Abstract
Although some intestinal epithelial cell lines are known to respond to lipopolysaccharide (LPS), understanding of the relationship between LPS responsiveness and the expression of LPS receptors or factors regulating LPS responsiveness of intestinal epithelial cell lines is incomplete. In this study, we demonstrate that commonly studied human intestinal epithelial cell lines can be classified into at least three different types on the basis of LPS responsiveness, Toll-like receptor-4 (TLR4) expression, and the effects of gamma interferon (IFN-γ) on LPS responsiveness. The first phenotype, which includes the HCT-116 and Caco-2 cell lines, is characterized by relative hyporesponsiveness to LPS and diminished expression of TLR4 protein. In these cells, IFN-γ does not induce LPS responsiveness. The second phenotype, which includes cell line SW480, exhibits a highly LPS-responsive phenotype and surface expression of TLR4 protein even in unprimed conditions. These lines are functionally similar to cells of monocytic lineage. In the third phenotype, which includes the HT-29 and Colo205 cell lines, TLR4 protein is largely present in the cytoplasmic fraction and the cells are hyporesponsive to LPS in an unprimed condition. However, priming of these cells with IFN-γ can induce LPS responsiveness through augmentation of LPS uptake and expression of MD-2 mRNA and intracellular TLR4 proteins. Finally, these findings suggest that the Th1 cytokine IFN-γ modulates LPS responsiveness through several mechanisms in intestinal epithelial cells and that these cells may comprise different subpopulations with distinct roles in innate immune responses.
Intestinal epithelial cells and their products, such as mucin and trefoil peptides, form a barrier that separates the host's internal milieu from the external environment (8, 35). In recent years, it has become clear that the intestinal epithelium also serves as the defensive frontline of the mucosal innate immune system in the gastrointestinal tract (16, 21). Lipopolysaccharides (LPS) produced by gram-negative bacteria and lipoprotein and peptidoglycan produced by gram-positive bacteria are abundant products of the normal flora that can induce innate immune responses. Although intestinal mucosa is constantly exposed to commensal bacteria and their components, the normal mucosa exhibits only minimal “physiologic” inflammation (4). However, invasion of pathogenic bacteria through epithelial cells or mechanical breaks in the continuous epithelial monolayer barrier (22) or the penetration of bacterial components typically results in an inflammatory response (18, 23).
Given their surface position, intestinal epithelial cells are poised to serve as sensors for luminal bacteria and, when appropriate, produce signals such as chemokines that can stimulate the recruitment of host inflammatory cells, including neutrophils, monocytes, and T cells, to sites of pathogen invasion (21). The clinical importance of the interaction between bacteria and intestinal mucosa is also underscored by recent studies of both human inflammatory bowel disease and genetic murine models of colitis (7, 21, 40). The presence of a luminal flora appears to be a required cofactor in the development of colitis in murine lines rendered susceptible through targeted disruption of a wide variety of genes (34, 38). These findings suggest that altered responses to luminal flora can result in chronic intestinal inflammation (33, 37).
In the past few years, it has also become clear that innate immune mucosal responses may play a central role in determining the character of host response to flora. Recent studies have demonstrated that many innate immune responses to pathogens are mediated by a family of highly conserved pattern recognition receptors (24-26). The Toll-like receptors (TLRs), a family composed of at least 10 mammalian homologs of Drosophila Toll, serve as pattern recognition receptors for various microbial products and can mediate production of proinflammatory cytokines (24-26). Thus, TLR4 functions as the main receptor for LPS from gram-negative bacteria and transduces signals through MyD88, interleukin-1 (IL-1) receptor-associated kinase, and TRAF6 to activate NF-κB and mitogen-activated protein kinase pathways (13, 17). The MD-2 protein is also required for effective LPS signal transduction, forming a complex with the extracellular domain of TLR4 (2, 9, 39).
Although previous studies, including those of our group and others, have demonstrated that some intestinal epithelial cell lines can respond to LPS (5), there has been no detailed analysis of the effect of T-cell-derived proinflammatory cytokines on TLR4 expression and LPS responsiveness in intestinal epithelial cells. The present study was initiated to define the role of proinflammatory cytokines in regulating LPS responsiveness in intestinal epithelial cells in order to define their integration into mucosal responses to products of luminal flora.
MATERIALS AND METHODS
Antibodies and reagents.
LPS (Escherichia coli serotype O25:B5; phenol extracted and ion-exchange chromatography purified; protein, <1%; RNA, <1%; catalog number L4524) was purchased from Sigma-Aldrich (Saint Louis, Mo.). Alexa Fluor 488-conjugated LPS from E. coli serotype O25:B5 was purchased from Molecular Probes, Inc. (Eugene, Oreg.). Recombinant human IL-1β and gamma interferon (IFN-γ) were purchased from R&D Systems (McKinley, Nebr.). The rabbit anti-TLR4 antiserum, mouse anti-TLR4 monoclonal antibody (MAb) (clone HTA-125), phycoerythrin (PE)-labeled anti-TLR4 MAb (clone HTA-125), and PE-labeled isotype control MAb (γ2a, κ; clone eBM2a) were purchased from eBioScience (San Diego, Calif.). PE-labeled anti-CD14 MAb (γ2a, κ; clone M5E2) was purchased from BD Biosciences (San Jose, Calif.).
Cell culture and cell stimulation assays.
The human colon cell lines HCT-116, Caco-2, HT-29, Colo205, and SW480 and the human monocytic cell line THP-1 were obtained from the American Type Culture Collection (Manassas, Va.). THP-1 cells were grown in RPMI 1640 (Cellgro Mediatech, Inc., Herndon, Va.) containing 10% fetal bovine serum (FBS) (Cellgro Mediatech, Inc.). HT-29 cells were grown in Dulbecco's modified Eagle's medium (DMEM; Cellgro Mediatech, Inc.) containing 10% FBS (Atlanta Biologicals Inc., Norcross, Ga.). Caco-2 cells were grown in DMEM containing 20% FBS. Colo205 and SW480 cells were grown in a 1:1 mixture of DMEM and Ham's F-12 medium (Cellgro Mediatech, Inc.) containing 10% FBS. All media were supplemented with 50 U of penicillin/ml and 50 μg of streptomycin/ml (Invitrogen, Carlsbad, Calif.). Cells were grown in 5% CO2 at 37°C within a humidified incubator and were grown to confluence in 6-well culture plates (BD Biosciences). Cells were pretreated with 10 ng of IFN-γ/ml or control medium for 12 h, washed, and stimulated with various concentrations of LPS or IL-1β with or without 10 ng of IFN-γ/ml. Supernatants were harvested after 18 h and stored at −20°C.
ELISA.
The IL-8 concentration in cell culture supernatant was determined by using the OptiIL-8 enzyme-linked immunosorbent assay (ELISA) set (BD Biosciences) according to the manufacturer's protocol.
RT-PCR and Northern blot analysis.
Total RNA was extracted by using TRIzol reagents (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized from 2 μg of total RNA by using Superscript II reverse transcriptase (RT) (Invitrogen). Aliquots (2 μl) of the product were subjected to 20, 25, 30, and 35 cycles of PCR amplification at 94°C for 1 min, 72°C for 40 s, and 55°C for 1 min using Taq DNA polymerase (Invitrogen). The forward and reverse PCR primers used for MD2 and glyceraldehyde-3-phoshate dehydrogenase (GAPDH) were 5′-GAA GCT CAG AAG CAG TAT TGG GTC-3′ and 5′-GGT TGG TGT AGG ATG ACA AAC TCC-3′ (MD2) and 5′-TCA TCT CTG CCC CCT CTG CT-3′ and 5′-CGA CGC CTG CTT CAC CAC CT-3′ (GAPDH), resulting in an amplification product of 422 bp for MD2 and 440 bp for GAPDH. The forward and reverse PCR primers were designed from different exons. Aliquots of the PCR products (10 μl) were analyzed by electrophoresis on 1.2% agarose gels.
For Northern blot analysis, 15 μg of total RNA was subjected to electrophoresis using 1% agarose-formaldehyde gels, followed by transfer to Nytran SuPerCharge membranes (Schleicher & Schuell, Keene, N.H.) by using the Turboblotter System (Schleicher & Schuell). Human cDNA probes (TLR4 and GAPDH) were generated by RT-PCR and cloned into vector pCR4-TOPO (Invitrogen) for use as hybridization probes for Northern blot analysis. Probes were labeled with [α-32P]dCTP by using Ready-To-Go DNA labeling beads (Amersham Biosciences Co., Piscataway, N.J.), followed by spin column removal of unincorporated nucleotides. Membranes were pretreated and hybridized in QuikHyb hybridization solution (Stratagene, La Jolla, Calif.) and washed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% sodium dodecyl sulfate (SDS) at room temperature for 5 min and then washed twice with 0.5× SSC containing 0.1% SDS at 65°C for 10 min. Membranes were exposed for 8 to 48 h at −80°C with intensifying screens.
Immunoprecipitation and immunoblotting.
Cells were grown in 6-well plates, stimulated with 10 ng of IFN-γ/ml for 18 h, and washed, and then 300 μl of lysis buffer (1% Triton X-100, phenylmethylsulfonyl fluoride, protease inhibitor, EDTA, Tris-HCl, NaCl [pH 7.4]) was added. After 10 min of incubation, cell-free lysates were obtained and stored at −80°C. The protein concentration was determined by using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, Calif.). For immunoprecipitation, 1 mg of protein was mixed with 2 μg of rabbit anti-TLR4 antibody (eBioscience) and 10 μl of Hitrap protein A Sepharose beads (Amersham Biosciences Co.). After overnight incubation at 4°C, immunocomplexes were washed with lysis buffer and solubilized in 30 μl of SDS sample buffer (1.25% SDS, 2.5% glycerol, 62.5 mM Tris-HCl [pH 6.8], 5% 2-mercaptoethanol). Immunoprecipitated proteins were separated on 4 to 20% Tris-glycine polyacrylamide gel (Invitrogen), and proteins were blotted onto polyvinylidene difluoride membranes. Membranes were blocked with 5% dry milk-0.1% Tween 20 in Tris-buffered saline for 2 h at room temperature and then incubated overnight at 4°C with biotinylated rabbit anti-TLR4 antibody (200 ng/ml) (eBioscience), followed by 1 h of incubation at room temperature with horseradish peroxidase-labeled rabbit anti-biotin antibody (1:1,000) (Cell Signaling, Beverly, Mass.). Staining was detected by using a Renaissance chemiluminescence kit (Perkin-Elmer Life Sciences, Inc., Boston, Mass.) and by exposure to radiographic film. For positive and negative controls, lysates were prepared from COS7 cells or COS7 cells transfected with pCMV1-FLAG-hTLR4 (kindly provided by R. Medzhitov, Yale University) by using Lipofectamine Plus reagents (Invitrogen). Protein expression was confirmed by Western blotting using anti-FLAG M2 MAb (Sigma-Aldrich).
Fluorescence-activated cell sorter (FACS) analysis.
Cell suspensions were prepared from confluent cells grown as monolayers in 6-well plates and detached by using 1% trypsin-0.02% EDTA. Cells (5 × 105 cells per sample) were washed with DMEM containing 2% FBS and used for direct immunofluorescence staining with PE-labeled anti-TLR4 MAb (clone HTA-125; eBioscience), PE-labeled anti-CD14 MAb (clone M5E2; BD Biosciences), or PE-labeled isotype control (γ2a, κ; eBioscience). The cells were analyzed on a FACScan TM (BD Biosciences). Forward and side scatter criteria were used to define single-cell populations.
LPS uptake assay.
Alexa Fluor 488-conjugated LPS from E. coli O55:B5 (Molecular Probes, Inc.) was preincubated in culture medium containing 10% FBS for 1 h at 37°C and added to cells grown in a 6-well plate at a concentration of 1 μg/ml. After the indicated incubation time, cells were detached from the surface by using 1% trypsin-0.02% EDTA. Cells (5 × 105 cells per sample) were washed with DMEM containing 2% FBS and stained by propidium iodide (1 μg/ml) to eliminate dead cells. The cells were analyzed on a FACScan TM (BD Biosciences). Forward and side scatter criteria were used to define single-cell populations.
For fluorescence microscopy observation, cells were cultured on Lab-Tek chamber slides (Nalge Nunc International Co., Naperville, Ill.) and Alexa Fluor 488-conjugated LPS (1 μg/ml) was added. After the indicated incubation time, cells were washed with cold PBS and fixed in a mixture of 50% ice-cold methanol and 50% ice-cold acetone for 5 min. After washing, 4′,6′-diamidino-2-phenylindole (DAPI) staining was performed for nuclear staining.
Statistical analysis.
Statistical significance between groups was assessed by Student's t test. Data are expressed as means ± standard deviations (SD). Triplicate determinations were performed in each experiment, and all experiments were repeated at least three times. A probability value of <0.05 was taken as the criterion for a significant difference.
RESULTS
Cell type-specific conversion of LPS responsiveness of human intestinal epithelial cells by IFN-γ.
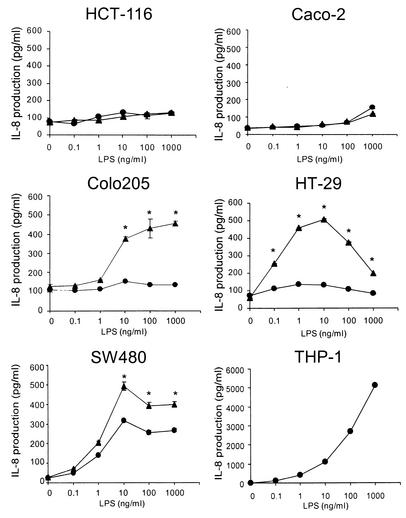
We first compared LPS responsiveness among five human intestinal epithelial cell lines (HCT-116, Caco-2, Colo205, HT-29, and SW480) which are commonly used in many laboratories and have been reported to be able to respond to IFN-γ (3, 20, 30, 36). These cell lines were stimulated with 0.1 to 1,000 ng of LPS/ml. After overnight culture, supernatants were collected and IL-8 activity in the supernatants was analyzed by ELISA. As shown in Fig. 1, most intestinal epithelial cell lines (HCT-116, Caco-2, HT-29, and Colo205) responded to LPS at concentrations of 1 μg/ml or greater. In contrast, significant IL-8 production was exhibited when SW480 cells were stimulated by concentrations of LPS as low as 1 ng/ml (Fig. 1). Although the absolute amount of IL-8 produced by SW480 cells was less than that from THP-1 cells, the concentrations of LPS needed to produce significant amounts of IL-8 were almost equivalent for these two cell lines. Next, we evaluated the effects of pretreatment with IFN-γ on IL-8 production in response to LPS. Although responsiveness to LPS in HCT-116 or Caco-2 cells did not change after priming with IFN-γ, pretreatment with IFN-γ resulted in a significant increase in LPS responsiveness in HT-29 and Colo205 cells. As shown in Fig. 1, significant IL-8 production was induced in HT-29 and Colo205 cells by stimulation with 0.1 and 10 ng of LPS/ml, respectively, after treatment with IFN-γ.
FIG. 1.
IFN-γ regulates LPS-dependent IL-8 production in human intestinal epithelial cells. Human intestinal epithelial (HCT-116, Caco-2, Colo205, HT-29, and SW480) or monocytic (THP-1) cells were incubated with IFN-γ (10 ng/ml) or medium for 12 h, washed, and stimulated with the indicated concentration of LPS with (epithelial cells only) (closed triangles) or without (closed circles) IFN-γ. Supernatants were harvested for measurement of IL-8 activity by ELISA as detailed in Materials and Methods. Each assay was carried out in triplicate, with results reported as means ± SD (error bars). *, P < 0.05 in comparison to respective control culture without IFN-γ.
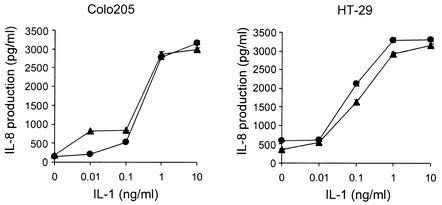
IL-1 is known to share signal transduction pathways with LPS downstream of MyD88 (27). In initial efforts to determine which steps in IL-8 production are involved in the IFN-γ-mediated enhancement of the LPS response in HT-29 and Colo205 cells, the effect of IL-1 with or without IFN-γ pretreatment on IL-8 production by HT-29 and Colo205 cells was evaluated. As shown in Fig. 2, although IL-1 induced concentration-dependent IL-8 production, this effect was unaffected by IFN-γ in either HT-29 or Colo205 cells. These results suggested that IFN-γ regulates events upstream of MyD88 to confer responsiveness to LPS.
FIG. 2.
IFN-γ does not enhance IL-1-induced IL-8 production from human intestinal epithelial cells. Cells were incubated with IFN-γ (10 ng/ml) or medium for 12 h, washed, and stimulated with IL-1β with (closed triangles) or without (closed circles) IFN-γ. IL-8 activity in supernatants was measured by ELISA as detailed in Materials and Methods. Each assay was carried out in triplicate, with results reported as means ± SD (error bars). *, P < 0.05 in comparison to respective control culture without IFN-γ.
Effects of IFN-γ on RNA expression of TLR4 and MD-2.
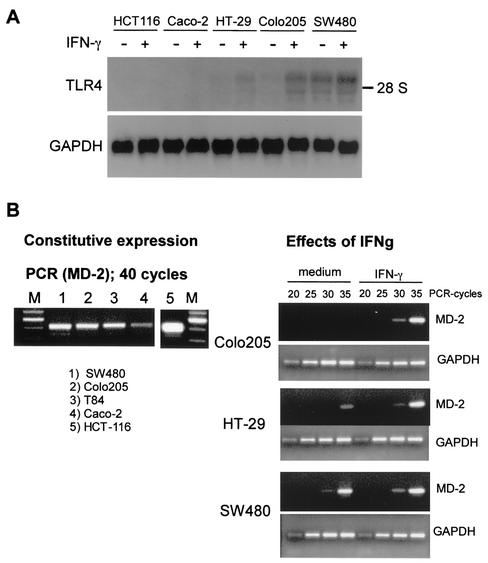
Using Northern blot analysis, we next examined the effect of IFN-γ on RNA expression of TLR4. As shown in Fig. 3A, intestinal epithelial cell lines (HT-29, Colo205, and SW480) expressed various amounts of TLR4 mRNA, and the expression of TLR4 mRNA was up-regulated by IFN-γ. The TLR4 mRNA levels in HT-29 and Colo205 cells were increased two- to fourfold following exposure to IFN-γ. Three distinct bands for TLR4 mRNA were observed by Northern blot analysis. Although we could not assess the functional differences among these bands in this study, these different bands reflect previously documented transcript variants for TLR4 (GenBank accession numbers U88880, NM_138554, NM_138556, NM_003266, and NM_138557). In contrast, TLR4 mRNA expression in HCT-116 and Caco-2 cells was not detected by Northern blotting even after IFN-γ treatment. Other Th1 and Th2 cytokines (IL-1, IL-6, tumor necrosis factor alpha, IL-4, IL-13, and transforming growth factor β) and growth factors (epidermal growth factor, hepatocyte growth factor, and fibroblast growth factor) also failed to induce TLR4 mRNA expression in these cells (data not shown). All epithelial cell lines evaluated (HCT-116, Caco-2, HT-29, Colo205, and SW480) expressed MD-2 mRNA, as detected by RT-PCR. IFN-γ up-regulated MD2 mRNA expression in both HT-29 and Colo205 cells (Fig. 3B). These results suggest that the relative hyporesponsiveness to LPS by HCT-116 and Caco-2 cells is due to relatively low steady-state concentrations of TLR4 mRNA.
FIG. 3.
IFN-γ up-regulates TLR4 and MD-2 mRNA in human intestinal epithelial cell lines. (A) Cells were cultured with IFN-γ (10 ng/ml) or medium for 6 h, and then total RNA was isolated. Northern blotting was performed as described in Materials and Methods, followed by hybridization with cDNA probes for TLR4 and GAPDH. (B) Cells were cultured with IFN-γ (10 ng/ml) or medium for 6 h, and then total RNA was isolated. MD-2 and GAPDH expression was assessed by RT-PCR using 2 μg of total RNA and primers.
Effects of IFN-γ on protein expression of TLR4.
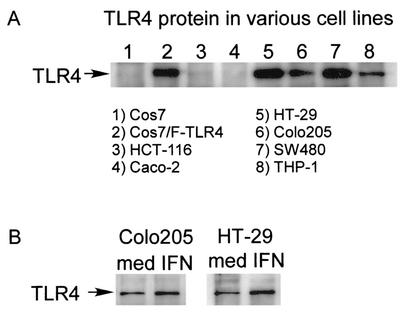
To determine whether the IFN-γ effects on TLR4 mRNA expression in intestinal epithelial cells are paralleled by changes in total protein and surface expression, we evaluated constitutive TLR4 protein in human intestinal epithelial cell lines by immunoprecipitation (IP)-Western analysis using anti-TLR4 polyclonal antibodies. Lysates from COS7 cells transfected with FLAG-tagged TLR4 plasmid served as a positive control. As shown in Fig. 4A, TLR4 protein was observed in lysates from SW480 and HT-29 cells. Significant expression of TLR4 protein was also present in lysates from Colo205 cells. IFN-γ up-regulated TLR4 protein expression in both Colo205 and HT-29 cells (Fig. 4B). TLR4 protein could not be detected with these techniques in HCT-116 and Caco-2 lysates with or without IFN-γ pretreatment.
FIG. 4.
Expression of TLR4 protein in intestinal epithelial cells and effects of IFN-γ on the expression of TLR4. (A) Whole-cell lysates of the cells were analyzed by an IP-Western system using polyclonal rabbit anti-TLR4 antibody. As a positive control, whole-cell lysates from pFLAG-TLR4-transfected Cos7 cells were used. (B) Cells were cultured with IFN-γ (10 ng/ml) or medium for 16 h. Whole-cell lysates of the cells were analyzed by an IP-Western system using polyclonal rabbit anti-TLR4 antibody.
Effects of IFN-γ on surface expression of TLR4 and CD14.
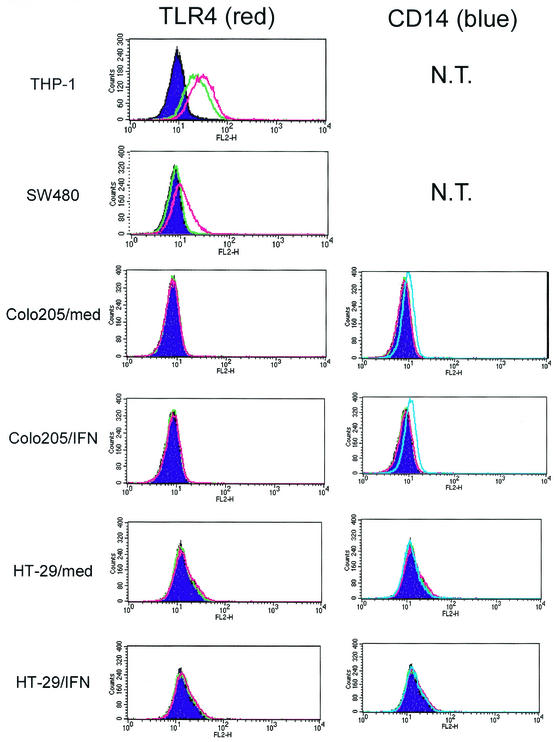
We next checked surface expression of TLR4 and CD14 by FACS analysis using PE-labeled MAbs against TLR4 and CD14. CD14 is known to directly bind to LPS and augments the LPS sensitivity of the TLR4-MD-2 complex in various cell types (6, 42). Surface CD14 expression was not detected in either HT-29 or SW480 cells with or without IFN-γ pretreatments (Fig. 5 and data not shown). Low but significant surface CD14 expression was observed in Colo205 cells. However, expression of CD14 in Colo205 cells was not changed after IFN-γ exposure. In contrast, surface expression of TLR4 at a concentration comparable to that present on monocytic cell lines was observed in SW480 cells. Surface expression of TLR4 was not altered by IFN-γ treatment. Although significant amounts of total TLR4 protein and MD2 mRNA were expressed in HT-29 or Colo205 cells after IFN-γ treatments, surface expression of TLR4 was not detected (Fig. 5).
FIG. 5.
Expression of TLR4 and CD14 by intestinal epithelial cells. Cells were cultured with IFN-γ (10 ng/ml) or medium for 16 h, collected, stained with PE-labeled anti-TLR4 MAb (red line), PE-labeled anti-CD14 MAb (blue line), or PE-labeled isotype control (green line), and analyzed by FACS as described in Materials and Methods. Filled purple areas show nonstaining control. Left panels show TLR4 staining, and right panels show CD14 staining. In all epithelial cell lines, significant background staining with isotype control could not be detected. However, significant background staining with isotype control MAb (green line) could be detected in THP-1 cells. This background staining seems to be mediated by Fc receptors expressed on THP-1 cells.
Effects of IFN-γ on LPS uptake into intestinal epithelial cells.
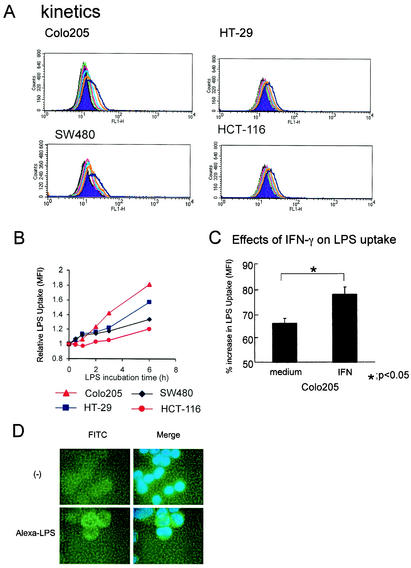
Recently, it has been reported that TLR4 protein is present in the Golgi apparatus, but not plasma membranes, in a murine small-intestinal epithelial cell line and colocalizes with internalized LPS (12). Therefore, although we detected surface TLR4 in some human colonic epithelial cell lines, we next examined whether human colonic intestinal epithelial cell lines demonstrate LPS uptake and whether IFN-γ can modulate this process. Alexa Fluor 488-conjugated LPS (1 μg/ml) was added to intestinal epithelial cells, followed by incubation at 37°C for 0.5, 1, 2, 3, or 6 h. Time-dependent incorporation of Alexa Fluor 488-conjugated LPS into cells was observed in all epithelial cells tested (HCT-116, Caco-2, HT-29, Colo205, and SW480). The rates of uptake varied among the cell lines (Fig. 6A and B). Fluorescence microscopy observation revealed that most of the incorporated Alexa Fluor 488-conjugated LPS was localized in the cytoplasm (Fig. 6D). LPS incorporation in Colo205 cells was stimulated by IFN-γ (Fig. 6C).
FIG. 6.
Internalization of LPS by human intestinal epithelial cells and effects of IFN-γ on LPS uptake. (A) Cells were incubated for 0.5 (green line), 1 (red line), 2 (light-blue line), 3 (orange line), or 6 (blue line) h with 1 μg of Alexa Fluor 488-conjugated LPS/ml and then analyzed by FACS as described in Materials and Methods. (B) The change in mean fluorescence intensity (MFI) after Alexa Fluor 488-conjugated LPS incubation is shown. (C) The effects of IFN-γ (10 ng/ml) on LPS uptake, with results reported as means ± SD (error bars). *, P < 0.05 in comparison to respective control culture without IFN-γ. (D) Fluorescence microscopy image of Colo205 cells after 3 and 6 h of incubation with 1 μg of Alexa Fluor 488-conjugated LPS/ml.
DISCUSSION
Under physiological conditions, intestinal epithelium appears to exhibit a state of hyporesponsiveness to the variety of products of commensal bacteria to which it is consistently exposed, such as LPS, lipoproteins, or peptidoglycan. However, intestinal epithelial cells can stimulate inflammatory responses through production of proinflammatory mediators such as IL-8 and nitric oxide in the context of infection by pathogenic bacteria or inflammatory bowel disease (15, 18, 22, 23). It is well recognized that inflammatory bowel disease is characterized by chronic dysregulated inflammation, and commensal bacteria may be a stimulus for these inflammatory responses (29). Furthermore, augmented production of Th1 cytokines, including IFN-γ, at sites of inflammatory bowel disease with inflammatory activity has been reported, suggesting that these Th1 cytokines may be involved in the change of responsiveness of intestinal epithelial cells to microbial products (10).
Although some, but not all, intestinal epithelial cell lines are known to respond to LPS, little is known about the relationship between LPS responsiveness and the expression of LPS receptors and other factors regulating LPS responsiveness. In this study, we demonstrate that IFN-γ modulates LPS responsiveness in intestinal epithelial cell lines and that commonly studied human intestinal epithelial cell lines can be classified into at least three different types on the basis of LPS responsiveness, TLR4 expression, and the effect of IFN-γ on LPS responsiveness. The heterogeneity in TLR4 expression and LPS responsiveness observed in these intestinal epithelial cell lines seems to reflect the heterogeneity of primary intestinal epithelial cells, because we observed that TLR4 and MD-2 mRNA were detected in most, but not all, primary intestinal epithelial cells isolated from freshly prepared biopsy samples (unpublished results).
The intestinal epithelial cell lines tested in this study, with the exception of SW480, were hyporesponsive to LPS stimulation, perhaps resembling primary cells in normal physiological conditions in vivo. Interestingly, among the cell lines studied, SW480 was responsive to LPS at nanogram range and expressed surface TLR4, as detected by FACS. Furthermore, the surface TLR4 on SW480 cells appears to be complexed with MD-2. However, the optimal LPS response of SW480 cells seems to be enhanced by soluble CD14, because SW480 cells lack detectable membrane CD14 even after IFN-γ stimulation. In contrast, HCT-116 and Caco-2 cells in the present study lacked TLR4 mRNA and TLR4 protein, although MD-2 mRNA was present in both cell lines. LPS responsiveness and TLR4 expression in either HCT-116 or Caco-2 cells were unaffected by IFN-γ stimulation. The observed lack of responsiveness to IFN-γ in both Caco-2 and HCT-116 cells cannot be attributed to defects in IFN-γ-induced signal transduction pathways in these cell lines, because IFN-γ can induce HLA class II expression and exert antiproliferative effects in both Caco-2 and HCT-116 cells (30, 36). A recent report demonstrated that the Caco-2 cell line acquires LPS responsiveness after transfection with both TLR4 and MD-2 cDNAs (2). These results suggest that the hyporesponsiveness to LPS in these cell lines seems to be caused by limited expression of TLR4. On the other hand, although HT-29 and Colo205 cells expressed amounts of TLR4 proteins and MD-2 mRNA that were almost comparable to those in SW480 cells, the former cell lines remained hyporesponsive to LPS unless primed. Recently, Randow and Seed found that TLR proteins (TLR1, TLR2, and TLR4) were retained intracellularly in a B-cell line in the absence of endoplasmic reticulum chaperone gp96 (32). These results suggest that surface expression of TLR4 may be specifically regulated after protein translation, although the contribution of gp96 in intracellular retention of TLR4 proteins in HT-29 and Colo205 cells is unknown.
In this paper, we found that IFN-γ could markedly enhance the sensitivity of cell response to LPS as assessed by production of IL-8 in both HT-29 and Colo205 cells. IL-1 was able to induce IL-8 production by both unprimed HT-29 and Colo205 cells, but this effect was not augmented by IFN-γ. These results suggest that the state of LPS responsiveness and the effect of IFN-γ in enhancing LPS responsiveness in these cell lines are mediated via events upstream of MyD88, because TLR4 and IL-1 receptor shared a common signal transduction pathway downstream of MyD88 (27). As shown in Fig. 3, IFN-γ augments TLR4 mRNA and protein expression in both HT-29 and Colo205 cells. Furthermore, MD-2 mRNA expression is also augmented by IFN-γ treatment. Recently, similar observations were reported by Abreu et al. (1). However, surface expression of TLR4 was not detected in either HT-29 or Colo205 cells even after IFN-γ treatments. Furthermore, IFN-γ-induced LPS response was not neutralized by the addition of anti-TLR4 MAb, using an MAb which has been reported to be able to neutralize LPS response in macrophages (39), a result confirmed by us in vitro (data not shown). These results suggest that HT-29 and Colo205 cells may respond to LPS after IFN-γ treatment through a mechanism independent of surface expression of TLR4. However, it is also possible that trypsin used to isolate cells leads to loss of surface TLR4 prior to FACS analysis, an effect which may render surface TLR4 undetectable in cell lines with intrinsically lower expression.
Recently, Hornef et al. found TLR4 in the Golgi apparatus that colocalized with internalized LPS in a murine small-intestinal epithelial cell line and that is highly responsive to LPS (12). The results of our study are consistent with those of this previous report and may suggest that internalized LPS can provide signals for IL-8 production through intracellular TLR4-MD-2 complexes in some human large-intestinal epithelial cell lines as well. Time-dependent uptake of fluorescence-labeled LPS and cytoplasmic transport could be observed in some human large-intestinal epithelial cell lines, and incorporation of LPS into cytoplasm was significantly augmented by IFN-γ in Colo205 cells (Fig. 6).
Internalization of LPS has been demonstrated in a number of studies (19, 41). Kitchens et al. reported that monocytes exhibit at least two different membrane-bound CD14-dependent LPS internalization pathways, one utilizing clathrin-coated pits and the other independent of clathrin-coated pits (19). Using HeLa cells, Thieblemont and Wright demonstrated that soluble CD14 also participated in the incorporation of LPS into cytoplasm (41). However, as demonstrated by most previous reports (5, 31), intestinal epithelial cell lines, except Colo205, lack surface CD14 expression in unprimed conditions and neither IFN-γ nor LPS stimulation induced surface CD14 expression on these cells (Fig. 5 and data not shown). In contrast, significant surface expression of CD14 was detected in Colo205 cells, but IFN-γ modulation of LPS uptake was not mediated via regulation of CD14 expression. Our present results demonstrate that intestinal epithelial cell lines may take up LPS through both surface TLR4-dependent and -independent pathways.
Recently, it has been demonstrated that NOD1/CARD4 and NOD2/CARD15, members of a subgroup of the CED4/Apaf-1 family of proteins characterized by a C-terminal leucine-rich repeat domain, function as cytoplasmic receptors for LPS and peptidoglycan components, respectively (11, 14, 28). NOD1 mRNA is widely expressed in various tissues (14). As expected, constitutive expression of NOD1 mRNA was observed by RT-PCR in all epithelial cell lines used in this study (data not shown). In contrast to NOD1, expression of NOD2 mRNA was initially reported to be macrophage specific (28). However, it has been found that some intestinal epithelial cells also express NOD2 mRNA and protein and that NOD2 mRNA is up-regulated by TNF-α, but not IFN-γ, in intestinal epithelial cells (11a). These results suggest that the contribution of NOD1/NOD2 to IFN-γ-enhanced IL-8 production observed in HT-29 and Colo205 cells is modest. Further studies are required to clarify the relative contributions of TLR and NOD systems to host responses to LPS derived from outside of cells and intracellular bacteria in human intestinal epithelial cells.
In aggregate, our results demonstrate that commonly used human intestinal epithelial cell lines can be classified into three different phenotypes according to LPS responsiveness and TLR4 expression pattern, likely reflecting heterogeneity in vivo. The first phenotype is characterized by hyporesponsiveness to LPS and diminished expression of TLR4 protein (HCT-116 and Caco-2). In these cells, pretreatment of IFN-γ does not induce LPS responsiveness. The second phenotype exhibits a highly LPS-responsive phenotype and a clear surface expression of TLR4 protein even in an unprimed condition (SW480). These cells are therefore functionally similar to those of monocytic lineage. In the last phenotype (HT-29 and Colo205), TLR4 protein is largely present in the cytoplasmic fraction and the cells are relatively hyporesponsive to LPS in unprimed condition. Interestingly, priming with IFN-γ can induce LPS responsiveness in this phenotype of cell lines through augmentation of LPS uptake and expression of MD-2 mRNA and intracellular TLR4 proteins.
Thus, the Th1 proinflammatory cytokine IFN-γ can modulate LPS responsiveness in intestinal epithelial cells through several mechanisms. These results provide insights into the mechanisms of LPS recognition in intestinal epithelial cells and into the processes through which Th1 cytokine affects LPS responsiveness in acute and chronic mucosal inflammation.
Acknowledgments
We thank R. Medzhitov (Yale University, New Haven, Conn.) for providing pCMV10FLAG-hTLR4.
This study was supported by grants from the National Institutes of Health (DK60049 and DK43351).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abreu, M. T., E. T. Arnold, L. S. Thomas, R. Gonsky, Y. Zhou, B. Hu, and M. Arditi. 2002. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J. Biol. Chem. 277:20431-20437. [DOI] [PubMed] [Google Scholar]
- 2.Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 167:1609-1616. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann-Leitner, E. S., and S. I. Abrams. 2000. Influence of interferon gamma on modulation of Fas expression by human colon carcinoma cells and their subsequent sensitivity to antigen-specific CD8+ cytotoxic T lymphocyte attack. Cancer Immunol. Immunother. 49:193-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boman, H. G. 2000. Innate immunity and the normal microflora. Immunol. Rev. 173:5-16. [DOI] [PubMed] [Google Scholar]
- 5.Cario, E., I. M. Rosenberg, S. L. Brandwein, P. L. Beck, H. C. Reinecker, and D. K. Podolsky. 2000. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164:966-972. [DOI] [PubMed] [Google Scholar]
- 6.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 7.Cong, Y., S. L. Brandwein, R. P. McCabe, A. Lazenby, E. H. Birkenmeier, J. P. Sundberg, and C. O. Elson. 1998. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J. Exp. Med. 187:855-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeWitt, R. C., and K. A. Kudsk. 1999. The gut's role in metabolism, mucosal barrier function, and gut immunology. Infect. Dis. Clin. North Am. 13:465-481, x. [DOI] [PubMed]
- 9.Dziarski, R., Q. Wang, K. Miyake, C. J. Kirschning, and D. Gupta. 2001. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to gram-positive and gram-negative bacteria and their cell wall components. J. Immunol. 166:1938-1944. [DOI] [PubMed] [Google Scholar]
- 10.Fais, S., M. R. Capobianchi, F. Pallone, P. Di Marco, M. Boirivant, F. Dianzani, and A. Torsoli. 1991. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. Kinetics of in vitro response to interferon gamma inducers. Gut 32:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Phipott. 2001. CARD4/NOD1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Hisamatsu, T., M. Suzuki, H. C. Reinecker, W. J. Nadeau, B. A. McCormick, and D. K. Podolsky 2003. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 124:993-1000. [DOI] [PubMed]
- 12.Hornef, M. W., T. Frisan, A. Vandewalle, S. Normark, and A. Richter-Dahlfors. 2002. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 195:559-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 14.Inohara, N., T. Koseki, L. del Peso, Y. Hu, C. Yee, S. Chen, R. Carrio, J. Merino, D. Liu, J. Ni, and G. Nunez. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274:14560-14567. [DOI] [PubMed] [Google Scholar]
- 15.Izzo, R. S., K. Witkon, A. I. Chen, C. Hadjiyane, M. I. Weinstein, and C. Pellecchia. 1993. Neutrophil-activating peptide (interleukin-8) in colonic mucosa from patients with Crohn's disease. Scand. J. Gastroenterol. 28:296-300. [DOI] [PubMed] [Google Scholar]
- 16.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 18.Kim, J. M., L. Eckmann, T. C. Savidge, D. C. Lowe, T. Witthoft, and M. F. Kagnoff. 1998. Apoptosis of human intestinal epithelial cells after bacterial invasion. J. Clin. Investig. 102:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitchens, R. L., P. Wang, and R. S. Munford. 1998. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J. Immunol. 161:5534-5545. [PubMed] [Google Scholar]
- 20.La, J., and J. C. Hua. 1995. Production of soluble HLA-class-I molecules by IFN-γ-induced colon-adenocarcinoma cells. Int. J. Cancer 60:576-581. [DOI] [PubMed] [Google Scholar]
- 21.Madara, J. L. 1997. Pathobiology of neutrophil interactions with intestinal epithelia. Aliment. Pharmacol. Ther. 11(Suppl. 3):57-62. [DOI] [PubMed] [Google Scholar]
- 22.Mashimo, H., D. C. Wu, D. K. Podolsky, and M. C. Fishman. 1996. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274:262-265. [DOI] [PubMed] [Google Scholar]
- 23.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Means, T. K., D. T. Golenbock, and M. J. Fenton. 2000. Structure and function of Toll-like receptor proteins. Life Sci. 68:241-258. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 26.Medzhitov, R., P. Preston-Hurlburt, and C. A. Janeway, Jr. 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388:394-397. [DOI] [PubMed] [Google Scholar]
- 27.Muzio, M., J. Ni, P. Feng, and V. M. Dixit. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278:1612-1615. [DOI] [PubMed] [Google Scholar]
- 28.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 29.Podolsky, D. K. 1991. Inflammatory bowel disease (1). N. Engl. J. Med. 325:928-937. [DOI] [PubMed] [Google Scholar]
- 30.Pollok, R. C., M. J. Farthing, M. Bajaja-Elliott, I. R. Sanderson, and V. McDonald. 2001. Interferon gamma induces enterocyte resistance against infection by intracellular Cryptoridium parvum. Gastroenterology 120:99-107. [DOI] [PubMed] [Google Scholar]
- 31.Pugin, J., C. C. Schurer-Maly, D. Leturcq, A. Moriarty, R. J. Ulevitch, and P. S. Tobias. 1993. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc. Natl. Acad. Sci. USA 90:2744-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randow, F., and B. Seed. 2001. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 3:891-896. [DOI] [PubMed] [Google Scholar]
- 33.Rembacken, B. J., A. M. Snelling, P. M. Hawkey, D. M. Chalmers, and A. T. Axon. 1999. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354:635-639. [DOI] [PubMed] [Google Scholar]
- 34.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A. C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 75:253-261. [DOI] [PubMed] [Google Scholar]
- 35.Sands, B. E., and D. K. Podolsky. 1996. The trefoil peptide family. Annu. Rev. Physiol. 58:253-273. [DOI] [PubMed] [Google Scholar]
- 36.Schiller, J. H., B. Storer, G. Bittner, and M. A. Horisberger. 1990. Characterization of the synergistic antiproliferative effects of interferon-gamma and tumor necrosis factor on human colon carcinoma cell lines. J. Interferon Res. 10:129-139. [DOI] [PubMed] [Google Scholar]
- 37.Schultz, M., and R. B. Sartor. 2000. Probiotics and inflammatory bowel diseases. Am. J. Gastroenterol. 95:S19-S21. [DOI] [PubMed] [Google Scholar]
- 38.Sellon, R. K., S. Tonkonogy, M. Schultz, L. A. Dieleman, W. Grenther, E. Balish, D. M. Rennick, and R. B. Sartor. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 66:5224-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutton, C. L., J. Kim, A. Yamane, H. Dalwadi, B. Wei, C. Landers, S. R. Targan, and J. Braun. 2000. Identification of a novel bacterial sequence associated with Crohn's disease. Gastroenterology 119:23-31. [DOI] [PubMed] [Google Scholar]
- 41.Thieblemont, N., and S. D. Wright. 1999. Transport of bacterial lipopolysaccharide to the Golgi apparatus. J. Exp. Med. 190:523-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright, S. D., R. A. Ramos, P. S. Tobias, R. J. Ulevitch, and J. C. Mathison. 1990. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249:1431-1433. [DOI] [PubMed] [Google Scholar]