton Dickinson, Mountain View, Calif.).
Immunoassay for IL-1β and IL-8.
Tissue culture cells (0.5 × 104) were seeded into 24-well plates, maintained in medium with 10% FBS for 16 to 20 h, washed in PBS, and then immersed in medium with 2.5% FBS and without antibiotics before coinfection. After 16 to 20 h of further growth, monolayers approximately 80% confluent were grown together with H. pylori (MOI, 100:1), H. pylori LPS (100 ng/ml), or E. coli-derived LPS (100 ng/ml) for 4 h at 37°C in 5% CO2. For antibody inhibition experiments, cells were pretreated with antibodies at different concentrations for 1 h at room temperature, followed by H. pylori infection for 4 h at 37°C in 5% CO2. As a positive control, TLR4 monoclonal antibody was incubated either alone or together with E. coli-derived LPS before cell supernatants were collected for chemokine analysis (17). Supernatants were then collected, centrifuged at 1,000 × g for 5 min, and employed in enzyme-linked immunosorbent assays (Biosource International, Inc. Camarillo, Calif.) as previously described (7).
Determination of bacterial CFU.
Tissue culture cells were coincubated with H. pylori (MOI, 100:1) for 2 h at 37°C. Cells were then washed five times with PBS to remove nonadherent bacteria. To recover adherent H. pylori, sterile water was used to lyse AGS cells (10 min) and dilutions were plated onto blood agar plates. Agar plates were incubated at 37°C for 4 days in microaerobic conditions, and CFU levels were determined.
Statistics
Results are expressed as means ± standard errors of the means (SEM). To compare mean values between two groups, the Student's t test was employed. An analysis of variance (ANOVA) program was used to determine differences between multiple groups (InStat; GraphPad Software Inc.).
RESULTS
Gastrointestinal cell lines express TLR4 and H. pylori infection upregulates TLR4 mRNA.
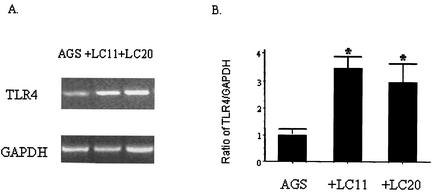
As shown in Fig. 1A, AGS cells constitutively expressed TLR4 mRNA. H. pylori infection induced increases in TLR4 transcription (Fig. 1A). However, there was no difference in TLR4 mRNA induction levels between the type I H. pylori strain, LC11 (cagA+, cagE+, VacA+), and the type II strain, LC20 (lacking cagA, lacking cagE, VacA−) (Fig. 1B).
FIG. 1.
H. pylori infection induces the transcription of TLR4. Total RNA was extracted from AGS cells following infection with H. pylori strain LC11 or LC20 for 2 h and analysis by RT-PCR using TLR4-specific primers. (A) A gel representative of three separate experiments shows TLR4 and GAPDH mRNA. (B) Levels of mRNA were semiquantitated by densitometry as the ratio of TLR4 to GAPDH. *, P < 0.01.
H. pylori infection upregulates the expression of glycosylated TLR4.
As shown in Fig. 2, indirect immune fluorescence microscopy confirmed that AGS cells expressed TLR4 on the cell surface. T84 cells served as a positive control (5). Preimmune sera and secondary antibody alone were negative (data not shown). Western blotting showed that 2 h of H. pylori infection upregulated TLR4 protein expression in whole-cell protein lysates obtained from both AGS and T84 epithelial cells (Fig. 3A). Both H. pylori strain LC11 and strain LC20 increased the amount of TLR4 in AGS cells. However, when H. pylori was incubated with MKN45 gastric epithelial cells for 6 h, strain LC11 increased glycosylated TLR4 levels, whereas strain LC20 did not (Fig. 3B). The upper bands seen in the panel result from the presence of the glycosylated (and thus functional) form of TLR4 (6, 25).
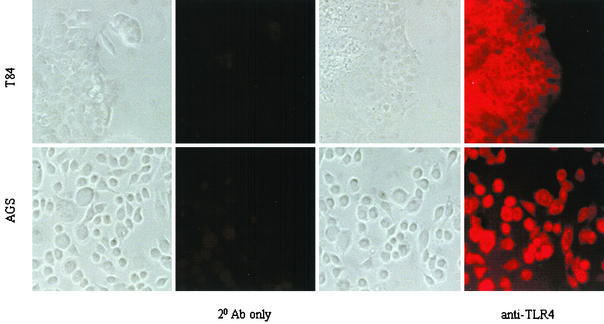
FIG. 2.
Human gastric (AGS) epithelial cells express TLR4 on the cell surface. Using TLR4 antibody followed by rhodamine-conjugated goat anti-rabbit antibody, expression of TLR4 was determined by fluorescence microscopy. Matching fields were taken under alternating phase-contrast and fluorescence microscopy. TLR4 expression was detected on the surfaces of both intestinal T84 cells (employed as a positive control [4]) and gastric AGS epithelial cells. The results shown are representative of three separate experiments.
FIG. 3.
H. pylori infection induces TLR4 protein expression in epithelial cells. (A) AGS and T84 cells were infected with H. pylori strains LC11 and LC20 for 2 h, and protein expression levels in Western blots of whole-cell protein lysates were semiquantified by densitometry calculating the ratio of TLR4 to β-actin. Results in uninfected cells were set to a value of 1. *, P < 0.05. Results shown are representative of three separate experiments. (B) Western blotting analysis of whole-cell protein lysates from MKN45 gastric epithelial cells infected with H. pylori strains LC11 and LC20 for 6 h at 37°C. LC11 infection increased glycosylated TLR4 (Gly-TLR4) expression, and both bacterial strains enhanced the expression of nonglycosylated TLR4 (TLR4) compared with that of uninfected gastric epithelia (O). N/S, nonspecific. The lower panel indicates levels of β-actin (employed as a control for protein loading) in each lane.
Secretion of IL-8 is induced by H. pylori.
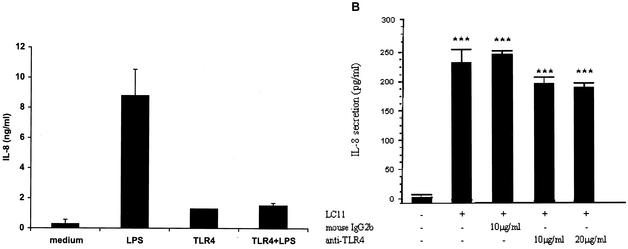
IL-8 secretion was measured and used as a marker to determine whether pylori activates NF-κB through TLR4. As shown in Fig. 4A, TLR4 antibody blocked IL-8 secretion in response to purified LPS. By contrast, neither polyclonal-TLR4 antiserum or monoclonal antibody against TLR4 (known to act as blocking antibodies [32]) inhibited chemokine secretion from AGS cells following H. pylori infection (Fig. 4B).
FIG. 4.
Monoclonal anti-TLR4 antibodies do not block H. pylori-induced IL-8 secretion from gastric epithelial cells. (A) The presence of TLR4 monoclonal antibody (HTA 125 clone; 100 μg/ml for 1 h at room temperature) prevented E. coli-derived LPS-induced IL-8 secretion from THP-1 cells. Medium, unstimulated cells without TLR4 antibody; LPS, macrophages stimulated with LPS (100 ng/ml for 4 h); TLR4, THP-1 cells incubated with the monoclonal antibody alone; TLR4+LPS, THP-1 cells challenged with E. coli LPS following incubation with the anti-TLR4 monoclonal antibody. Concentrations of IL-8 in culture supernatants were measured by immunoassay (P < 0.05 by ANOVA). (B) AGS cells were incubated with antibodies and their isotype controls (1 h) followed by infection with H. pylori for 4 h at 37°C. No difference was observed between H. pylori-infected cells and infected cells treated with TLR4 antibodies and isotype controls. Data are presented as means ± SEM of three separate experiments. ***, P < 0.001 by ANOVA.
LPS-induced chemokine secretion requires membrane-bound CD14 in gastric epithelial cells.
To determine whether TLR4 mediates signaling responses to H. pylori infection, IL-8 secretion from differentiated-monocyte (macrophage-like) THP-1 cells was compared with that from AGS cells. As shown in Fig. 5A, LPSs derived from both H. pylori and E. coli induced levels of chemokine secretion from THP-1 cells comparable with that from live bacteria. In contrast, little IL-8 secretion was induced by purified H. pylori- and E. coli-derived LPS in AGS cells, even though both cell lines express TLR4 (15). According to the results of flow cytometry, the expression of membrane-bound CD14 (mCD14), the LPS coreceptor, exhibited by THP-1 cells (Fig. 5B) was higher than the minimal mCD14 expression seen with AGS cells (Fig. 5C).
FIG. 5.
H. pylori LPS induces IL-8 secretion from THP-1 cells but not from AGS cells. (A) AGS and THP-1 cells were either infected with H. pylori (MOI, 100:1) or treated with purified H. pylori- or E. coli-derived LPS (100 ng/ml) for 4 h at 37°C. Levels of IL-8 in cell-free tissue culture medium supernatants were measured by immunoassay. Data are presented as means ± SEM of three separate experiments. ***, P < 0.001 by ANOVA. (B) mCD14 staining of THP-1 and AGS cells measured by flow cytometry. The black line depicts the negative control (R-PE conjugate mouse IgG2a), and the grey line shows R-PE-conjugated monoclonal anti-human mCD14 staining. THP-1 cells (lower graph), but not AGS cells (upper graph), expressed CD14.
TLR4 mediates H. pylori binding to CHO-transfected cells.
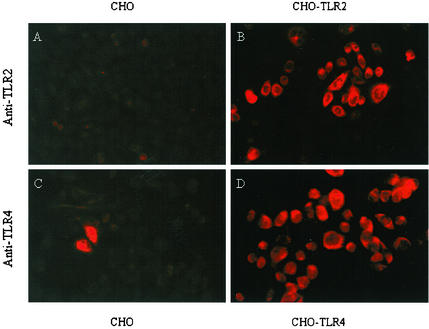
Transfected CHO cells (22) were used to study whether H. pylori can employ TLRs as a mechanism for adherence. Nontransfected CHO cells expressed low levels of TLR2 and TLR4 (Fig. 6A and C), whereas immunostaining confirmed that TLR2-transfected cells expressed TLR2 (Fig. 6B) and TLR4-transfected cells expressed abundant TLR4 (Fig. 6D). To determine whether TLR2 or TLR4 expression promoted H. pylori adhesion, CHO, CHO TLR2-, and CHO TLR4-transfected cells were infected with H. pylori at an MOI of 100:1 for 2 h.
FIG. 6.
TLR2- and TLR4-transfected CHO cells express TLRs. CHO cells were stained with polyclonal immune serum against either TLR2 or TLR4 followed by rhodamine-conjugated secondary antibody and examination under fluorescence microscopy. Expression of TLR2 was prominent on CHO-TLR2 cells (B) but not on nontransfected CHO cells (A). Compared with that on naive CHO cells (C), surface expression of TLR4 was readily evident on CHO-TLR4 cells (D).
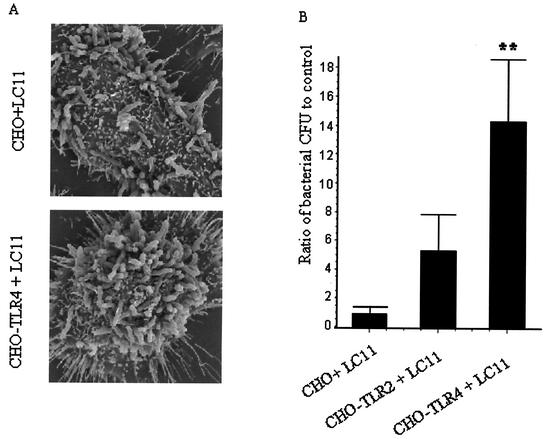
Scanning electron microscopy showed higher levels of H. pylori adherence to CHO-TLR4 cells compared with that to nontransfected CHO cells (Fig. 7A). To complement the scanning electron microscopy findings, CFU levels of H. pylori adherent to CHO, CHO-TLR2, or CHO-TLR4 cells were determined following bacterial infection. As shown in Fig. 7B, H. pylori adherence to TLR4-transfected CHO cells was higher than that of both TLR2-transfected CHO cells and nontransfected CHO cells (P < 0.001).
FIG. 7.
H. pylori adheres to TLR4-transfected CHO cells. (A) Scanning electron microscopy demonstrated that levels of H. pylori binding to CHO-TLR4-transfected cells after 2 h of infection (lower photomicrograph) were higher than those of binding to untreated CHO cells infected with the gastric pathogen for the same time period (upper photomicrograph). (B) Bacterial CFU of H. pylori recovered from CHO cell lysates after 2 h of infection. Data are presented as means ± SEM of three separate experiments. **, P < 0.01 by ANOVA.
DISCUSSION
This study demonstrates that human gastric epithelial cells constitutively express TLR4 mRNA. In addition, levels of TLR4 mRNA were increased following infection with clinical isolates of H. pylori independent of the presence or absence of the cag pathogenicity island. Protein expression of glycosylated TLR4 in response to H. pylori infection was also enhanced in three gastrointestinal cell lines (AGS, MKN45, and T84). We confirmed previous studies which indicated that TLR4 does not mediate H. pylori-induced IL-8 secretion in gastric epithelia (20), a finding that contrasts with studies employing macrophages of mouse origin (21). In addition, using overexpression of hTLR2 and hTLR4 in CHO cells, we demonstrated that TLR4 expression promotes increased binding of H. pylori to cell surfaces.
These data confirm previous results indicating that gastric cancer epithelial cell lines express TLR4 (21; S. Ishihara, M. Rumi, Y. Kadowaki, Y. Miyaoka, N. Yoshino, H. Yuki, H. Sato, C. F. Ortega-Cava, and Y. Kinoshita, Digestive Disease Week of the American Gastroenterological Association, Gastroenterology 122:A-428, abstr. T1179, 2002). In addition, Park et al. indicated in a preliminary report (J. Y. Park, D. S. Han, C. S. Eun, Y. C. Jeon, and J. S. Hahm, Digestive Disease Week of the American Gastroenterological Association, Gastroenterology 122:A534, abstr. W932, 2002) that TLR4 protein expression is increased in gastric tissue biopsies obtained from H. pylori-positive patients compared with that for uninfected controls.
Activation of TLRs induces NF-κB activation to mediate both chemokine and cytokine production (31). Therefore, IL-8 was chosen as an inducible marker for monitoring results after H. pylori infection of tissue culture cells. However, neither polyclonal nor monoclonal anti-TLR4 antibodies, which have previously been described as blocking antibodies (32), inhibited H. pylori-induced IL-8 secretion from AGS cells. In contrast to findings for murine macrophages in which activation of NF-kB is TLR4 dependent (21), the findings of the present study and of a previous study (20) indicate that activation of nuclear transcription responses controlling chemokine production in gastric epithelia is independent of TLR4.
Kawahara et al. (18) showed that purified LPS derived from a type I H. pylori strain induces the expression of TLR4, but not TLR2, in guinea pig gastric parietal cells. Taken together, these findings are consistent with the results of the present study, which show an increase in TLR4 expression, but an impaired chemokine response, in human gastric epithelial cell lines after H. pylori infection.
Adherence of H. pylori to host epithelial cells is a critical first step in virulence (11). H. pylori reportedly binds to a variety of host cell molecules, including phosphatidyl ethanolamine (14), N-acetylneuraminyllactose (10), GM3 ganglioside (27), sulfatides (16), the Lewisb antigen (12, 28), lactotetraosylceramide (30), major histocompatibility class II antigens (13) and α5β1 integrin, which leads to its internalization in gastric epithelial cells (29). However, it is not clear what events occur downstream of these interactions. Herein, we employed complementary techniques, including scanning electron microscopy and determination of bacterial CFU, to demonstrate that TLR4-transfected cells demonstrate increases in H. pylori adherence compared with both TLR2-transfected cells and nontransfected CHO cells. The recent finding that expression of TLR4 is increased in gastric epithelia of H. pylori-infected individuals indicates that the surface-exposed antigen is a biologically plausible receptor potentially available for mediating microbial adhesion (Park et al., Gastroenterology 122:A534, 2002).
In summary, in this study we have found that H. pylori infection induces transcription and translation of TLR4 in a variety of gastrointestinal epithelial cells. In addition, in an overexpression model, H. pylori utilizes TLR4 as a receptor for binding to the host cell surface. These results contribute to a better understanding of H. pylori-host cell interactions.
Acknowledgments
This study was supported by fellowship awards from the Canadian Institutes of Health Research (CIHR)-University Industry (Canadian Association of Gastroenterology, AstraZeneca Canada, Altona Pharma). P.J.M.C. is the recipient of a CIHR/Canadian Digestive Health Foundation Doctoral Award. P.M.S. is the recipient of a Canadian Research Chair in Gastrointestinal Disease.
Editor: V. J. DiRita
REFERENCES
- 1.Akira, S., K. Hoshino, and T. Kaisho. 2000. The role of Toll-like receptors and MyD88 in innate immune responses. J. Endotoxin Res. 6:383-387. [PubMed] [Google Scholar]
- 2.Blaser, M. J., and D. E. Berg. 2001. Helicobacter pylori genetic diversity and risk of human disease. J. Clin. Investig. 107:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cario, E., D. Brown, M. McKee, K. Lynch-Devaney, G. Gerken, and D. K. Podolsky. 2002. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am. J. Pathol. 160:165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cario, E., and D. K. Podolsky. 2000. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect. Immun. 68:7010-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 6.da Silva Correia, J., and R. J. Ulevitch. 2002. MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 277:1845-1854. [DOI] [PubMed] [Google Scholar]
- 7.Day, A. S., N. L. Jones, J. T. Lynett, H. A. Jennings, C. A. Fallone, R. Beech, and P. M. Sherman. 2000. cagE is a virulence factor associated with Helicobacter pylori-induced duodenal ulceration in children. J. Infect. Dis. 181:1370-1375. [DOI] [PubMed] [Google Scholar]
- 8.Dreskin, S. C., G. W. Thomas, S. N. Dale, and L. E. Heasley. 2001. Isoforms of Jun kinase are differentially expressed and activated in human monocyte/macrophage (THP-1) cells. J. Immunol. 166:5646-5653. [DOI] [PubMed] [Google Scholar]
- 9.Dytoc, M., B. Gold, M. Louie, M. Huesca, L. Fedorko, S. Crowe, C. Lingwood, J. Brunton, and P. M. Sherman. 1993. Comparison of Helicobacter pylori and attaching-effacing Escherichia coli adhesion to eukaryotic cells. Infect. Immun. 61:448-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, D. G., T. K. Karjalainen, D. J. Evans, Jr., D. Y. Graham, and C. H. Lee. 1993. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J. Bacteriol. 175:674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans, D. J., Jr., and D. G. Evans. 2000. Helicobacter pylori adhesins: review and perspectives. Helicobacter 5:183-195. [DOI] [PubMed] [Google Scholar]
- 12.Falk, P., K. A. Roth, T. Borén, T. U. Westblöm, J. I. Gordon, and S. Normark. 1993. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. USA 90:2035-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan, X., H. Gunasena, Z. Cheng, R. Espejo, S. E. Crowe, P. B. Ernst, and V. E. Reyes. 2000. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J. Immunol. 165:1918-1924. [DOI] [PubMed] [Google Scholar]
- 14.Gold, B. D., M. Huesca, P. M. Sherman, and C. A. Lingwood. 1993. Helicobacter mustelae and Helicobacter pylori bind to common lipid receptors in vitro. Infect. Immun. 61:2632-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, Q., S. Akashi, K. Miyake, and H. R. Petty. 2000. Lipopolysaccharide induces physical proximity between CD14 and Toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J. Immunol. 165:3541-3544. [DOI] [PubMed] [Google Scholar]
- 16.Kamisago, S., M. Iwamori, T. Tai, K. Mitamura, Y. Yazaki, and K. Sugano. 1996. Role of sulfatides in adhesion of Helicobacter pylori to gastric cancer cells. Infect. Immun. 64:624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson, H., C. Hessle, and A. Rudin. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawahara, T., S. Teshima, A. Oka, T. Sugiyama, K. Kishi, and K. Rokutan. 2001. Type I Helicobacter pylori lipopolysaccharide stimulates Toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect. Immun. 69:4382-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 20.Maeda, S., M. Akanuma, Y. Mitsuno, Y. Hirata, K. Ogura, H. Yoshida, Y. Shiratori, and M. Omata. 2001. Distinct mechanism of Helicobacter pylori-mediated NF-kappa B activation between gastric cancer cells and monocytic cells. J. Biol. Chem. 276:44856-44864. [DOI] [PubMed] [Google Scholar]
- 21.Maeda, S., H. Yoshida, K. Ogura, Y. Mitsuno, Y. Hirata, Y. Yamaji, M. Akanuma, Y. Shiratori, and M. Omata. 2000. H. pylori activates NF-κB through a signaling pathway involving IκB kinases, NF-κB-inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology 119:97-108. [DOI] [PubMed] [Google Scholar]
- 22.Medvedev, A. E., P. Henneke, A. Schromm, E. Lien, R. Ingalls, M. J. Fenton, D. T. Golenbock, and S. N. Vogel. 2001. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J. Immunol. 167:2257-2267. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 19:423-474. [DOI] [PubMed] [Google Scholar]
- 24.Opitz, B., N. W. Schroder, I. Spreitzer, K. S. Michelsen, C. J. Kirschning, W. Hallatschek, U. Zahringer, T. Hartung, U. B. Gobel, and R. R. Schumann. 2001. Toll-like receptor -2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappa B translocation. J. Biol. Chem. 276:22041-22047. [DOI] [PubMed] [Google Scholar]
- 25.Ronnett, G. V., and M. D. Lane. 1981. Post-translational glycosylation-induced activation of aglycoinsulin receptor accumulated during tunicamycin treatment. J. Biol. Chem. 256:4704-4707. [PubMed] [Google Scholar]
- 26.Sharma, S. A., M. K. Tummuru, G. G. Miller, and M. J. Blaser. 1995. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect. Immun. 63:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slomiany, B. L., J. Piotrowski, A. Samanta, K. VanHorn, V. L. Murty, and A. Slomiany. 1989. Campylobacter pylori colonization factor shows specificity for lactosylceramide sulfate and GM3 ganglioside. Biochem. Int. 19:929-936. [PubMed] [Google Scholar]
- 28.Su, B., P. M. Hellström, C. Rubio, J. Célik, M. Granström, and S. Normark. 1998. Type I Helicobacter pylori shows Lewis(b)-independent adherence to gastric cells requiring de novo protein synthesis in both host and bacteria. J. Infect. Dis. 178:1379-1390. [DOI] [PubMed] [Google Scholar]
- 29.Su, B., S. Johansson, M. Fällman, M. Patarroyo, M. Granström, and S. Normark. 1999. Signal transduction-mediated adherence and entry of Helicobacter pylori into cultured cells. Gastroenterology 117:595-604. [DOI] [PubMed] [Google Scholar]
- 30.Teneberg, S., I. Leonardsson, H. Karlsson, P.-A. Jovall, J. Angstrom, D. Danielsson, I. Manslund, A. Ljung, T. Wadstrom, and K.-A. Karlsson. 2002. Lactotetraosylceramide, a novel glycosphingolipid receptor for Helicobacter pylori, present in human gastric epithelium. J. Biol. Chem. 277:19709-19719. [DOI] [PubMed] [Google Scholar]
- 31.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, J. E., A. Warris, E. A. Ellingsen, P. F. Jørgensen, T. H. Flo, T. Espevik, R. Solberg, P. E. Verweij, and A. O. Aasen. 2001. Involvement of CD14 and Toll-like receptors in activation of human monocytes by Aspergillus fumigatus hyphae. Infect. Immun. 69:2402-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]