Abstract
Phagocytosis and mechanisms of killing of Aspergillus fumigatus conidia by murine alveolar macrophages (AM), which are the main phagocytic cells of the innate immunity of the lung, were investigated. Engulfment of conidia by murine AM lasts 2 h. Killing of A. fumigatus conidia by AM begins after 6 h of phagocytosis. Swelling of the conidia inside the AM is a prerequisite for killing of conidia. The contributions of NADPH oxidase and inducible nitric oxide synthase to the conidicidal activity of AM were studied using AM from OF1, wild-type and congenic p47phox−/− 129Sv, and wild-type and congenic iNOS−/− C57BL/6 mice. AM from p47phox−/− mice were unable to kill A. fumigatus conidia. Inhibitors of NADPH oxidase that decreased the production of reactive oxidant intermediates inhibited the killing of A. fumigatus without altering the phagocytosis rate. In contrast to NADPH oxidase, nitric oxide synthase does not play a role in killing of conidia. Corticosteroids did not alter the internalization of conidia by AM but did inhibit the production of reactive oxidant intermediates and the killing of A. fumigatus conidia by AM. Impairment of production of reactive oxidant intermediates by corticosteroids is responsible for the development of invasive aspergillosis in immunosuppressed mice.
Invasive aspergillosis (IA) is one of the most severe infectious diseases in immunocompromised patients, especially in solid-organ and bone marrow transplant recipients. There has been a substantial increase in the number of patients at risk for developing IA due to the increased number of transplantations, the development of new intensive chemotherapy regimens for hematological diseases and solid tumors, AIDS, and the increased use of immunosuppressive regimens for treating autoimmune disease. As a consequence, during the past 30 years, the incidence of IA has dramatically increased (7, 19, 35). Diagnosis is difficult and often too late, treatment is ineffective, and, as a consequence, mortality is high.
One of the most striking conclusions of a literature survey on Aspergillus fumigatus, the main causal agent of IA, is how little we know about the pathobiological factors of this organism (13). This is especially true for the early stages of disease development. Following inhalation of airborne A. fumigatus conidia, as with most airborne particles or microorganisms entering the respiratory tract, the normal host is protected by pulmonary innate immunity, including phagocytosis by alveolar macrophages (AM), the major resident phagocytic cells in the respiratory tract. Establishment of IA occurs in immunocompromised patients as the fungus escapes from the AM and invades tissues (13). Data on phagocytosis and killing of A. fumigatus conidia by AM are scarce and even contradictory (16, 32). Furthermore, the molecular and biochemical mechanisms responsible for conidial killing by AM of the immunocompetent host have not been identified. This lack of basic understanding of the role of AM in IA may prevent the advancement of new treatments. The murine model offers a reasonable approach to the study of IA, since it has been already used to (i) investigate the virulence of various A. fumigatus mutants, (ii) evaluate the efficacy of various anti-A. fumigatus drugs, and (iii) analyze the T-cell and cytokine responses against A. fumigatus infection (3, 4, 11). In addition, the use of mutant mice is extremely helpful for elucidation of fundamental physiopathological mechanisms in infectious diseases, including respiratory diseases such as IA (1, 24).
In this study, several outbred and inbred transgenic mouse strains that are resistant or susceptible to A. fumigatus were used to analyze the phagocytosis and killing of A. fumigatus conidia by AM. We show that (i) AM play an essential role in clearing A. fumigatus conidia from the lung, (ii) engulfment of conidia by AM is not affected by immunosuppression, (iii) reactive oxidant intermediates (ROI) are essential for killing of the conidia once they have swollen inside the AM, and (iv) ROI production is altered by treatment of mice with corticosteroids.
MATERIALS AND METHODS
Fungal strains.
The A. fumigatus clinical isolate CBS 144.89 was maintained on 2% malt extract agar slants at 22°C. Conidial suspensions were prepared, and conidia were labeled with fluorescein isothiocyanate (FITC), as described previously (9, 34). Swollen conidia were obtained by incubating 2 × 105 conidia/ml in RPMI 1640 supplemented with 20% heat-inactivated fetal calf serum (FCS), penicillin (100 U/ml), and streptomycin (100 U/ml) at 37°C for 3 h. Swollen conidia were extensively washed and resuspended in PBS-0.1% Tween 20. Paraformaldehyde (p-FA)-fixed conidia were prepared following a 2-h incubation in 3% p-FA at room temperature. The conidia were then washed three times in PBS-Tween, incubated for 10 min in 50 mM NH4Cl to quench the remaining aldehydes, and finally washed three times with PBS-Tween.
Mouse strains and immunosuppression regimens.
Several wild-type and mutant mouse models were used, as follows: (i) 32- to 34-g, 6- to 8-week-old male outbred Swiss OF1 mice (Iffa Credo, Saint-Germain sur l'Arbresle, France), (ii) 8- to 12-week-old wild-type and p47phox−/− 129Sv mice (the latter are deficient in the p47phox NADPH oxidase unit gene), bred at the animal facilities at University College and kindly provided by J. Roes (Department of Immunology and Immunopathology, University College London, London, United Kingdom), and (iii) 6- to 8-week-old wild-type and inducible nitric oxide synthase (iNOS)-deficient C57BL6 mice, bred at the University of Salamanca (Salamanca, Spain). C57BL6 mice (CERJ, Le Genest Saint Isle, France) were used for irradiation experiments.
For immunosuppression by corticosteroids, 25 mg of cortisone acetate (Sigma, St. Louis, Mo.) was injected intraperitoneally twice, at 5 and 2 days before collection of AM for in vitro experiments and, alternatively, at day 3 and immediately after intranasal inoculation (day 0) for in vivo experiments. Total-body irradiation was given as a single exposure by using a source of 137Cs (IBL 637; CIS Bio International) at a dose rate of approximately 0.7 Gy/min, for a total dose of 7.5 Gy. Mice were infected 3 days after irradiation. Irradiated mice received enrofloxacin (Baytril; Bayer) in their drinking water to approximate a dosage of 0.4 mg/g of body weight/day in order to prevent bacterial infection associated with irradiation-induced neutropenia.
Reagents and antibodies.
FITC, mouse and goat sera, p-FA, horseradish peroxidase (HRP), zymosan A, superoxide dismutase (SOD), and phenylarside oxide (PAO) were obtained from Sigma. Texas Red goat anti-rabbit immunoglobulin was purchased from Jackson ImmunoResearch Laboratory. RPMI 1640 medium with glutamine and with or without phenol red, heat-inactivated FCS, penicillin, and streptomycin were purchased from Gibco BRL (Cergy Pontoise, France). Diphenylene iodonium chloride (DPI), luminol, and lucigenin were purchased from Calbiochem.
AM.
AM were harvested from mouse lungs with 0.5 ml of ice-cold Ca2+- and Mg2+-free PBS(8 to 50 times) through an 18-gauge plastic catheter inserted into the trachea after cervical dissection. Cellular subpopulations were analyzed with Diff Quick (Dade Behing, Marburg, Germany). Cells were separated from lavage fluid by centrifugation at 400 × g for 8 min at 4°C and were then washed, and AM were suspended at a concentration of 2 × 106/ml of RPMI 1640 supplemented with penicillin (100 U/ml), streptomycin (100 U/ml), and 5% heat-inactivated FCS. Aliquots of 250 μl, containing 5 × 105 cells, were added to 8-well Permanox slides (Lab-Tek; Nalge Nunc International Corp., Naperville, Ill.). The cells were allowed to adhere for 60 to 90 min at 37°C under a humidified atmosphere with 5% CO2. All wells were then washed three times with RPMI 1640. The viability of the AM preparations was higher than 99% as judged by trypan blue exclusion.
Phagocytosis and ingestion assay.
Phagocytosis assays were performed as described previously (9). Briefly, after addition of FITC-labeled conidia, 8-well slides were centrifuged at 400 × g for 1 min, and cultures were incubated at 37°C under a 5% CO2 atmosphere with 80% humidity. At different times following ingestion, 3% p-FA-fixed AM were incubated with a tetramethyl rhodamine isothiocyanate (TRITC)-labeled anti-conidium rabbit polyclonal antibody (34). Preincubation and antibody dilution were carried out in a mixture of 5% goat and 5% mouse serum (vol/vol) in PBS. Only undamaged cells with Hoechst stain-positive nuclei (stained after permeabilization with 0.05% saponin followed by a 5-min incubation in a solution of Hoechst 33342 [Molecular Probes, Eugene, Oreg.] at 10 μg/ml) were counted. Three phagocytic indexes were calculated. The total percentage of internalized conidia was calculated as (number of FITC-positive, Texas red-negative conidia/number of FITC-positive conidia) × 100. The percentage of macrophages that had ingested at least one conidium was calculated as (number of AM with at least one FITC-positive, Texas red-negative conidium/total number of AM) × 100. The mean number of conidia per AM was calculated as the number of FITC-positive, Texas red-negative conidia divided by the number of AM with at least one intracellular conidium.
Mouse infection assays.
Before infection, each mouse was anesthetized by intramuscular injection of 0.1 ml of a solution containing 10 μg of ketamine (Mérial, Lyon, France)/ml and 2 μg of xylazine (Bayer, Leverkusen, Germany)/ml. Twenty five microliters of an FITC-labeled conidial suspension of A. fumigatus in PBS-0.1% Tween 20 at 4 × 106 and 4 × 108 conidia/ml was inoculated intranasally by using an automatic pipetting device. Survival of mice was monitored, or the mice were used as a source of AM to investigate conidial killing.
Killing experiments.
AM containing FITC-labeled conidia recovered by centrifugation from bronchoalveolar lavage fluid of infected mice or AM monolayers infected in vitro were lysed with 0.2 ml of water, left overnight at 4°C, and supplemented with 200 μl of a medium containing 4% glucose, 2% Mycopeptone (Biokar, Beauvais, France), and 0.1% chloramphenicol. The percentage of killing (number of nongerminated spores per 100 counted FITC-labeled conidia) in the culture well after 6 to 8 h of incubation at 37°C was assessed under a fluorescent microscope. Control wells containing only A. fumigatus conidia showed that the percentage of germination of the conidia used was always >95%.
Electron microscopy.
AM were fixed overnight at 4°C with 2.5% glutaraldehyde in Sörensen buffer, postfixed for 30 min in aqueous 1% osmium tetraoxide, and embedded in Epon resin (27). Ultrathin (50- to 60-nm-thick) sections were stained with 4% uranyl acetate followed by lead citrate.
Measurement of ROI produced by AM.
A total of 3 × 105 AM adhering to a 96-well plate (Greiner Cellstar) for 60 to 90 min in RPMI 1640 medium without phenol red and supplemented with 5% heat-inactivated FCS were used for ROI assays. After wells were washed three times with serum-free RPMI 1640 medium, AM were infected with A. fumigatus conidia at a conidium/AM ratio of 1:1 in RPMI 1640 supplemented with 20% FCS. For measurements of ROI at different times of infection, the supernatant was discarded and replaced with RPMI 1640 medium containing 20% FCS, 50 μM luminol, and 5 U of HRP per well. For zymosan assays, opsonised zymosan (0.5 mg/ml) was added to noninfected AM at the same time as the chemiluminescent probes. Measurements were performed on a Victor2 luminometer (EGG Wallac). The time of measurement of ROI was 10 s per well for an entire hour. Production of ROI was estimated by the height of the measurement curve observed during the assay and was expressed as relative light units (RLU).
Statistical analysis.
Data were analyzed by one- and/or two-way analysis of variance, and mouse survival was estimated by the Kaplan-Meier method using software from Abacus.
RESULTS
Engulfment of conidia by AM of immunocompetent mice.
Internalization of A. fumigatus conidia involved filopodia that contacted, progressively surrounded, and engulfed the conidium both in vivo (Fig. 1) and in vitro. AM internalized A. fumigatus conidia rapidly in vitro, with 30% of the conidia internalized after 15 min of incubation with AM. After 2 h of incubation, 85% of conidia were phagocytosed by AM from immunocompetent mice (Fig. 2a). The mean number of conidia internalized per AM remained constant over time at 2.5 conidia per AM at a conidium/AM ratio of 1:1 (Fig. 2b). This result indicates that the most active AM internalized two to three conidia very quickly, and a second burst in phagocytosis by AM followed. The process continued until all conidia were engulfed. Accordingly, Fig. 2c shows that the number of macrophages with at least one conidium increased over time. No significant differences in internalization were seen in the three phagocytic indexes when resting, swollen, or p-FA-fixed conidia were ingested, and similar indices were found when a 5 or 20% FCS concentration was used (data not shown).
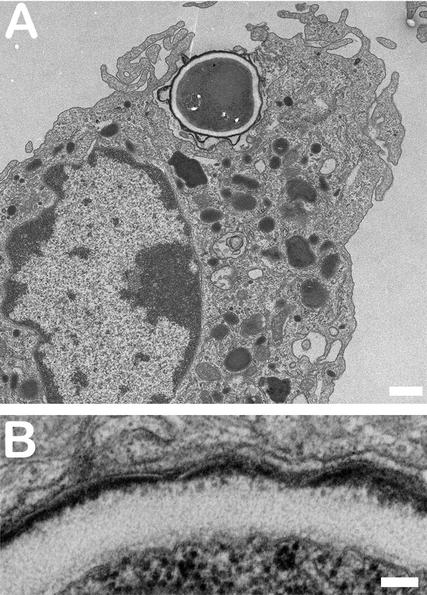
FIG. 1.
Engulfment of A. fumigatus conidia by AM from immunocompetent OF1 mice. Mice were infected in vivo with 107 conidia, and AM were collected 90 min after infection. (A) Low magnification showing conidial engulfment by AM filopodia. Bar, 1 μm. (B) High magnification showing tight contact between the outer conidial cell wall and the membrane of the phagosome. Note that the electron-lucent layer of the cell wall is a single layer. Bar, 100 nm.
FIG. 2.
Internalization of A. fumigatus conidia by mouse AM in vitro. AM and conidia were incubated at a conidium/AM ratio of 1:1 in the presence of 5% FCS. (a) Percentage of total conidia internalized by AM. (b) Mean number of conidia internalized per AM. (c) Percentage of AM that have internalized at least one conidium. Data are means ± standard errors based on at least three experiments.
Conidial swelling inside AM of immunocompetent mice and inhibition of germination.
Three hours after engulfment in vitro, the conidia swell within the AM phagosome, while the conidial cell wall remains in tight apposition with the phagolysosome membrane (Fig. 3A). Swelling always preceeds conidial germination. A double-layered cell wall, characteristic of the first stage of swelling of the conidia, was seen under the electron microscope (Fig. 3B). The diameter of resting conidia was 2.3 ± 0.05 μm. After 2 h in the AM, the conidial diameter remained unchanged (2.3 ± 0.03 μm). After 6 h, all conidia in the AM were swollen, with an average diameter significantly higher than that of resting conidia (2.9 ± 0.03 μm) (P < 0.01). However, their average diameter remained lower than that of conidia swollen in RPMI medium alone, where conidial diameters reached 2.9 ± 0.04 and 4.2 ± 0.1 μm after 2 and 6 h of incubation in the culture medium, respectively (P < 0.01). None of the phagocytosed conidia germinated (Fig. 3C).
FIG. 3.
(A) Swollen conidium inside an AM phagosome after a 24-h in vivo infection in an immunocompetent mouse. In a swollen conidium, the ultrastructure of the conidium organelles is well preserved (whereas fixation and inclusion of a resting conidium is always damaging; see Fig. 1 for comparison). Bar, 350 nm. (B) The cell wall of a swollen conidium has a double electron-lucent layer under the electron-dense outer melanin layer that is in direct contact with the phagolysosome membrane. Bar, 450 nm. (C) Fluorescence view of FITC conidia internalized by AM after 6 h of incubation. Note that the intracellular swollen conidia (long arrows) do not germinate, whereas the extracellular nonphagocytosed conidia produce a germ tube (short arrows). Bar, 12 μm.
Killing of conidia. (i) In vitro.
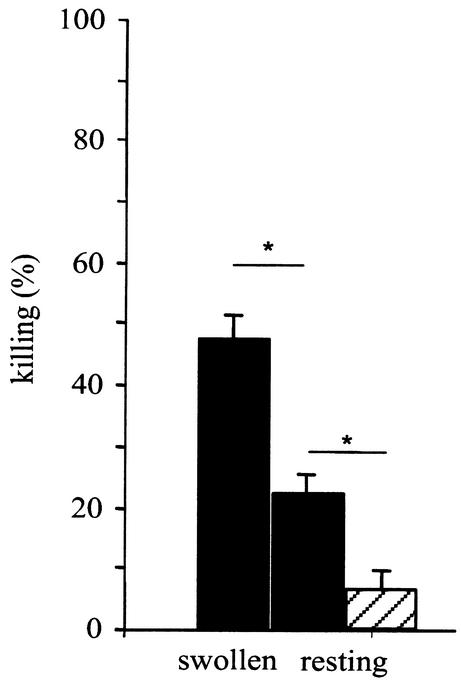
In vitro killing assays were limited to a period of 6 h postinfection in order to avoid any putative perturbation of AM killing by the germination of extracellular conidia (Fig. 3C). After a 6-h incubation, in vitro killing of resting conidia reached 6.6% at a 1:1 conidium/AM ratio (Fig. 4). This result showed that the inhibition of germ tube formation in the AM phagosome was mainly fungistatic, since the germinative capacity of the swollen conidia that remained inside the AM was only partially affected. Since it was difficult to monitor the fate of conidia engulfed by AM in vitro for more than 6 h, attempts to increase the killing rate in vitro were made by modifying the conidium/AM ratio and by using germinated swollen conidia, as suggested by others (15). A reduction in the number of conidia ingested per macrophage was associated with an increase in killing from 6.6 to 22% when a conidium/AM ratio of 1:1 to 1:10 was used (Fig. 4). Forty-seven percent of swollen conidia versus 22% of resting conidia were killed after 6 h of incubation in vitro at a 1:10 conidium/AM ratio (Fig. 4).
FIG. 4.
Percentages of swollen and resting conidia killed by AM after a 6-h incubation in vitro. Filled bars, conidium/AM ratio of 1:10; hatched bar, conidium/AM ratio of 1:1. Data shown are means from three experiments. Error bars, standard errors. ∗, P < 0.05.
(ii) In vivo.
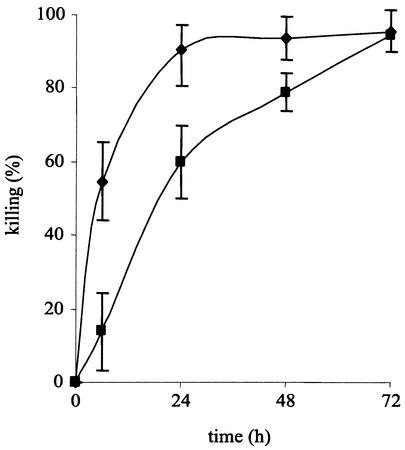
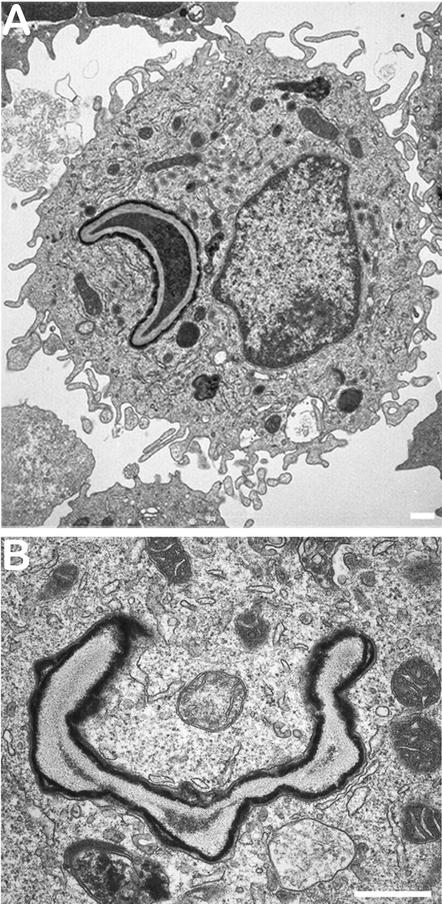
Three days were required for an immunocompetent mouse infected with 107 conidia to kill more than 90% of the conidia (Fig. 5). A similar level of killing occurred at 24 h postinfection with an inoculum of 105 conidia (Fig. 5). Dead conidia had a half-moon shape (easily seen with FITC-labeled conidia) (Fig. 6). After a few days, only the cell wall ghosts remained in the AM (Fig. 6).
FIG. 5.
In vivo conidial killing estimated in murine AM recovered from mice infected intranasally with 105 (♦) and 107 (▪) conidia. Values are means of at least three experiments ± standard errors.
FIG. 6.
Dead conidia inside AM after in vivo infection of immunocompetent OF1 mice with 107 conidia. (A) Conidium inside AM after a 24-h infection. Note the moon crescent shape of the conidium. Bar, 500 nm. (B) Conidium ghost after a 48-h infection. Bar, 400 nm.
AM control a low-dose inoculum of A. fumigatus.
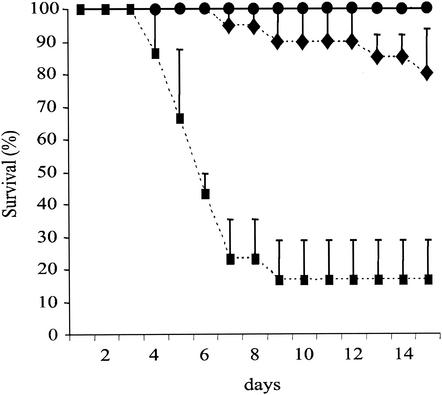
The first 4 ml of bronchoalveolar lavage fluids of mice before infection contained (1.0 ± 0.2) × 105 AM and (0.9 ± 0.1) × 103 polymorphonuclear neutrophils. After a 24-h infection of immunocompetent mice with 105 conidia, the number of AM counted in the bronchoalveolar lavage fluid increased only slightly, to reach (1.6 ± 0.6) × 105 AM, whereas the number of polymorphonuclear neutrophils remained low (2 × 104 ± 0.2 × 104). In contrast, an inoculum of 107 conidia induced an important recruitment of neutrophils to the lung. In the bronchoalveolar lavage fluids of mice infected with 107 conidia, (1.2 ± 0.4) × 106 AM and (7 ± 2.8) × 106 polymorphonuclear neutrophils were counted. Mice irradiated with 7.5 Gy did not have neutrophils, and their AM counts were similar to those of nonirradiated control mice. Moreover, the killing capacity of the AM from irradiated mice was similar to that for control mice: in vitro killing rates were 28 and 36% after 6 h, and in vivo killing rates reached 92 and 95% after 24 h with an inoculum of 105 conidia for irradiated and control mice, respectively. Very few irradiated mice developed experimental aspergillosis when they were infected with 105 conidia (Fig. 7). This result indicated that AM were able to clear an inoculum of 105 conidia from the lung almost completely. When the killing ability of the AM was impaired by cortisone acetate, 80 to 90% of the mice were killed with an inoculum of 105 conidia (Fig. 7), while the proportions of AM and neutrophils in cortisone acetate-treated mice were similar to those in control immunocompetent mice (data not shown).
FIG. 7.
Survival curve of mice intranasally inoculated with 105 conidia. Symbols: •, control mice; ♦, irradiated mice; ▪, cortisone acetate-treated mice.
ROI and AM during A. fumigatus infection.
The luminol-peroxidase method was validated by using zymosan, a known inducer of oxidative stress. The luminescence signal was 10 times higher than the signal obtained with control AM. The signal was abolished after addition of 20 μg of SOD/ml and either 0.1 μM DPI or 0.2 μM PAO; DPI and PAO are two known inhibitors of NADPH oxidase-dependent reactions. In addition, no signal was obtained after challenge of AM from p47phox−/− mice with zymosan (data not shown).
(i) Production of ROI by AM after A. fumigatus phagocytosis.
Kinetic studies showed that maximal ROI production occurred after 3 h of phagocytosis, when conidia had swollen inside the AM. Levels of ROI production at 30 min, 3 h, and 6 h postinfection were 870, 2,350, and 970 RLU, respectively, whereas levels of ROI production by noninfected AM at the same times were 700, 1,230, and 270 RLU, respectively. Viable conidia were essential to induce ROI production, since p-FA-fixed conidia did not trigger ROI production. Phagocytosis of swollen conidia was associated with higher production of ROI than phagocytosis of resting conidia. High ROI production was correlated to an elevated level of killing (Fig. 8). The specificity of the luminol-HRP reaction following phagocytosis of A. fumigatus conidia was confirmed by (i) the abolition of the luminescence signal after addition of SOD, DPI, and PAO and (ii) the lack of signal in AM from p47phox−/− mice infected with conidia (data not shown).
FIG. 8.
Production of ROI (filled bars) and in vitro killing of resting or swollen conidia (open bars) by AM. “AM” represents the noninfected control. AM were infected with either p-FA-fixed swollen conidia (Sco p-FA), resting conidia (Rco), or swollen conidia (Sco) at a conidium/AM ratio of 1:1 for ROI production and 1:10 for the killing assay. Data are means of at least three replicates. Error bars, standard errors. ∗, P < 0.001.
(ii) Inhibition of ROI suppresses conidial killing by AM.
A dramatic decrease in killing of A. fumigatus conidia both in vitro and in vivo was seen with AM from p47phox−/− mice, which do not produce ROI (Table 1). In in vivo experiments with p47phox−/− mice, intracellular conidia were viable and germinated inside the AM (data not shown). Moreover, addition of the NADPH oxidase inhibitors DPI and PAO to the incubation mixture significantly reduced in vitro killing (Table 1). The phagocytosis indices at 60 min were similar for AM from both p47phox+/+ and p47phox−/− mice or outbred mice with or without DPI and PAO (data not shown). Killing of A. fumigatus conidia by AM of iNOS-deficient mice was not altered: killing levels for wild-type and iNOS−/− mice were similar, reaching 50% ± 9% and 45% ± 2% in vitro and 95% ± 5% and 89% ± 4% in vivo, respectively. At 60 min postinfection, the phagocytosis indices were similar for AM populations of parental and iNOS knockout mice (data not shown). These data showed that killing was specifically associated with ROI and not with nitric oxide intermediates.
TABLE 1.
Rate of killing of A. fumigatus conidia by AM from outbred mice in the presence or absence of NADPH oxidase inhibitors and by AM from p47phox−/− mice
| Mouse strain and condition | % Killinga |
|---|---|
| OF1 | |
| Infection in vitro (swollen conidia) | |
| control | 62 ± 5 |
| DPI | 13 ± 5* |
| PAO | 36 ± 4* |
| 129Sv | |
| Infection in vitro (swollen conidia) | |
| p47phox+/+ | 31 ± 1 |
| p47phox−/− | 4 ± 1* |
| Infection in vivo (resting conidia) | |
| p47phox+/+ | 75 ± 6 |
| p47phox−/− | 10 ± 1* |
Conditions for killing experiments were a 6-h infection in vitro (ratio of AM to swollen conidia, 10:1) and a 24-h infection in vivo with 105 conidia. *, P < 0.01 for comparison of infected AM with NADPH oxidase inhibitors against infected AM without NADPH inhibitors and for comparison of p47phox−/− against p47phox+/+ mice.
Effects of a corticosteroid on the phagocytic and killing capacities of mouse AM.
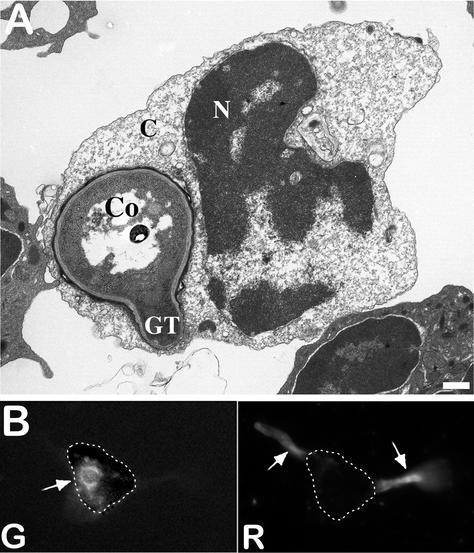
No significant differences were seen in the phagocytosis indices (percentage of internalized conidia, mean number of conidia per phagocytosing AM, and percentage of phagocytosing AM) of cortisone acetate-treated mice and control mice at 15, 30, 60, 120, and 240 min postinfection (data not shown). In contrast, killing of conidia by AM from cortisone acetate-treated mice was impaired. Double immunofluorescence labeling and electron microscopy observations showed that germination of conidia occurred intracellularly in the AM of corticosteroid-treated mice (Fig. 9). Germ tubes were produced inside the phagolysosomes of AM from cortisone acetate-treated mice without disruption of the phagolysosomal membrane which surrounded the fungal cell wall. Further growth of the germ tube resulted in disruption of the vacuolar membrane, followed by an outgrowth and death of the AM (Fig. 9).
FIG. 9.
(A) Germinating conidium inside an AM of a cortisone acetate-treated mouse. Note the germ tube (GT) emerging from the conidium (Co) and the alterations in the AM nucleus (N) and cytoplasmic organelles (C). Bar, 350 nm. (B) Fluorescence images. (Right) Mycelia grow out of an AM immunodecorated with an anti-A. fumigatus antiserum conjugated with Texas Red (R). (Left) The cell wall of a germinated conidium labeled with FITC prior to infection is still seen intracellularly (G), but the mycelial portion that is intracellular is not labeled with the antiserum. Dotted lines indicate the outline of the AM.
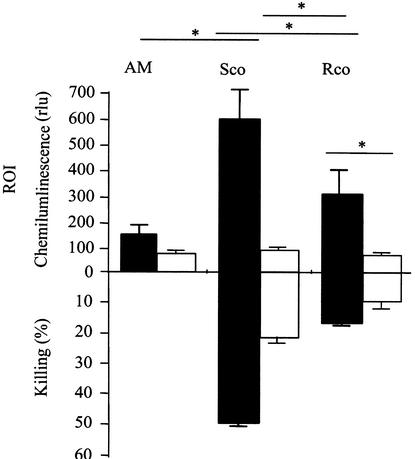
In vivo, cortisone acetate treatment induced a limited but significant reduction (P < 0.01) in the killing of conidia. At 24 h after in vivo infection, 50% of conidia of a 105-conidium inoculum were killed in cortisone acetate-treated mice versus 85% in control mice. A similar effect of cortisone acetate treatment was seen when killing assays were performed in vitro. Swollen conidia remained more sensitive to killing than resting conidia (Fig. 10). Release of extracellular ROI was not detected in AM from cortisone acetate-treated mice infected with conidia (Fig. 10). The lack of detection of ROI does not mean absence of intracellular production of ROI. Indeed, incubation of cortisone acetate-treated AM with 0.5 mg of zymosan/ml resulted in a production of ROI (414 ± 1 RLU) that reached 30% of the level produced by control AM from immunocompetent mice (1,256 ± 94 RLU). Since this release of ROI is inhibited by SOD, our results show that even though cortisone acetate-treated mice did not secrete extracellular ROI following infection with conidia, these cells were still able to produce intracellular ROI. Although ROI were detected in smaller amounts than in naive AM, the amount of ROI produced by the AM of cortisone acetate-treated mice was sufficient to kill a significant percentage of the conidia phagocytosed.
FIG. 10.
Effects of glucocorticoids on the production of ROI and ex vivo killing of swollen conidia (Sco) and resting conidia (Rco) by AM. ROI production was measured after a 3-h infection with swollen or resting conidia and was estimated by the luminol-HRP method as described above (conidium/AM ratio, 1:1). Killing was assessed after a 6-h infection in vitro (conidium/AM ratio, 1:10). ∗, P < 0.05. “AM” represents the control without conidia. AM were from immunocompetent (filled bars) or cortisone acetate-treated (open bars) mice.
DISCUSSION
The primary observations of our study on the phagocytosis and killing of A. fumigatus conidia by murine AM, summarized in Fig. 11, are as follows. (i) Internalization of conidia requires 2 h and is not affected by glucocorticosteroid or reactive oxidant inhibitors. (ii) After engulfment, the first stage of conidial germination, conidial swelling, is not affected. (iii) Killing of swollen conidia is directly associated with ROI production. (iv) Total inhibition of NADPH oxidase resulted in nearly 100% conidial germination. (v) A partial reduction in ROI production following glucocorticoid administration to mice is sufficient to allow the germination of A. fumigatus in AM of cortisone-treated mice.
FIG. 11.
Steps in the phagocytosis and killing of A. fumigatus conidia by murine AM.
Internalization of conidia was fast, and around 90% of the conidial population was engulfed by murine AM after 2 h of infection. Similar rates of engulfment have been reported for human and rabbit macrophages derived from monocytes (25, 26, 31). In contrast to the situation for most bacterial pathogens, viable A. fumigatus organisms are not essential for efficient engulfment, as evidenced by the fact that the same phagocytic index scores were calculated whether p-FA-fixed conidia or viable resting conidia were used (10). One striking result was the heterogeneity of the AM population in its capacity to ingest conidia. The most avid AM will ingest two to three conidia each. However, after ingestion of two to three conidia, their ability to engulf conidia seems reduced. A second population of less active AM then phagocytoses the remaining conidia. When a conidium/AM ratio of 5:1 was used, >95% of AM contained at least one conidium, showing that all AM have the capacity to phagocytose conidia. This pattern of kinetics suggests a heterogeneity in the AM population (14).
The data reported in the literature for the killing of A. fumigatus conidia by macrophages are extremely heterogeneous (Table 2). Several explanations may account for the variability reported in Table 2. First, the methods and strains used to estimate the viability of the conidia are different. Second, the duration of incubation varies from 1 to 30 h. Our data show that 6 h is the maximal incubation time for estimation of killing in vitro. In contrast to previous reports, we were not able to remove all extracellular nonphagocytosed conidia that would germinate after 6 h of incubation, producing a mycelial mat that would eventually alter the AM layer. Third, the macrophages used have different origins both in terms of the host (mouse, rabbit, or human) and in terms of body location (alveolar, peritoneal, and monocyte-derived macrophages), and it has been shown previously that the origin of the macrophages greatly influences conidial killing (32). The high percentage of killing after a few hours reported from other studies seems overestimated, however, since swelling of the conidium is an absolute requirement for inducing the production of ROI, which are responsible for conidial killing. Since intracellular swelling of the conidium takes >4 h in the AM, only low killing rates can be expected after 6 h of phagocytosis, as reported by Levitz et al. (16).
TABLE 2.
Percentages of killing of A. fumigatus conidia by macrophages reported in the literature
| Authors (reference) | Macrophage origina | Method(s)b | Time of infection (h); killing (%) |
|---|---|---|---|
| Roilides et al. (30) | Rabbit | CFU count | 7; 55 |
| Madan et al. (17) | Human | MTT | 1; 50 |
| Robertson et al. (29) | Human | Germination | 3; 25 |
| Schaffner et al. (32) | Rabbit | CFU count | 6; 35 |
| 24; 85 | |||
| Morgenstern et al. (24) | Mouse | CFU count | 6; 86 |
| Levitz et al. (16) | Rabbit | Germination | 6; <10 |
| Michaliszyn et al. (22) | Human | PI | 18; 25 |
| Mouse | 18; 45 | ||
| Meier-Osusky et al. (21) | Human monocytes | CFU count | 18; 50 |
| Roilides et al. (30) | Human MDM | CFU count | 7; 42 |
| Jahn et al. (10) | Human MDM | CFU count, PI | 12; 15 |
| Waldorf et al. (36) | Mouse | Germination | 15; 20 |
| Germination | 18; 70 | ||
| Marr et al. (20) | THP-1 cell line | CFU | 4; 60 |
| FUN-1 | 6; 90 | ||
| Brummer et al. (2) | Mouse | CFU | 2.5; 38 |
MDM, monocyte-derived macrophages.
MTT, tetrazolium salt colorimetric assay; PI, propidium iodide staining; FUN-1, cell stain (Molecular Probes, Eugene, Org.).
A review of the literature has shown that no standardized method existed to quantify the production of ROI by AM (12); moreover, in our hands, the classically used ferricytochrome c reduction method was not sensitive enough to detect ROI produced by AM (data not shown). To palliate this disadvantage, a sensitive luminescence method was developed based on the addition of exogenous HRP to luminol to compensate for the lack of endogenous myeloperoxidase in the AM. In contrast to previous studies (24, 31, 32), we demonstrate here that the ROI are essential components of the AM in the killing of A. fumigatus conidia. Several lines of evidence support the role of ROI: (i) a similar increase in ROI was seen when the luminol-peroxidase mixture was replaced by lucigenin at a 50 μM concentration (5) (data not shown); (ii) IA is the primary cause of death in patients suffering from chronic granulomatous disease (37); (iii) an increase in ROI production after phagocytosis of heat-killed A. fumigatus conidia has been documented previously (26, 33); (iv) inhibition of NADPH oxidase following the use of chemical inhibitors or disruption of the encoding gene in mice induces a decrease in the killing of conidia by AM; and (v) inhibition by corticoids of ROI production, also reported by others (6, 18), has been associated with a reduction in intracellular killing of conidia by macrophages. The mechanisms of killing of conidia by ROI are unknown. ROI may be directly toxic to swollen conidia inside the phagolysosome, or ROI could act as a cofactor for other toxic reagents that kill conidia (15, 28). Among these toxic molecules, cationic peptides (16) or phagolysosomial enzymes such as proteases and chitinases (8, 9, 12, 23), activation of which could be associated with acidification of the phagolysosome after phagocytosis, could play a role in the killing of the conidia.
Acknowledgments
We are very grateful to R. Calderone for appropriate comments during the editing of our manuscript and to J. P. Debeaupuis for preparing the illustrations.
B. Philippe was supported by grants from “Vaincre la Mucoviscidose” and CANAM/APHP.
Editor: T. R. Kozel
REFERENCES
- 1.Bellamy, R. 1999. The natural resistance-associated macrophage protein and susceptibility to intracellular pathogens. Microbes Infect. 1:23-27. [DOI] [PubMed] [Google Scholar]
- 2.Brummer, E., A. Maqbool, and D. A. Stevens. 2001. Protection of bronchoalveolar macrophages by granulocyte-macrophage colony-stimulating factor against dexamethasone suppression of fungicidal activity for Aspergillus fumigatus conidia. Med. Mycol. 39:509-515. [DOI] [PubMed] [Google Scholar]
- 3.Cacciapuoti, A., D. Loebenberg, E. Corcoran, F. Menzel, Jr., E. L. Moss, Jr., C. Norris, M. Michalski, K. Raynor, J. Halpern, C. Mendrick, B. Arnold, B. Antonacci, R. Parmegiani, T. Yarosh-Tomaine, G. H. Miller, and R. S. Hare. 2000. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob. Agents Chemother. 44:2017-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cenci, E., A. Mencacci, A. Bacci, F. Bistoni, V. P. Kurup, and L. Romani. 2000. T cell vaccination in mice with invasive pulmonary aspergillosis. J. Immunol. 165:381-388. [DOI] [PubMed] [Google Scholar]
- 5.Chateau, M. T., H. Rabesandratana, and R. Caravano. 1996. Differentiated U937 cells and human monocytes exhibit a differential production of extracellular oxygen species: O2•− excretion versus H2O2 diffusion. FEMS Immunol. Med. Microbiol. 13:19-28. [DOI] [PubMed] [Google Scholar]
- 6.De Castro, C. M., R. Manhaes de Castro, A. Fernandes de Medeiros, A. Queiros Santos, W. T. Ferreira e Silva, and J. Luis de Lima Filho. 2000. Effect of stress on the production of O2− in alveolar macrophages. J. Neuroimmunol. 108:68-72. [DOI] [PubMed] [Google Scholar]
- 7.Groll, A. H., P. M. Shah, C. Mentzel, M. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto, S., K. Nomoto, and T. Yokokura. 1986. The role of superoxide anion and lysosomal enzymes in anti-listerial activity of elicited peritoneal macrophages. Scand. J. Immunol. 24:429-436. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim-Granet, O., B. Philippe, H. Boleti, E. Boisvieux-Ulrich, D. Grenet, M. Stern, and J. P. Latgé. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71:891-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahn, B., A. Rampp, C. Dick, A. Jahn, M. Palmer, and S. Bhakdi. 1998. Accumulation of amphotericin B in human macrophages enhances activity against Aspergillus fumigatus conidia: quantification of conidial kill at the single-cell level. Antimicrob. Agents Chemother. 42:2569-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaton-Ogay, K., S. Paris, M. Huerre, M. Quadroni, R. Falchetto, G. Togni, J. P. Latgé, and M. Monod. 1994. Cloning and disruption of the gene encoding an extracellular metalloprotease of Aspergillus fumigatus. Mol. Microbiol. 14:917-928. [DOI] [PubMed] [Google Scholar]
- 12.Johansson, A., and C. Dahlgren. 1992. Differentiation of human peripheral blood monocytes to macrophages is associated with changes in the cellular respiratory burst activity. Cell Biochem. Funct. 10:87-93. [DOI] [PubMed] [Google Scholar]
- 13.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehnert, B. E., Y. E. Valdez, R. J. Sebring, N. M. Lehnert, G. C. Saunders, and J. A. Steinkamp. 1990. Airway intra-luminal macrophages: evidence of origin and comparisons to alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 3:377-391. [DOI] [PubMed] [Google Scholar]
- 15.Levitz, S., and R. Diamond. 1985. Mechanism of resistance of Aspergillus fumigatus conidia to killing by neutrophils in vitro. J. Infect. Dis. 152:33-42. [DOI] [PubMed] [Google Scholar]
- 16.Levitz, S. M., M. E. Selsted, T. Ganz, R. I. Lehrer, and R. D. Diamond. 1986. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J. Infect. Dis. 154:483-489. [DOI] [PubMed] [Google Scholar]
- 17.Madan, T., P. Eggleton, U. Kishore, P. Strong, S. S. Aggrawal, P. U. Sarma, and K. B. Reid. 1997. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect. Immun. 65:3171-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maridonneau-Parini, I., M. Errasfa, and F. Russo-Marie. 1989. Inhibition of O2− generation by dexamethasone is mimicked by lipocortin I in alveolar macrophages. J. Clin. Investig. 83:1936-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 20.Marr, K. A., M. Koudadoust, M. Black, and S. A. Balajee. 2001. Early events in macrophage killing of Aspergillus fumigatus conidia: new flow cytometric viability assay. Clin. Diagn. Lab. Immunol. 8:1240-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier-Osusky, I., G. Schoedon, F. Blauer, M. Schneemann, and A. Schaffner. 1996. Comparison of the antimicrobial activity of deactivated human macrophages challenged with Aspergillus fumigatus and Listeria monocytogenes. J. Infect. Dis. 174:651-654. [DOI] [PubMed] [Google Scholar]
- 22.Michaliszyn, E., S. Senechal, P. Martel, and L. de Repentigny. 1995. Lack of involvement of nitric oxide in killing of Aspergillus fumigatus conidia by pulmonary alveolar macrophages. Infect. Immun. 63:2075-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsuyama, M., R. Ohara, K. Amako, K. Nomoto, and T. Yokokura. 1986. Ontogeny of macrophage function to release superoxide anion in conventional and germfree mice. Infect. Immun. 52:236-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgenstern, D. E., M. A. Gifford, L. L. Li, C. M. Doerschuk, and M. C. Dinauer. 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J. Exp. Med. 185:207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murayama, T., R. Amitani, Y. Ikegami, R. Nawada, W. J. Lee, and F. Kuze. 1996. Suppressive effects of Aspergillus fumigatus culture filtrates on human alveolar macrophages and polymorphonuclear leucocytes. Eur. Respir. J. 9:293-300. [DOI] [PubMed] [Google Scholar]
- 26.Nessa, K., L. Palmberg, U. Johard, P. Malmberg, C. Jarstrand, and P. Camner. 1997. Reaction of human alveolar macrophages to exposure to Aspergillus fumigatus and inert particles. Environ. Res. 75:141-148. [DOI] [PubMed] [Google Scholar]
- 27.Phillips, D. M., R. Pearce-Pratt, X. Tan, and V. R. Zacharopoulos. 1992. Association of mycoplasma with HIV-1 and HTLV-I in human T lymphocytes. AIDS Res. Hum. Retrovir. 8:1863-1868. [DOI] [PubMed] [Google Scholar]
- 28.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 29.Robertson, M. D., K. M. Kerr, and A. Seaton. 1989. Killing of Aspergillus fumigatus spores by human lung macrophages: a paradoxical effect of heat-labile serum components. J. Med. Vet. Mycol. 27:295-302. [DOI] [PubMed] [Google Scholar]
- 30.Roilides, E., A. Dimitriadou-Georgiadou, T. Sein, I. Kadiltsoglou, and T. J. Walsh. 1998. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect. Immun. 66:5999-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaffner, A. 1985. Therapeutic concentrations of glucocorticoids suppress the antimicrobial activity of human macrophages without impairing their responsiveness to gamma interferon. J. Clin. Investig. 76:1755-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaffner, A., H. Douglas, A. I. Braude, and C. E. Davis. 1983. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect. Immun. 42:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahan, T. A., W. G. Sorenson, and D. M. Lewis. 1994. Superoxide anion production in response to bacterial lipopolysaccharide and fungal spores implicated in organic dust toxic syndrome. Environ. Res. 67:98-107. [DOI] [PubMed] [Google Scholar]
- 34.Sturtevant, J., and J. P. Latgé. 1992. Participation of complement in the phagocytosis of the conidia of Aspergillus fumigatus by human polymorphonuclear cells. J. Infect. Dis. 166:580-586. [DOI] [PubMed] [Google Scholar]
- 35.Wald, A., W. Leisenring, J. A. van Burik, and R. A. Bowden. 1997. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J. Infect. Dis. 175:1459-1466. [DOI] [PubMed] [Google Scholar]
- 36.Waldorf, A. R., S. M. Levitz, and R. D. Diamond. 1984. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 150:752-760. [DOI] [PubMed] [Google Scholar]
- 37.Winkelstein, J. A., M. C. Marino, R. B. Johnston, Jr., J. Boyle, J. Curnutte, J. I. Gallin, H. L. Malech, S. M. Holland, H. Ochs, P. Quie, R. H. Buckley, C. B. Foster, S. J. Chanock, and H. Dickler. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79:155-169. [DOI] [PubMed] [Google Scholar]