Abstract
Sera and peripheral blood mononuclear cells (PBMC) from patients displaying different clinical symptoms as well as from normal uninfected individuals (NI) were used to evaluate the humoral and cellular responses of Chagas' disease patients to Trypanosoma cruzi-derived paraflagellar rod proteins (PFR). Our results show that sera from both asymptomatic Chagas' disease patients (ACP) and cardiac Chagas' disease patients (CCP) have higher levels of antibodies to PFR than sera from NI. Immunoglobulin G1 (IgG1) and IgG3 were the main Ig isotypes that recognized PFR. We also tested three recombinant forms of PFR, named rPAR-1, rPAR-2, and rPAR-3, by Western blot analysis. Sera from seven out of eight patients with Chagas' disease recognized one of the three rPAR forms. Sera from 75, 50, and 37.5% of Chagas' disease patients tested recognized rPAR-3, rPAR-2, and rPAR-1, respectively. PFR induced proliferation of 100 and 70% of PBMC from ACP and CCP, respectively. Further, stimulation of cells from Chagas' disease patients with PFR enhanced the frequencies of both small and large CD4+ CD25+ and CD4+ CD69+ lymphocytes, as well as that of small CD8+ CD25+ lymphocytes. Finally, we evaluated the ability of PFR to elicit the production of gamma interferon (IFN-γ) by PBMC from patients with Chagas' disease. Fifty percent of the PBMC from ACP as well as CCP produced IFN-γ upon stimulation with PFR. PFR enhanced the percentages of IFN-γ-producing cells in both CD3+ and CD3− populations. Within the T-cell population, large CD4+ T lymphocytes were the main source of IFN-γ.
Chagas' disease, caused by infection with the protozoan parasite Trypanosoma cruzi, is a major public health problem in Latin America. Approximately 20 million people are infected, and an additional 100 million individuals are at risk of infection. Reductions in these numbers are expected, since major advances in control of the insect vectors and in methods for screening blood banks have been made, resulting in substantial decreases in frequencies of T. cruzi transmission in several countries of South America (28, 33, 34). The acute phase of Chagas' disease lasts 1 to 3 months, until parasite replication is controlled by the host immune response. The fate of chronic patients is unpredictable. About 20 to 30% of the patients develop cardiomyopathy (16, 17) of variable severity, while in 8 to 10% the disease evolves to a digestive disorder characterized by pathological dilatations of the esophagus and/or colon (megaesophagus and/or megacolon) (32). The cardiac and digestive forms of the disease are always associated with tissue parasitism. Most patients (60 to 70%), however, are apparently asymptomatic and are referred to as indeterminate, a condition characterized by no clinical manifestation but positive serology and parasitology tests (3, 6, 22).
Chemotherapeutic agents are of limited effectiveness in treating T. cruzi infection in humans (11, 31), and most patients present side effects during treatment. No effective anti-T. cruzi vaccines are available for humans, and potential vaccines have been tested only in experimental animal models of Chagas' disease. One particular vaccine candidate that has been shown to be very effective in protecting mice against T. cruzi infection is the paraflagellar rod proteins (PFR) (18, 19, 35). The PFR preparation is composed of four distinct proteins, as determined by direct amino acid sequence analysis, immunological analysis with PFR-specific monoclonal antibodies, and analyses of the genes that encode these four proteins (9). The PFR are major structural components of the T. cruzi flagellum, identified as a complex lattice of filaments and expressed by T. cruzi at all stages where flagella are present, including the infective-stage trypomastigotes and metacyclics (9). Further, PFR are highly conserved among different T. cruzi strains (unpublished data). It is noteworthy that the amino acid sequences and ultrastructural characteristics of PFR are not related to any of the major filamentous systems of eukaryotic cells, such as microfilaments, microtubules, or intermediate filaments (5), and thus are unlikely to elicit autoimmune responses. While the protective immunological mechanisms elicited by immunization of mice with PFR have been determined, essentially nothing is known about the immunogenicity of PFR in humans. In this study, we evaluate the immunogenicity of purified PFR in humans by accessing both humoral and cellular responses of clinically defined Chagas' disease patients, naturally infected with T. cruzi. Our results show that the majority of patients with Chagas' disease develop both humoral and cellular responses to PFR, with dominant responses for PAR-2 and PAR-3.
MATERIALS AND METHODS
Patients.
The sera and peripheral blood mononuclear cells (PBMC) analyzed in this study were obtained from patients with Chagas' disease followed up by Manoel Otávio Da Costa Rocha. All the Chagas' disease patients were chronically infected with T. cruzi and came from different areas of the state of Minas Gerais, Brazil. The patients were assigned to groups according to clinical criteria based on physical examination and cardiac examinations (i.e., chest X-ray, electrocardiogram, echocardiography, ergometry, Holter monitoring, and autonomic test). The main groups were (i) the indeterminate group, composed of asymptomatic patients (ACP), and (ii) the cardiac group, containing patients displaying various degrees of cardiac disturbance (CCP). The CCP were further divided into three subgroups according to the severity of the disease, with cardiac forms CCP1, CCP2, and CCP3 having less, intermediate, and more severe damage, respectively. Age- and sex-matched seronegative individuals from the same community were used as controls. All individuals participating in the study gave informed consent. Diagnosis of patients with Chagas' disease was based on indirect immunofluorescence assay (IFI) and enzyme-linked immunosorbent assay (ELISA). The age range for the patients was 20 to 60 years.
Preparation of native T. cruzi antigens.
PFR were purified as previously described by Saborio and coworkers (26, 35). Briefly, 1011 T. cruzi Peru strain epimastigotes were harvested by centrifugation, washed in phosphate-buffered saline (PBS), and lysed in 0.1 M Tricine (pH 8.5) containing 1% Nonidet P-40. The pellet was extracted with high-salt buffer consisting of 0.1 M Tricine, 1 M NaCl, and 1% Triton X-100 by using sonication. This crude flagellar pellet was successively extracted with 2.0 and 6.0 M urea in 10 mM Tricine (pH 8.5). The resulting supernatant contained approximately 50% PFR and 50% tubulin. PFR were separated from tubulin by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and recovered by electroelution using a Prep Cell (model 491; Bio-Rad Laboratories, Richmond, Calif.). Fractions containing the PFR were extensively dialyzed against PBS, concentrated in a Centricon concentrator (Amicon, Beverly, Mass.), and sterilized by filtration through a 0.45-μm-pore-size filter. Protein concentrations in the highly purified PFR samples were determined by use of a protein assay (Pierce Chemical Corp., Rockford, Ill.), and purity was assessed by SDS-PAGE.
The epimastigote antigen (EPI-Ag) was prepared from epimastigotes of the Colombian strain of T. cruzi. Briefly, epimastigote forms were grown at 28°C in cell-free liver infusion tryptose medium supplemented with 10% fetal calf serum. Live parasites were collected from liquid culture, washed three times in ice-cold PBS, resuspended at a final concentration of 109 parasites/ml, and then submitted to three cycles of freeze-thawing at −70 and 37°C, respectively, followed by five 30-s rounds of sonication. The resulting homogenate was then centrifuged at 10,000 × g for 30 min, and the supernatant EPI-Ag was used as the antigen preparation. The protein concentration of parasite extracts was determined by the Bradford method using bovine serum albumin (BSA) as a standard. EPI-Ag was frozen at −70°C until use.
Preparation of rPAR proteins derived from T. cruzi.
The entire PAR-1, PAR-2, and PAR-3 coding regions were cloned into plasmid expression vector pTrcHis for expression in Escherichia coli. Recombinant proteins were produced and purified as described elsewhere (9). Briefly, recombinant PAR (rPAR) proteins were partially purified from E. coli cell lysates by fast protein liquid affinity chromatography using Ni+-activated columns. Final purification of rPAR proteins was accomplished by preparative SDS-PAGE on a Bio-Rad Prep Cell.
Sources of antibodies used for ELISA, immunoblotting, cytokine measurements, and fluorescence-activated cell sorter (FACS) analysis.
Monoclonal anti-human immunoglobulin G1 (IgG1), IgG2, IgG3, and IgG4 antibodies (clones 8c/6-39, HP-6014, HP-6050, and HP-6025, respectively) for ELISA were purchased from Sigma (St. Louis, Mo.). Fc-specific, peroxidase-linked anti-human IgG (clone 6017) also was obtained from Sigma. Monoclonal antibodies against human interleukin-10 (IL-10), tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and IL-4 (clones JES3-19F1, MAb1, NIB42, and 8D4-8, respectively) and biotinylated antibodies against IL-10, TNF-α, IFN-γ, and IL-4 (clones JES3-12G8, MAb11, 4S.B3, and MP4-25D2, respectively), used in the capture and detection assays, respectively, were purchased from Becton and Dickinson Biosciences (San Diego, Calif.). Also purchased from this company were fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies specific to the cell surface proteins CD3, CD4, and CD8, as well as phycoerythrin (PE)-conjugated monoclonal antibodies specific to CD25 and CD69. A PE-labeled IFN-γ-specific antibody for intracellular staining was also purchased from Becton and Dickinson Biosciences.
Quantification of serum antibodies against PFR.
Maxisorp 96-well ELISA plates (Nalge-Nunc) were coated with PFR or EPI-Ag (100-μl aliquots containing 10 μg of protein/ml in PBS) and incubated overnight at 4°C. Coated plates were blocked with 1% albumin for 2 h at 37°C; then serum samples from Chagas' disease patients or noninfected individuals (NI), diluted (1:20 and 1:100 for IgG isotypes and total IgG, respectively) in PBS-0.05% Tween 20, were added and incubated for 1 h at 37°C. Plates were washed with PBS and reacted with peroxidase-conjugated anti-human IgG (Sigma Chemical Co.) for 1 h at room temperature. Bound antibody was measured by reaction with 2,2′-azino-di-3-ethylbenzthiazoline sulfonate (ABTS) (Sigma Chemical Co.), followed by detection at 405 nm in an automated ELISA plate reader.
Immunoblotting.
PFR and rPAR proteins subjected to one-dimensional SDS-PAGE were transferred to nitrocellulose paper by using the Mini-Proteam (Bio-Rad). The blots were soaked with 2% casein-PBS-Tween 20 for 1 h at room temperature to block free binding sites and were then incubated overnight at 4°C with serum samples from Chagas' disease patients or NI at a 1:1,000 dilution. Blots were washed three times with PBS-Tween 20 and then incubated with peroxidase-conjugated anti-human IgG. After being rinsed with 0.05 M carbonate-bicarbonate buffer (pH 9.6), blots were incubated with the ECL Plus system (Amersham, Little Chalfont, Buckinghamshire, England) and exposed to X-ray films (15) for detection of the antigen-antibody complex.
In vitro proliferation analysis.
In vitro proliferative responses of PBMC were determined according to the protocol described by Gazzinelli et al. (13). Briefly, PBMC from Chagas' disease patients or NI were obtained by separating whole blood over Histopaque (Sigma Chemical Co.) and washing three times with medium. Cells were counted and cultured in the presence or absence of different stimuli for 3 or 5 days at a concentration of 2.5 × 105 cells/well in 96-well plates (Corning Glass Works, Corning, N.Y.). Stimuli used in the cultures included PFR and EPI-Ag (at a final concentration of 10 μg/ml for 5 days), and as a positive control, we employed the mitogen PHA (Phaseolus vulgaris lectin) (at a final concentration of 5 μg/ml for 3 days). After the incubation period, cultures were exposed to 0.5 mCi of [3H]thymidine for 6 h and then harvested, and the incorporated radioactivity was measured in a scintillation counter. All cultures were performed in triplicate. The above concentrations of antigens were determined by performing titration experiments (data not shown).
Cytokine measurements.
IFN-γ, IL-4, IL-10, and TNF-α concentrations in supernatants from PBMC cultures, collected after 5 days of stimulation, were measured by sandwich ELISAs with the monoclonal antibodies listed above. The assay was performed according to the manufacturer's suggested protocol. Briefly, 96-well plates were coated with 2 μg of the anti-cytokine capture antibody/ml diluted in coating buffer (0.1 M Na2PO4, pH 9.0) and were incubated overnight at 4°C. Plates were blocked by 1 h of incubation with blocking buffer containing 1% BSA and were then washed with PBS-Tween; 100-μl aliquots of culture supernatant were then added and incubated overnight at 4°C. Plates were washed, 100-μl aliquots of the biotinylated anti-cytokine detection antibody (2 μg/ml) diluted in blocking buffer-Tween were added, and plates were incubated for 1 h at room temperature. Peroxidase-conjugated strepavidin, diluted 1:2,000 in blocking buffer-Tween, was added to the plates and incubated for 30 min at room temperature. After plates were washed, bound antibodies were detected by using the ABTS detection system described above. Plates were washed three times with PBS-Tween following each incubation.
Immunostaining for flow cytometric analysis.
Peripheral blood was drawn from volunteers, and PBMC were obtained by density centrifugation as described above and then analyzed for cell surface markers and intracellular cytokine expression. Briefly, 2.5 × 106 PBMC were cultured in 24-well plates in 1-ml cultures for 20 h with either medium alone, PFR or EPI-Ag (10 μg/ml), or PHA (at 5 μg/ml). During the last 4 h of culture, brefeldin A (1 μg/ml), which impairs protein secretion by the Golgi apparatus, was added to the cultures. The cells were then washed and resuspended in ice-cold PBS plus azide, stained with FITC-labeled antibodies specific for surface markers, and fixed in 2% formaldehyde. The fixed cells were then permeabilized with a solution of saponin and stained for 30 min at 4°C by using anti-IFN-γ monoclonal antibodies directly conjugated with PE (IFN-γ). FITC- and PE-labeled Ig control antibodies and a control with unstimulated PBMC were included in all experiments. Preparations were then washed and fixed as described above and analyzed by use of a FACScalibur (Becton Dickinson), selecting the lymphocyte population. In all cases, 30,000 gated events were acquired for later analysis. Lymphocytes were analyzed for their intracellular cytokine expression patterns and frequencies and for surface markers in a number of ways by using the Cell Quest program.
Statistical analysis.
The Student t test was used for final determinations of the significance of differences in ELISA results, PBMC proliferation, and IFN-γ production between patients with Chagas' disease and individuals from the control group. Differences were considered significant when P was less than 0.05.
RESULTS
Antibodies specific for PFR in sera from patients with Chagas' disease.
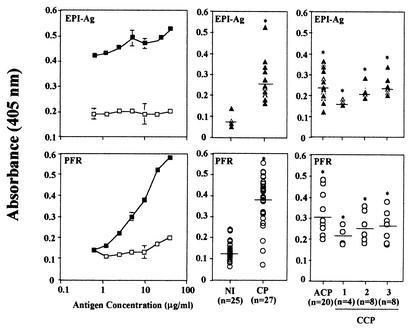
Figure 1 presents the results obtained in the ELISA, showing the titration curves of total EPI-Ag (top left) and PFR (bottom left) as well as a quantitative analysis comparing levels of anti-EPI-Ag (top center and right) and anti-PFR (bottom center and right) antibodies present in sera from Chagas' disease patients and NI. The ideal concentration of PFR for use in ELISAs was determined to be 10 to 20 μg/ml. Our results show that the majority of the patients with Chagas' disease (80%) yielded positive results when we employed PFR in an ELISA. In contrast, none of the NI displayed positive results in the PFR ELISA. No difference was observed among levels of PFR-specific antibodies in sera from ACP and CCP displaying different degrees of severity of cardiac disease (i.e., CCP1, CCP2, and CCP3).
FIG. 1.
ELISA employing PFR distinguishes sera from Chagas' disease patients (CP) from sera from NI. (Left) Curves of concentrations of EPI-Ag and PFR in ELISAs employing pools of CP and NI sera. Total IgG antibodies specific to EPI-Ag (top) and PFR (bottom) in sera (1:100) of NI (□) and CP (▪) are shown. (Center) Levels of antibodies specific to EPI-Ag (▴) (top) and PFR (○) (bottom) present in individual sera from NI and CP. (Right) Levels of antibodies specific to EPI-Ag (▴) (top) and PFR (○) (bottom) present in sera from individual CP grouped according to the severity of Chagas' disease. Asterisks indicate statistical significance for comparisons between groups (P < 0,05). Each point represents the mean absorbance of duplicates.
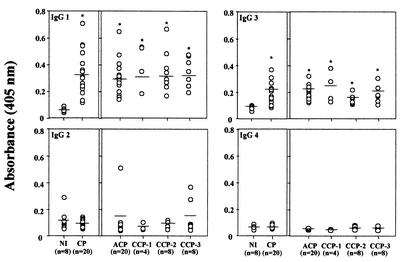
IgG1 and IgG3 are the main isotypes specific for PFR in sera from patients with Chagas' disease.
We extended our studies to determine the main Ig isotypes present in the sera from Chagas' disease patients that were involved in the recognition of PFR. Figure 2 shows that IgG1 (top left) and IgG3 (top right) were the main Ig isotypes from Chagas' disease patient sera that recognized PFR. Again, no difference was observed among different clinical forms of Chagas' disease or among patients with different degrees of severity of the cardiac form of the disease.
FIG. 2.
Measurement of anti-PFR Ig isotypes, subclasses IgG1, IgG2, IgG3, and IgG4, present in sera from NI and in sera from patients with Chagas' disease (CP). Asterisks indicate statistic significance for comparisons between groups (P < 0,05). Each data point represents the mean absorbance of duplicate wells.
Immunoreactivity to PFR and rPAR.
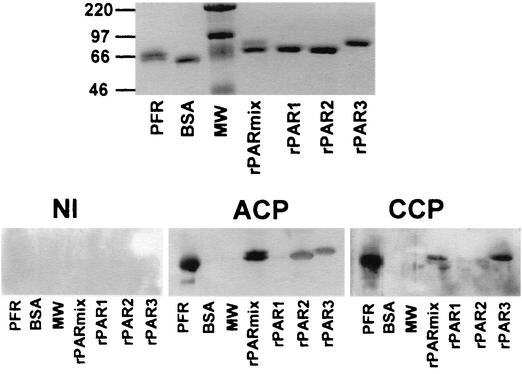
We also tested E. coli-derived rPARs in an ELISA. Differences between reactivities of sera from Chagas' disease patients and NI were not as clear (data not shown) as those observed with the native PFR, which contain all four forms of PAR. Nonetheless, rPAR-1, rPAR-2, and rPAR-3 were clearly recognized by antibodies present in sera from Chagas' disease patients, as shown in the immunoblot presented in Fig. 3. The top panel shows a polyacrylamide gel with PFR, BSA, a molecular weight ladder, a mixture of rPARs, and the individual rPAR forms rPAR-1, rPAR-2, and rPAR-3. The bottom panel shows results of Western blotting with the same experimental set using sera from one NI (left), one ACP (middle), and one CCP (right). Densitometric analyses of Western blots were performed, and results fivefold higher than the average results obtained with sera from NI were considered positive. While sera from eight Chagas' disease patients tested recognized native PFR, none from the NI did so. Sera from two out of four NI showed only minimal recognition of rPARs. Sera from seven out of eight Chagas' disease patients recognized the rPAR mixture. Sera from 75, 50, and 37.5% of the Chagas' disease patients tested recognized rPAR-3, rPAR-2, and rPAR-1, respectively. In agreement with the ELISA results, recognition of native PFR was always more intense than that of rPARs. We were unable to test the highly purified rPAR-4 due to its insolubility in the recombinant form.
FIG. 3.
Specific recognition of rPAR proteins by antibodies present in sera from patients with Chagas' disease. (Top) Specific bands of native PFR, a mixture containing various rPARs (rPARmix), each individual rPAR, BSA (control), and a molecular weight (MW) ladder run in an SDS-PAGE gel and silver stained. (Bottom) Nitrocellulose sheets blotted with a polyacrylamide gel containing PFR, BSA (unrelated control protein), rPARmix, rPAR-1, rPAR-2, and rPAR-3, and developed with individual sera from NI, ACP, and CCP.
Native PFR do elicit cellular responses in the majority of PBMC from patients with Chagas' disease.
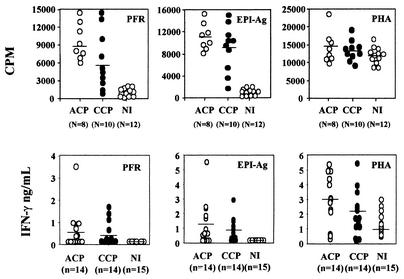
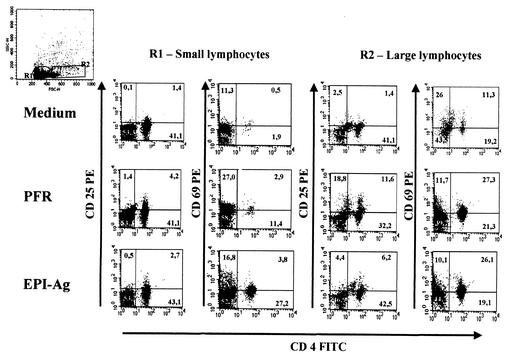
We considered proliferative results higher than the average for NI plus 3 standard errors to be positive. As shown in Fig. 4, 100 and 90% of PBMC from ACP and CCP, respectively, proliferated upon stimulation with native EPI-Ag. We considered PFR quite immunogenic, because 100 and 70% of PBMC from ACP and CCP, respectively, proliferated upon stimulation with native PFR. We also determined the phenotype of T cells proliferating in response to PFR stimulation. Our results show that stimulation of PBMC from Chagas' disease patients with PFR enhanced the frequencies of both small and large CD4+ CD25+ and CD4+ CD69+ lymphocytes, as well as that of small CD8+ CD25+ lymphocytes (Fig. 5 and Table 1).
FIG. 4.
Specific stimulation of PBMC from Chagas' disease patients with PFR. Shown are proliferation of PBMC (top panels) and IFN-γ synthesis (bottom panels) by PBMC (2.5 × 105/well) from NI, ACP, and CCP after stimulation with PFR (left panels), EPI-Ag (center panels), or PHA (right panels). Incorporation of [3H]thymidine (top panels) and levels of IFN-γ in supernatants (bottom panels) were measured after 5 days of PBMC stimulation with PFR (10 μg/ml) or EPI-Ag (10 μg/ml) or after 3 days of stimulation with PHA (5 μg/ml). Each data point for thymidine incorporation represents mean counts per minute from triplicate wells, and each cytokine absorbance value is the mean from duplicate wells.
FIG. 5.
CD4+ T cells are the main lymphocyte subset responsive to PFR. Representative histograms show frequencies of various T-cell populations in PBMC of Chagas' disease patients after 20 h of culture with either PFR, EPI-Ag, or medium alone. Frequencies of cells expressing the indicated surface markers were determined by using antibodies directly conjugated with either PE (y axis) or FITC (x axis) as described in Materials and Methods. Top left histogram represents forward- and side-scatter gates for R1 (small lymphocytes) and R2 (large lymphocytes).
TABLE 1.
Increases in percentages of double-positive T cells after culture with PFR or EPI-Ag
| Antigena | % (Fold increase) of lymphocytesb expressing the indicated surface markers
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Small lymphocytes (R1)
|
Large lymphocytes (R2)
|
|||||||
| CD4/CD25 | CD4/CD69 | CD8/CD25 | CD8/CD69 | CD4/CD25 | CD4/CD69 | CD8/CD25 | CD8/CD69 | |
| None | 2.2 ± 0.1 | 2.0 ± 0.0 | 1.0 ± 0.6 | 0.7 ± 0.3 | 5.4 ± 1.6 | 1.5 ± 0.6 | 2.5 ± 1.1 | 0.1 ± 1.1 |
| PFR | 4.7 ± 1.4 (2.1) | 5.8 ± 5.7 (2.8) | 2.7 ± 3.0 (2.7) | 0.5 ± 0.5 (0.7) | 12.5 ± 9.3 (2.3) | 3.6 ± 3.5 (2.3) | 3.6 ± 2.8 (1.4) | 0.1 ± 0.1 (0.8) |
| None | 2.0 ± 0.1 | 2.0 ± 0.0 | 1.9 ± 0.6 | 0.3 ± 0.3 | 3.1 ± 1.6 | 1.7 ± 1.6 | 1.0 ± 1.1 | 1.7 ± 1.1 |
| Epi-Ag | 5.1 ± 3.1 (2.5) | 4.2 ± 2.8 (2.1) | 9.4 ± 10.2 (5.0) | 0.2 ± 0.3 (0.7) | 6.7 ± 3.6 (2.1) | 3.1 ± 9.6 (1.8) | 0.7 ± 0.9 (0.7) | 1.6 ± 2.5 (0.9) |
None, medium alone. PBMC of the same patients were cultured in the absence of antigens.
Percentages are means ± standard deviations from media of PBMC from eight patients. Total lymphocytes in top right quadrants of histograms (double-positive cells) were quantified.
We also evaluated the ability of PFR to elicit production of cytokines by PBMC from patients with Chagas' disease. Among the cytokines tested, the main ones produced by PBMC from Chagas' disease patients stimulated with PFR were IFN-γ and TNF-α. Increased levels of IFN-γ production in response to PFR were observed for 50% of ACP (7 of 14; average, 0.56 ± 0.93 ng/ml) and 50% of CCP (7 of 14; average, 0.39 ± 0.56 ng/ml). Upon stimulation with EPI-Ag, 71.4% of PBMC from ACP (10 of 14; average, 1.1 ± 1.5 ng/ml) and 85.7% of PBMC from CCP (12 of 14; average, 0.7 ± 0.8 ng/ml) produced IFN-γ. One hundred percent (28 patients) of PBMC stimulated with PHA produced large amounts of IFN-γ (average, 2.8 ng/ml). Another cytokine produced by PBMC from Chagas' disease patients stimulated with PFR was TNF-α. Forty percent of PBMC from ACP (6 of 15; average, 0.3 ± 0.6 ng/ml) and 36.3% of PBMC from CCP (4 of 11; average, 0.2 ± 0.3 ng/ml) stimulated with PFR produced TNF-α; 20% of ACP (3 of 15; average, 0.07 ± 0.1 ng/ml) and 45.4% of CCP (5 of 11; average, 0.4 ± 0.2 ng/ml) PBMC produced TNF-α upon stimulation with EPI-Ag. PBMC from 26 patients stimulated with PHA produced larger amounts of TNF-α (average, 1.9 ng/ml) than PBMC from NI. Other cytokines (i.e., IL-4 and IL-10) were also assessed concomitantly, but their levels were undetectable under our conditions.
Large CD4+ T lymphocytes are the main source of IFN-γ in PBMC stimulated with native PFR.
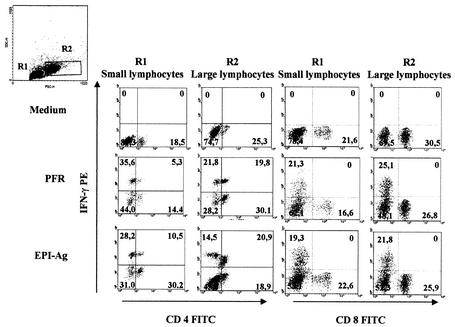
Finally, we analyzed the phenotype of cell populations within the PBMC that produce IFN-γ upon stimulation with PFR. Corroborating the results with ELISA, PBMC from 50% of Chagas' disease patients (three of six) tested by intracellular staining were found to produce IFN-γ upon stimulation with PFR. As shown by the results of intracellular cytokine staining (Fig. 6), large CD4+ T lymphocytes were the main source of IFN-γ in PBMC from Chagas' disease patients. Cells from gates R1 and R2 represent small and large lymphocytes, respectively. It is shown at the R1 and R2 gates that the main cell populations producing IFN-γ were either single-positive (CD4− IFN-γ+) or double-positive (CD4+ IFN-γ+) T cells. As shown in Fig. 6 (right panels), CD4-negative IFN-γ-producing cells were also CD8 negative. Further investigation showed that this cell subset was also CD3 negative (data not shown), indicating that these were not T cells. A significant proportion of IFN-γ-producing cells were also CD25 positive. Under the experimental conditions used here, the small and large CD8+ T lymphocytes were not a major source of IFN-γ.
FIG. 6.
Large CD4+ T lymphocytes are the main source of IFN-γ in PBMC from patients with Chagas' disease. Representative histograms show frequencies of various T-cell populations in PBMC from one out of three Chagas' disease patients after 20 h of culture with either PFR, EPI-Ag, or medium alone. Frequencies of cells expressing the indicated surface markers and intracellular cytokines were determined by use of antibodies directly conjugated with either FITC (x axis) or PE (y axis) as described in Materials and Methods. Top left histogram represents forward- and side-scatter gates for R1 (small lymphocytes) and R2 (large lymphocytes).
DISCUSSION
Natural transmission of Chagas' disease in areas of endemicity has decreased due to the use of insecticide for control of the hematophagous vectors. However, the vectors have not been eliminated, and the possibilities of interruption of the vector control program and acquisition of resistance to insecticide are worrisome (28). In addition, T. cruzi transmission has increased in countries where natural infection is absent, due to the expanding exodus from areas of endemicity of individuals who act as blood or organ donors (33). Both nitrofurans and nitroimidazoles (i.e., Nifurtimox and Benznidazole, respectively), which are used for treatment, cure only 60 to 70% of Chagas' disease patients in the acute phase of infection. Further, the chemotherapy of Chagas' disease is even less effective for patients in the chronic phase. These compounds show no efficacy in some of the cases, since some T. cruzi strains are naturally resistant to these drugs (8). In addition, interruption of therapy is often necessary, because some side effects are not tolerated after long-term administration (7). New drugs are currently being tested in experimental models and clinical trials but are not commercially available to treat patients with Chagas' disease (31). In this scenario, an effective vaccine against T. cruzi may represent one alternative for the prevention and control of Chagas' disease. Although no vaccine is available at the moment, a few T. cruzi antigens have been identified as potential candidates for vaccine development. In experimental models, parasite-derived antigens such as ASP-1, ASP-2, CRP, cruzipain, Tc52, PFR, TolA-like surface protein, trans-sialidase, and TSA-1 (4, 10, 12, 18-21, 27, 29, 35-37) have been shown to induce efficient protective immunity against T. cruzi infection.
We have been working with the PFR as a potential vaccine candidate against T. cruzi infection. During the process of transformation from trypomastigote to amastigote, the flagellum of the tyrpomastigote is reduced in length by about 90%. This degradative process occurs within the host cell cytoplasm; thus, the catabolic products of proteins found within the flagellum should be readily available for entry into the major histocompatibility complex class I presentation pathway as well. We believe that this is an important aspect of the ability of PFR to elicit protective immunity, as CD8+ T lymphocytes have been shown to be an important component of protective immunity against T. cruzi infection (25, 30). Further, PFR have no amino acid sequence homology, ultrastructural similarity, or immunological cross-reactivity with tubulin, actin, intermediate filament proteins, or other proteins present in mammalian cells (5, 9, 26). Therefore, PFR are unlikely to elicit an autoimmune response and thus may be safely used as an immunogen in a vaccine or immunotherapeutic protocols for prophylaxis or treatment of human Chagas' disease, respectively.
An initial study with a mouse model showed that immunization with PFR induced anti-PAR antibodies and protected mice against challenge with a virulent strain of T. cruzi (35). Additional experiments demonstrated that protective immunity elicited by vaccination with PFR was dependent on T cells rather than B cells (18, 19). Thus, B-cell-deficient mice immunized with PFR and subsequently challenged with a lethal inoculum of T. cruzi presented reduced parasitemia and 100% survival. Further, it was shown that vaccination with PFR elicits parasite-specific CD4+ Th1 lymphocytes that act as a major source of IFN-γ for macrophage activation (18, 19). Once activated, macrophages produce high levels of reactive nitrogen intermediates, which are responsible for controlling parasite replication inside host cells (14, 18, 24). Additional evidence also suggests that immunoprotection induced by vaccination with PFR is in part mediated by CD8 Tc1 cells (37).
The efficiencies of different adjuvants as vehicles for native PFR and various rPARs in immunization against T. cruzi have also been evaluated. Freund's (subcutaneous) adjuvant and alum adjuvant have been shown to elicit larger amounts of IFN-γ and of IL-2 and antibodies, respectively (19). Only the former adjuvant formulation induced protective immunity in mice immunized with PFR, which was associated with a strong Th1-type response. Immunization with PFR and rIL-12 simultaneously adsorbed to alum also resulted in induction of a Th1 response associated with protective immunity (37). We have also verified the immunogenicity of rPAR-1 and rPAR-2 alone or in combination with different adjuvants (i.e., alum, Freund's adjuvant, QS-21, Ribi-700, adenovirus, and rIL-12). It has been shown that the rPARs are also able to induce protective immunity against T. cruzi infection (37).
However, there was no information regarding the immunogenicity of PFR in humans. In order to address this question, we decided to investigate the levels of humoral and cellular responses to PFR in sera and PBMC from Chagas' disease patients naturally infected with T. cruzi. By ELISA, anti-PFR antibodies were found in 95% of T. cruzi-infected patients tested. Western blot analysis showed that 37.5, 50.0, and 75.0% of sera from patients with Chagas' disease recognized rPAR-1, rPAR-2, and rPAR-3, respectively. In addition, PFR elicited proliferative responses of variable intensity in PBMC from most (85%) patients infected with T. cruzi. These findings indicate that in addition to being immunogenic for most patients, the PFR epitopes are probably conserved among various T. cruzi isolates. In fact, our unpublished results demonstrate that genes encoding the various PFR are highly conserved among different strains of T. cruzi.
Our studies also show that the majority of patients with Chagas' disease presented high levels of IgG1 and IgG3 Ig isotypes specific to PFR. Because IFN-γ is the main cytokine that promotes isotype switching to the IgG1 and IgG3 subclasses, our results indicate that during natural infection with T. cruzi, PFR elicit a type 1 cellular response. This hypothesis is further supported by the dominant IFN-γ response and lack of IL-4 production by PFR-stimulated PBMC from ACP and CCP. We also show that among T cells, the CD4+ CD8− population is the main subset responsible for IFN-γ synthesis after stimulation with PFR proteins. However, there is a CD3− CD4− CD8− cell population that apparently contributed to IFN-γ production. Consistent with these data are findings from other groups showing that PBMC from T. cruzi-infected patients in response to other T. cruzi antigens such as cruzipain, trans-sialidase, and B13 (1, 2, 23) are predominantly of type 1 and are characterized by high levels of IFN-γ production. These findings indicate that natural infection with T. cruzi favors the development of a Th1 response to the parasite antigens.
At this point major ethical and logistic barriers make the testing of a vaccine against Chagas' disease in humans difficult. However, the repeated experiments showing protective immunity elicited by the native PFR in rodent models (18, 19, 35, 37) and the pronounced results demonstrating the immunogenicity of PFR in humans are encouraging for testing of the vaccine in a primate model.
Acknowledgments
We thank Juliana Assis of the Laboratory of Immunology, CPqRR, FIOCRUZ, for help with FACS analysis.
This work was supported by U.S. Public Health Service grant TW01104 from the National Institutes of Health. R.T.G. is a Research Fellow from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and is supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG; EDT 24000).
Editor: J. M. Mansfield
REFERENCES
- 1.Abel, L. C., L. V. Rizzo, B. Ianni, F. Albuquerque, F. Bacal, D. Carrara, E. A. Bocchi, H. C. Teixeira, C. Mady, J. Kalil, and E. Cunha-Neto. 2001. Chronic Chagas' disease cardiomyopathy patients display an increased IFN-γ response to Trypanosoma cruzi infection. J. Autoimmun. 17:99-107. [DOI] [PubMed] [Google Scholar]
- 2.Arnholdt, A. C., M. R. Piuvezam, D. M. Russo, A. P. Lima, R. C. Pedrosa, S. G. Reed, and J. Scharfstein. 1993. Analysis and partial epitope mapping of human T cell responses to Trypanosoma cruzi cysteinyl proteinase. J. Immunol. 151:3171-3179. [PubMed] [Google Scholar]
- 3.Brener, Z., and R. T. Gazzinelli. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol. 114:103-110. [DOI] [PubMed] [Google Scholar]
- 4.Costa, F., G. Franchin, V. L. Pereira-Chioccola, M. Ribeirão, S. Schenkman, and M. M. Rodrigues. 1998. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16:768-774. [DOI] [PubMed] [Google Scholar]
- 5.de Souza, W., and T. Souto-Padron. 1980. The paraxial structure of the flagellum of trypanosomatidae. J. Parasitol. 66:229-236. [PubMed] [Google Scholar]
- 6.Dias, J. C. P. 1989. The indeterminate form of human chronic Chagas' disease: a clinical epidemiological review. Rev. Soc. Bras. Med. Trop. 22:147-156. [DOI] [PubMed] [Google Scholar]
- 7.DoCampo, R., and S. N. J. Moerno. 1985. Biochemical toxicology of anti-parasitic compounds used in the chemoprophylaxis of American trypanosomiasis (Chagas' disease). Rev. Biochem. Toxicol. 7:159-204. [Google Scholar]
- 8.Filardi, L. S., and Z. Brener. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 81:755-759. [DOI] [PubMed] [Google Scholar]
- 9.Fouts, D. L., G. A. Stryker, K. S. Gorski, M. J. Miller, T. V. Nguyen, R. A. Wrightsman, and J. E. Manning. 1998. Evidence for four distinct major protein components in the paraflagellar rod of Trypanosoma cruzi. J. Biol. Chem. 273:21846-21855. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura, A. E., S. S. Kinoshita, V. L. Pereira-Chioccola, and M. M. Rodrigues. 2001. DNA sequences encoding CD4+ and CD8+ epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene. Infect. Immun. 69:5477-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvão, L. M., R. M. Nunes, J. R. Cançado, Z. Brener, and A. U. Krettli. 1993. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans. R. Soc. Trop. Med. Hyg. 87:220-223. [DOI] [PubMed] [Google Scholar]
- 12.Garg, N., and R. L. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., V. M. Leme, J. R. Cancado, G. Gazzinelli, and J. Scharfstein. 1990. Identification and partial characterization of Trypanosoma cruzi antigens recognized by T cells and immune sera from Chagas' disease patients. Infect. Immun. 58:1437-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., I. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of IFN-γ-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by IL-10 and TGF-β. Eur. J. Immunol. 22:2501-2506. [DOI] [PubMed] [Google Scholar]
- 15.Giraldo, M., H. Cannizzaro, M. A. J. Ferguson, I. Almeida, and R. T. Gazzinelli. 2000. Fraction of membrane components from tachyzoite forms of Toxoplasma gondii: differential recognition by immunoglobulin M (IgM) and IgG present in sera from patients with acute and chronic toxoplasmosis. J. Clin. Microbiol. 38:1453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higuchi, M. L., T. Brito, M. M. Reis, A. Barbosa, G. Bellotti, A. C. Pereira-Barreto, and F. Pileggi. 1997. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovasc. Pathol. 2:101-106. [DOI] [PubMed] [Google Scholar]
- 17.Jones, E. M., D. G. Colley, S. Tostes, E. R. Lopes, C. L. Vnencak-Jones, and T. L. McCurley. 1993. Amplification of a Trypanosoma cruzi DNA sequence from inflammatory lesions in human chagasic cardiomyopathy. Am. J. Trop. Med. Hyg. 48:348-357. [DOI] [PubMed] [Google Scholar]
- 18.Miller, M. J., R. A. Wrightsman, G. A. Stryker, and J. E. Manning. 1997. Protection of mice against Trypanosoma cruzi by immunization with paraflagellar rod proteins requires T cell, but not B cell, function. J. Immunol. 158:5330-5337. [PubMed] [Google Scholar]
- 19.Miller, M. J., R. A. Wrightsman, and J. E. Manning. 1996. Trypanosoma cruzi: protective immunity in mice immunized with paraflagellar rod proteins is associated with a T-helper type 1 response. Exp. Parasitol. 84:156-167. [DOI] [PubMed] [Google Scholar]
- 20.Ouaissi, A., E. Guilvard, Y. Delneste, G. Caron, G. Magistrelli, N. Herbault, N. Thieblemont, and P. Jeannin. 2002. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via Toll-like receptor 2, and confers protection against lethal infection. J. Immunol. 168:6366-6374. [DOI] [PubMed] [Google Scholar]
- 21.Quanquin, N. M., C. Galaviz, D. L. Fouts, R. A. Wrightsman, and J. E. Manning. 1999. Immunization of mice with a TolA-like surface protein of Trypanosoma cruzi generates CD4+ T-cell-dependent parasiticidal activity. Infect. Immun. 67:4603-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rassi, A., A. O. Luquetti, A. Rassi, Jr., S. G. Rassi, and A. G. Rassi. 1992. Chagas disease—clinical features, p. 81-101. In S. Wendel, Z. Brener, M. Camargo, and A. Rassi (ed.), Chagas disease (American trypanosomiasis): its impact on transfusion and clinical medicine. Proceedings of the Congress of the International Society of Blood Transfusion, Brazil '92. Brazilian Society of Hemathology and Hemotherapy, Sao Paulo, Brazil.
- 23.Ribeirão, M., V. L. Pereira-Chioccola, L. Renia, A. F. Filho, S. Schenkman, and M. M. Rodrigues. 2000. Chagasic patients develop a type 1 immune response to Trypanosoma cruzi trans-sialidase. Parasite Immunol. 22:49-53. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues, M. M., M. Ribeirão, and S. B. Boscardin. 2000. CD4 Th1 but not Th2 clones efficiently activate macrophages to eliminate Trypanosoma cruzi through a nitric oxide dependent mechanism. Immunol. Lett. 73:43-50. [DOI] [PubMed] [Google Scholar]
- 25.Rottenberg, M. E., M. Bakhiet, T. Olsson, K. Kristensson, T. Mak, H. Wigzell, and A. Orn. 1993. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect. Immun. 61:5129-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saborio, J. L., J. M. Hernandez, S. Narayanswami, R. Wrightsman, E. Palmer, and J. E. Manning. 1989. Isolation and characterization of paraflagellar proteins from Trypanosoma cruzi. J. Biol. Chem. 264:4071-4075. [PubMed] [Google Scholar]
- 27.Schnapp, A. R., C. S. Eickhoff, D. Sizemore, R. Curtiss III, and D. F. Hoft. 2002. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect. Immun. 70:5065-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schofield, C. J., and J. C. P. Dias. 1999. The Southern Cone Initiative against Chagas disease. Adv. Parasitol. 42:1-27. [DOI] [PubMed] [Google Scholar]
- 29.Sepulveda, P., M. Hontebeyrie, P. Liegeard, A. Mascilli, and K. A. Norris. 2000. DNA-based immunization with Trypanosoma cruzi complement regulatory protein elicits complement lytic antibodies and confers protection against Trypanosoma cruzi infection. Infect. Immun. 68:4986-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338-340. [DOI] [PubMed] [Google Scholar]
- 31.Urbina, J. A. 2001. Specific treatment of Chagas disease: current status and new developments. Curr. Opin. Infect. Dis. 14:733-741. [DOI] [PubMed] [Google Scholar]
- 32.Vago, A. R., A. M. Macedo, S. J. Adad, D. D. Reis, and R. Corrêa-Oliveira. 1996. PCR detection of Trypanosoma cruzi in esophageal tissues of patients with chronic digestive Chagas' disease. Lancet 348:891-892. [DOI] [PubMed] [Google Scholar]
- 33.Wendel. S. 1998. Transfusion-transmitted Chagas' disease. Curr. Opin. Hematol. 5:406-411. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 1995. Tropical disease research. Progress 1975-94. Highlights 1993-94, p. 125-134. In Twelfth Programme Report of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. World Health Organization, Geneva, Switzerland.
- 35.Wrightsman, R. A., M. J. Miller, J. L. Saborio, and J. E. Manning. 1995. Pure paraflagellar rod protein protects mice against Trypanosoma cruzi infection. Infect. Immun. 63:122-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrightsman, R. A., B. D. Dawson, D. L. Fouts, and J. E. Manning. 1994. Identification of immunodominant epitopes in Trypanosoma cruzi trypomastigote surface antigen-1 protein that mask protective epitopes. J. Immunol. 153:3148-3154. [PubMed] [Google Scholar]
- 37.Wrightsman, R. A., and J. E. Manning. 2000. Paraflagellar rod proteins administered with alum and IL-12 or recombinant adenovirus expressing IL-12 generates antigen-specific responses and protective immunity in mice against Trypanosoma cruzi. Vaccine 18:1419-1427. [DOI] [PubMed] [Google Scholar]