Abstract
Sepsis induces an early inflammatory cascade initiated by the innate immune response. This often results in the development of multisystem organ failure. We examined the role of CD40, a costimulatory molecule that is integral in adaptive immunity, by using a murine model of polymicrobial sepsis. CD40 knockout (KO) mice had delayed death and improved survival after cecal ligation and puncture (CLP). In addition, they had less remote organ injury as manifested by reduced pulmonary capillary leakage. The improvements in survival and remote organ dysfunction in CD40 KO mice were associated with reduced interleukin-6 (IL-6) and IL-10 levels in serum and bronchoalveolar lavage fluid compared to the levels in wild-type (WT) controls. Furthermore, in contrast to WT mice, CD40 KO mice had no induction of the Th1 cytokines IL-12 and gamma interferon in serum or lungs after CLP. The alterations in cytokine production in CD40 KO mice were associated with similar changes in transcription factor activity. After CLP, CD40 KO mice had attenuated activation of nuclear factor κB and signal transducer and activator of transcription 3 in both the lung and the liver. Finally, WT mice had increased expression of CD40 on their alveolar macrophages. These data highlight the importance of CD40 activation in the innate immune response during polymicrobial sepsis and the subsequent development of remote organ dysfunction.
Sepsis and septic shock affect nearly 500,000 people per year, and the mortality rate is approximately 35 to 45% (43). Mortality in sepsis is frequently the result of the development of multisystem organ failure, including acute respiratory distress syndrome and acute lung injury (ALI) (42, 43).
The development of multisystem organ failure is associated with increased production of proinflammatory cytokines in both serum and bronchoalveolar lavage fluid (BALF), including increased production of interleukin-6 (IL-6), IL-1β, and tumor necrosis factor alpha (TNF-α) (28, 29). However, immunomodulatory therapy directed at inhibition of these cytokines has been largely unsuccessful (1, 31). One explanation for the failures is the redundancy in the action of these cytokines. Consequently, investigators are now focusing on upstream mediators capable of controlling numerous proinflammatory pathways (9, 12, 15, 26).
Production of proinflammatory cytokines in the early phases of sepsis-induced ALI (in the first 24 to 48 h) is controlled in part by the innate immune response (7). Prior to the development of either cellular or humoral immunity, mediators in the innate immune response, such as Toll-like receptor 4 (TLR4), are capable of binding bacterial products. This results in cellular activation, including activation of nuclear factor κB (NF-κB) and production of IL-6, IL-12, TNF-α, and IL-1β (25, 46, 48). However, TLR4 knockout (KO) mice, while protected from endotoxemia, have no survival advantage in murine models of polymicrobial sepsis, suggesting that additional receptors play an important role in the innate immune response during sepsis (12).
CD40 (tumor necrosis factor receptor superfamily member 5) is a 48-kDa protein expressed primarily on B cells, macrophages, dendritic cells, vascular endothelial cells, and fibroblasts (39). CD40 expression is regulated at the transcriptional level, and both signal transducer and activator of transcription 1 (STAT-1) and NF-κB are capable of increasing expression (30, 38). CD40 is classically activated by binding to CD154 (a CD40 ligand) present on the surfaces of T cells, macrophages, or platelets (18, 39). Activation of CD40 results in induction of a number of transcription factors, including NF-κB and STAT-3 (4, 14). This results in induction of numerous cytokines, including IL-6 and IL-10 (11, 13, 22). CD40 is also a potent inducer of T-helper type 1 (Th1) cytokines. This is accomplished through stimulation of IL-12 production, which in turn drives production of gamma interferon (IFN-γ) (21, 27).
Based on these observations, investigators have focused on the role of CD40 in the development of a Th1 response in diseases such as tuberculosis and in immunologic disorders, including chronic transplant rejection and systemic lupus erythematosis (17, 23, 47). The role of CD40 in acute inflammatory disorders is less well characterized. CD40 plays a prominent role in intracellular infection models, such as Trypanazoma cruzi infection, toxoplasmosis, and malaria, specifically in controlling the Th1 cytokine response (10, 33, 39). However, the role of CD40 in acute pneumococcal pneumonia, caused by a common extracellular bacterial pathogen, appears to be limited to the generation of appropriate humoral immunity with no effect on the innate immune response (20).
The ability of CD40 to regulate inflammation and specifically its ability to control multiple mediators integral to the host inflammatory response, such as IL-12, IL-6, and NF-κB, suggest that CD40 plays a prominent early role in polymicrobial sepsis. Consequently, we investigated whether CD40 KO mice have an attenuated response to sepsis and induction of remote organ dysfunction, as manifested by measurement of mortality, capillary leakage, transcription factor regulation, and proinflammatory cytokine production.
MATERIALS AND METHODS
Mice.
Female CD40 KO mice (Tnfrsf5tmlKik) and appropriate strain controls (wild-type [WT] C57BL/6) were purchased from Jackson Laboratories (Bar Harbor, Maine). The mice were allowed free access to food and water and were maintained on 12-h light-dark cycle in accordance with animal care guidelines. The animal use committee of New York University approved all studies.
CLP.
Mice were acclimatized for 5 to 7 days prior to use. Cecal ligation and puncture (CLP) was performed by using a modification of the procedure originally described by Wichterman et al. (44). Briefly, mice were anesthetized with 2% isoflourane, and a 1- to 1.5-cm midline laparotomy was performed. The cecum was ligated and punctured once through and through with a 19-gauge needle. The cecum was replaced, and the abdomen was closed with 4-0 silk sutures. All mice received 1 ml of warm 0.9% saline and were allowed free access to food and water postoperatively. Sham-operated mice underwent an identical procedure without ligation of the cecum or puncture.
Bronchoalveolar lavage and lung interstitial cell isolation.
After euthanasia with CO2 and cervical dislocation, the lungs were lavaged with three 1-ml aliquots of cold phosphate-buffered saline (PBS). BALF was centrifuged, and supernatants were divided into aliquots and stored at −70°C until they were analyzed. Pelleted cells were then used for flow cytometry (see below).
Interstitial cells were isolated as previously described (32). Briefly, the pulmonary circulation was flushed via the right ventricle with PBS, and the lungs were excised en bloc, minced, and placed into RPMI 1640 containing 20 U of type IV collagenase per ml, 50 μM type I DNase, and 50 μM β-mercaptoethanol (Sigma, St. Louis Mo.). Samples were disrupted by passage through a 21-gauge needle. After centrifugation at 1,000 × g for 15 min at 4°C, the cell pellet was removed and resuspended in RPMI 1640. The remaining cells were harvested, and whole-cell extracts were obtained by NP-40 extraction as described below.
Livers were flushed in situ with PBS via the right ventricle. Each liver was removed, snap frozen in liquid nitrogen, and stored at −70°C until it was used.
Peritoneal macrophage isolation.
Female C57BL/6 mice or CD40 KO mice were injected with 3 ml of 3% thioglycolate (Sigma) intraperitoneally. Peritoneal macrophages were harvested by peritoneal lavage 72 h later. The cells were resuspended in RPMI 1640 (Bio-Whittaker, Walkersville, Md.) with 10% fetal calf serum. The cells were plated in 96-well plates and incubated for 24 h with either saline or 100 ng of lipopolysaccharide (LPS) (Sigma) per ml. Cell culture supernatants were collected at stored at −70°C until further analysis.
Cytokine analysis.
IL-6, IL-10, and IL-12 (p40) were assayed by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Minneapolis, Minn.). All experiments were performed in duplicate. Myeloperoxidase (MPO) activity was determined with liver homogenates by using a commercially available ELISA (Calbiochem, San Diego, Calif.).
Transcription factor analysis.
Lung interstitial cells or whole livers were incubated in NP-40 lysis buffer in PBS with protease inhibitors as previously described (19). An electrophoretic mobility shift assay (EMSA) for NF-κB was performed by using the probe 5′ TGGGCTGGGGAATCCCGCTAA 3′ as described previously (19). Specific competition analysis was performed with a 200-fold excess of unlabeled oligonucleotide. Supershift analyses for p65 and p50 fractions of NF-κB were performed with a specific antibody (Santa Cruz, Santa Cruz, Calif.). Nuclear extraction from lung interstitial cells and immunoblotting of NF-κB were performed as previously described (19).
Immunoblotting for Tyr-phosphorylated STAT-3 and total STAT-3 was performed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis by utilizing a 10% polyacrylamide gel. All lanes were normalized for protein content with 30 to 50 μg of protein/lane. Membranes were probed with anti-phospho-STAT-3 or anti-STAT-3 (Cell Signal Technology, Beverly, Mass.). Immunoblotting for C/EBPβ was performed as previously described (19). Membranes were developed by using the ECL Plus detection system (Amersham, Piscataway, N.J.).
Evans blue permeability.
Evans blue permeability was determined by using a modification of the procedure described previously (16). Briefly, mice were injected with 160 mg of Evans blue dye (dissolved in PBS; Sigma) per kg. After 1 h the blood and lungs of the mice were collected. The lungs were homogenized in PBS and 2 volumes of formamide (Sigma) and incubated for 18 h at 60°C. Homogenates were centrifuged at 5,000 × g for 30 min, and the supernatants were removed for Evans blue concentration. The Evans blue concentration was determined spectrophotometrically by using the corrected absorbance at 620 nm (A620corr). A620corr was calculated by using the following formula: A620corr = A620 − (1.436 × A740 + 0.03).
The degree of capillary leakage was defined as the ratio of the amount of Evans blue in lung homogenate to the amount of Evans blue in serum.
Immunofluorescent labeling and flow cytometry.
BALF cells were incubated with 0.8% NH4Cl at 4°C for 7 to 10 min to remove erythrocytes and then centrifuged in the cold at 750 × g for 10 min. Prechilled cell dissociation buffer (Sigma) containing 5% fetal calf serum was added to the pellets, and the cells were resuspended to a concentration of 1 × 106 to 2 × 106 cells/ml.
BALF cell suspensions were incubated with a pretitrated mixture of unconjugated and phycoerythrin-conjugated anti-CD16/CD32 (clone 2.4G2) on ice for 15 min to block nonspecific FcR-mediated binding of monoclonal antibodies, after which a cocktail containing allophycocyanin-conjugated anti-CD45 (clone 30-F11) and fluorescein isothiocyanate-conjugated anti-CD40 (clone HM40-3) was added. Irrelevant monoclonal antibodies conjugated to the same fluorophores were used to determine nonspecific cell surface binding. All monoclonal antibodies were purchased from BD Pharmingen (San Diego, Calif.). Following 20 min of incubation on ice, BALF cells were washed with cold PBS and fixed with 1% formaldehyde in DPBS at 4°C. Labeled cells were analyzed with a Becton Dickinson FACSCalibur used according to the manufacturer's specifications. Data were acquired in the list mode and were subsequently processed by using CellQuest software. Forward-scattered light (size) and 900 angle-scattered light (granularity) intensities at 488 nm were used to exclude debris and select for alveolar macrophages. The mean fluorescein isothiocyanate intensity per cell was determined for ≥5,000 allophycocyanin-phycoerythrin events within the alveolar macrophage-size gate in each specimen.
Histology.
After euthanasia, lungs were insufflated via tracheal cannulation with 1 ml of 1% low-melting-point agarose (Sigma). The lungs were removed en bloc and fixed in 10% formalin. Five-micrometer sections were obtained and stained with hematoxylin and eosin. Five random high-power fields were assessed for interstitial cellularity, hemorrhaging, and fibrin deposition and were graded by using a scale from 0 (no injury) to 3 (severe injury). All slides were reviewed by a pathologist blinded to the source of the samples.
Statistics.
All numerical data were expressed as means ± standard errors of the means. P values were derived from a two-tailed Mann-Whitney test or a log-rank test for survival analysis by utilizing the Graphpad Prism statistical software (Graphpad, San Diego, Calif.).
RESULTS
CD40 KO mice had attenuated mortality and lung injury after CLP.
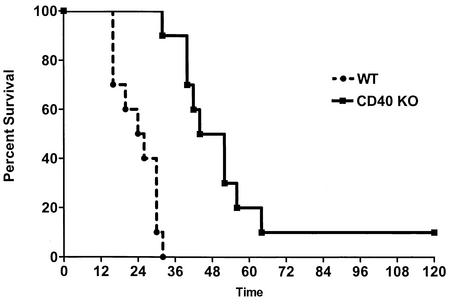
Like other workers, we used a large-gauge needle (19 gauge) for CLP to induce overwhelming sepsis (40). We observed that WT mice were susceptible to CLP; the mean survival time was 24 h, and 100% mortality occurred by 32 h. In contrast, CD40 KO mice were protected from overwhelming sepsis, had a mean survival time of 48 h, and exhibited 90% mortality at 5 days (Fig. 1). The difference was highly statistically significant (P < 0.0001). Sham-operated mice had a normal appearance and no mortality, as did controls that were not operated on.
FIG. 1.
CD40 KO mice have improved survival after CLP. WT or CD40 KO mice were subjected to CLP and were monitored for survival for 5 days. Time zero represents the time of surgery. When compared to the WT, the P value was <0.0001 (10 mice per group).
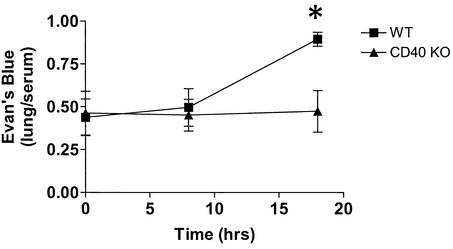
We used albumin leakage in the lungs as evidence of early acute lung injury and thus evidence of remote organ dysfunction. Evans blue dye extravasation is frequently used to quantify the degree of albumin leakage as this dye binds to albumin with the same affinity as I131 (16, 35). At 8 h, there were slight, statistically insignificant increases in Evans blue accumulation in the lungs of WT mice compared to the accumulation in the lungs of CD40 KO mice (Fig. 2). By 18 h, when the survival curves began to separate (71% survival for WT mice versus 100% survival for CD40 KO mice), the WT mice exhibited increased pulmonary capillary albumin leakage after CLP compared to the leakage in controls that were not operated on (0.89 ± 0.13 versus 0.41 ± 0.13; P = 0.006). In contrast, Evans blue accumulation in CD40 KO mice subjected to CLP did not differ from Evans blue accumulation in mice that were not operated on, and CD40 KO mice subjected to CLP exhibited marked attenuation in albumin leakage in the lungs compared to the leakage in WT mice (0.47 ± 0.12 versus 0.89 ± 0.13; P < 0.02) (Fig. 2).
FIG. 2.
CD40 KO mice have attenuated Evans blue extravasation in the lung compared to that of WT mice after CLP. The WT (▪) or CD40 KO (▴) mice were subjected to CLP or a sham operation. The mice were given 160 mg of Evans blue per kg, and the lung/serum Evans blue ratio was calculated. The WT mice had a significant greater lung/serum Evans blue ratio than the CD40 KO mice at 18 h. An asterisk indicates that the P value is 0.02. There were five to eight mice per group.
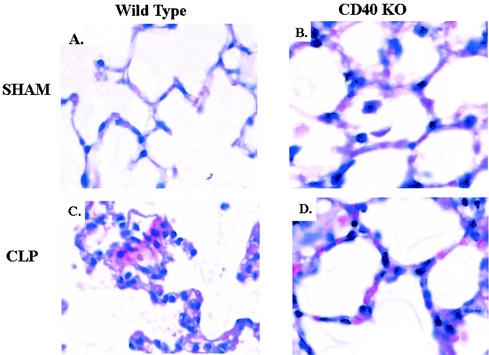
We next investigated whether the changes in albumin leakage were associated with pathological lung changes. Compared to the results for sham-operated mice, mice subjected to CLP exhibited markedly increased interstitial cellularity, increased fibrin deposition, and marked interstitial thickening (Fig. 3A and C). The differences were statistically significant when the results were graded by performing a blinded pathological assessment (Table 1). In contrast, CD40 KO mice subjected to CLP exhibited reductions in both interstitial cellularity and fibrin deposition compared to the results for WT mice (Fig. 3C and D). The differences were statistically significant when the data were quantified by blinded assessment (Table 1). In addition, there was no statistical difference for either measurement between CD40 KO mice subjected to CLP and controls that were not operated on.
FIG. 3.
CD40 KO mice have attenuation in histologic lung injuries compared to the injuries in WT mice after CLP. CD40 KO or WT mice were subjected to CLP or a sham operation. At 18 h, lungs were fixed and stained with hematoxylin and eosin. Magnification, ×40. (A) WT mouse after sham operation. (B) CD40 KO mouse after sham operation. (C) WT mouse after CLP. (D) CD40 KO mouse after CLP.
TABLE 1.
Blinded pathological assessment of five random high-power fields graded on a scale from 0 to 3 (no damage to maximal injury)
| Treatment | Interstitial cellularity | Hemorrhage | Fibrin deposition |
|---|---|---|---|
| WT CLP | 1.33 ± 0.16 | 0.73 ± 0.18 | 0.6 ± 0.13 |
| CD40 KO CLPa | 0.87 ± 0.17b | 0.47 ± 0.17 | 0.2 ± 0.11c |
CD40 KO mice exhibited reduced interstitial cellularity and fibrin deposition compared to the interstitial cellularity and fibrin deposition of WT mice after CLP.
P = 0.05.
P = 0.02.
Finally, we used MPO to specifically assay for inflammation in the liver. CD40 KO mice had reduced MPO levels in the liver compared to the levels in WT mice after CLP (233 ± 23 versus 327 ± 23 μg/ml; P = 0.03; five mice per group). Together, these data demonstrate that CD40 KO mice are protected from lethality and the development of multisystem organ failure after CLP.
CD40 KO mice had attenuated production of inflammatory cytokines during polymicrobial sepsis.
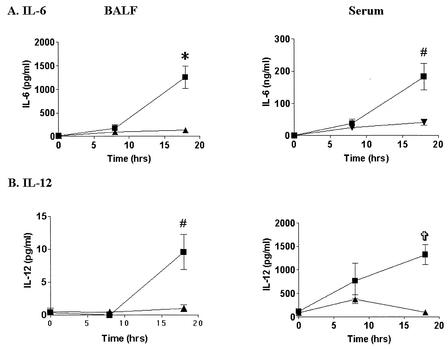
To examine the mechanism of protection of CD40 KO mice from CLP, we measured BALF and serum levels of IL-6 and IL-12. Eight hours after CLP, WT mice exhibited significantly higher levels of BALF IL-6 (171 ± 81 versus 8.5 ± 4 pg/ml; P = 0.004) and serum IL-6 (37.5 ± 14 versus 0.11 ± .01 ng/ml; P = 0.01) and IL-12 (767 ± 137 versus 113 ± 5 pg/ml; P = 0.08) than the controls (Fig. 4). There were no differences in the cytokine levels in BALF or serum between controls that were not operated on and sham-operated controls (data not shown). In contrast, CD40 KO mice had reduced production of both cytokines at 8 h, although the differences were not statistically significant. By 18 h, WT mice exhibited a continued increase in the IL-6 and IL-12 levels in BALF compared to the levels in sham-operated mice (Fig. 4). In contrast, CD40 KO mice exhibited marked reductions in the levels of BALF and serum IL-6 compared to the levels in WT mice (Fig. 4A). The results were even more impressive with IL-12, as CD40 KO mice had only minimal induction in the BALF or serum (Fig. 4B). However, LPS-stimulated peritoneal or alveolar macrophages from CD40 KO mice exhibited induction of IL-12 similar to that exhibited by macrophages from WT mice (512 ± 52 versus 602 ± 52 pg/ml; n = 4), implying that macrophages from CD40 KO mice are capable of normal IL-12 production in response to other stimuli of the innate immune response.
FIG. 4.
CD40 KO mice have attenuated production of IL-6 and IL-12 in BALF and serum compared to the production in WT mice after CLP. WT (▪) or CD40 KO (▴) mice were subjected to CLP or a sham operation. BALF and serum were collected at 18 h. Cytokines were assayed by ELISA. (A) IL-6 induction was attenuated in BALF and serum of CD40 KO mice compared with the induction in WT mice 18 h after CLP. (B) IL-12 p40 induction was attenuated in serum in CD40 KO mice compared with the induction in WT mice 18 h after CLP. An asterisk indicates that the P value is 0.0001; a number sign indicates that the P value is 0.005; and a cross indicates that the P value is 0.001. There were 7 to 11 mice per group.
We then investigated whether the attenuation of cytokine production was associated with increased production of IL-10. Similar to the IL-6 results, CD40 KO mice exhibited significant reductions in IL-10 production in BALF (31 ± 9.4 versus 144 ± 44 pg/ml; P = 0.008) and serum (4.6 ± 1.6 versus 13.8 ± 2.8 ng/ml; P = 0.02) 18 h after CLP.
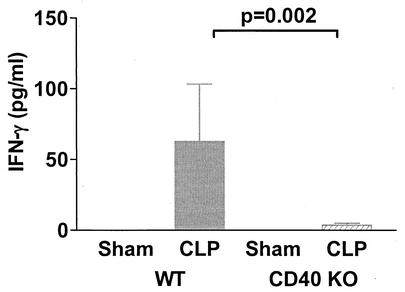
Finally, we sought to determine whether the impairment of IL-12 production in CD40 KO mice was associated with a similar reduction in IFN-γ production. There were no detectable levels of IFN-γ in BALF from either WT or CD40 KO mice before or after CLP (data not shown). However, CD40 KO mice subjected to CLP exhibited significant attenuation of serum IFN-γ levels compared to the levels in WT mice at 18 h (Fig. 5). There was no detectable difference in IFN-γ levels between CLP and sham-operated CD40 KO mice.
FIG. 5.
CD40 KO mice have attenuated serum IFN-γ levels after CLP. WT or CD40 KO mice were subjected to CLP, and serum was collected at 18 h for analysis by ELISA. CD40 KO mice had marked attenuation of IFN-γ induction after CLP compared to the induction in WT mice. There were approximately five to seven mice per group.
CD40 KO mice had altered transcription factor activity after CLP.
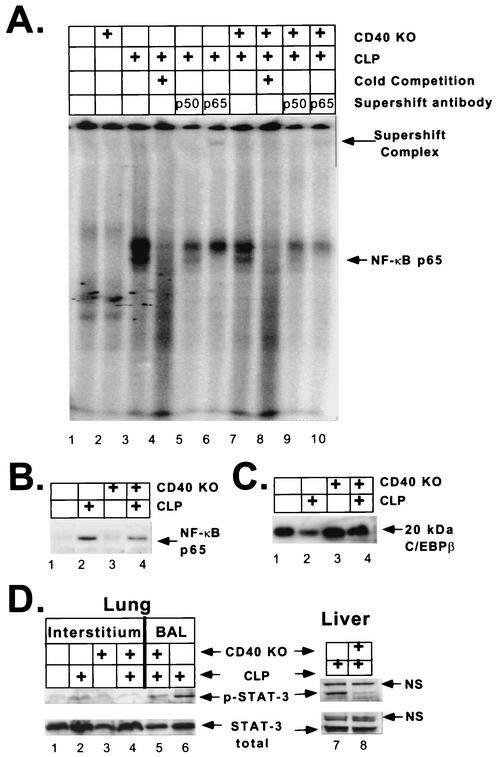
We next sought to determine whether changes in proinflammatory cytokine production after CLP was associated with altered transcription factor activity. To do this, we used NF-κB and STAT-3 as they are both regulated by CD40 activation (4, 39, 48). Furthermore, along with C/EBPβ (NF-IL-6), they are both involved in the transcriptional control of IL-6 and IL-10 (24, 37, 46, 48). After CLP, WT mice exhibited an increase in NF-κB DNA binding activity in lung interstitial cells compared to the activity in sham-operated controls (Fig. 6A, compare lanes 1 and 3). In contrast, CD40 KO mice subjected to CLP had attenuated NF-κB DNA binding activity compared to the activity of WT mice (Fig. 6A, compare lanes 3 and 7). The specificity of the DNA binding complexes was confirmed by specific competition with excess unlabeled oligonucleotide (Fig. 6A, compare lanes 3 and 4). The faster-migrating complex (lower band) was determined to be an NF-κB-containing complex by a supershift analysis with anti-p65 antibodies (Fig. 6A, compare lane 3 with lanes 5 and 6). The more slowly migrating complex (upper band) did not supershift with either anti-p50 or anti-p65 antibody (Fig. 6A, compare lane 3 with lanes 5 and 6). Phosphorimager analysis confirmed that there was a quantitative twofold reduction in NF-κB DNA binding activity in CD40 KO mice compared to the activity in WT mice after CLP. Whole-liver homogenates produced a similar pattern, indicating that the effects were systemic and not localized to the lung (data not shown). To confirm the results obtained with the EMSA, we obtained nuclear extracts from lung interstitial cells in an independent experiment. Immunoblotting for the p65 subunit of NF-κB showed that there was nuclear translocation after CLP in WT mice (Fig. 6B, lanes 1 and 2). Similar to the EMSA results, CD40 KO mice had a twofold reduction in p65 nuclear translocation compared to the translocation in WT mice (Fig. 6B, compare lanes 2 and 4). To asses whether the changes in NF-κB were due to degradation of transcription factors in the samples, the same interstitial cell extracts that were used in the experiment whose results are shown in Fig. 6A were assayed for C/EBPβ expression. Lung interstitial cells from septic WT mice exhibited downregulation of the 20-kDa isoform of C/EBPβ (Fig. 6C, lanes 1 and 2). In contrast to the NF-κB results, septic CD40 KO mice had larger amounts of the 20-kDa isoform of C/EBPβ (Fig. 6C, lanes 2 and 4). This suggests that the reduction in the amount of NF-κB in CD40 KO mice was not due to nonspecific degradation of transcription factors.
FIG. 6.
CD40 KO mice have attenuated transcription factor activity in lung interstitial cells compared to the activity in WT mice after CLP. WT or CD40 KO mice were subjected to CLP or a sham operation. (A) Lung interstitial cells were harvested, and the whole-cell extract was assayed for NF-κB by EMSA. WT and CD40 KO mice exhibited no NF-κB activity at the baseline (lanes 1 and 2). WT mice exhibited significant induction of NF-κB after CLP, which was reduced in CD40 KO mice (lanes 3 and 7). The specificity of the DNA binding complexes was confirmed by specific competition with excess unlabeled oligonucleotide (lanes 3 and 4). The lower band was determined to be an NF-κB-containing complex by supershift analysis with either anti-p65 or anti-p50 antibodies (compare lane 3 with lanes 5 and 6). All lanes contained pooled samples from two or three mice, and samples were normalized for protein. (B) Nuclear extracts from lung interstitial cells were assayed for the p65 subunit of NF-kB by immunoblotting. WT mice showed upregulation of p65 nuclear translocation after CLP (lanes 1 and 2). This was attenuated approximately twofold in CD40 KO mice (lanes 2 and 4). (C) Whole-cell extracts from lung interstitial cells were assayed for C/EBPβ by immunoblotting. WT mice exhibited a loss of C/EBPβ after CLP compared to the amount of C/EBPβ in controls that were not operated on (lanes 1 and 2). In contrast, there was little change in C/EBPβ in CD40 KO mice after CLP (lanes 3 and 4). (D) Lung interstitial cells were assayed for phosphorylated and total STAT-3 by immunoblotting. CD40 KO mice had attenuated STAT-3 phosphorylation in lung interstitial cells compared to the phosphorylation in WT mice after CLP (upper band) (compare lanes 3 and 4), and there was little difference in the total STAT-3 (lower band). There was no difference in the STAT-3 levels in sham-operated controls (lanes 1 and 2). Similar results were obtained with BALF cells (lanes 5 and 6). Finally, similar results were obtained with liver homogenates (lanes 7 and 8). Furthermore, the appearance of a nonspecific band (NS) provided an additional control for protein loading. In all cases, lanes were normalized for total protein. Lung interstitial cells from three to five mice were pooled.
Finally, we investigated the role of STAT-3. Like other members of the STAT family, STAT-3 is activated by phosphorylation and thus is capable of DNA binding (24). Figure 6D shows that WT mice had increased STAT-3 phosphorylation in NP-40 extracts from lung interstitial cells after CLP (lanes 1 and 2). Similar results were obtained with an EMSA (data not shown). CD40 KO mice exhibited a twofold reduction in STAT-3 phosphorylation compared to the STAT-3 phosphorylation in WT mice in lung interstitial cells (lanes 2 and 4), in BALF cells (lanes 6 and 5), and in livers (lanes 7 and 8). The total STAT-3 was not appreciably changed in any of the lanes, suggesting that the changes were due solely to alterations in STAT-3 phosphorylation. Additionally, the liver extracts produced a nonspecific band with both phospho-STAT-3 and total-STAT-3 antibodies. This nonspecific band was the same in all lanes, further demonstrating that the changes were not due to uneven loading of lanes. These data suggest that CD40 activation is involved in STAT-3 phosphorylation during polymicrobial sepsis.
CD40 was upregulated in alveolar macrophages after CLP.
Multiple inflammatory disorders of the lung dependent upon generation of an adaptive immune response are associated with increased expression of CD40. Therefore, we examined whether CD40 expression in BALF cells was altered in the CLP model of sepsis-induced ALI. Flow cytometric analyses of BALF cells 18 h after CLP in WT mice revealed that there was a twofold increase in the surface density of CD40 on alveolar macrophages, as measured by the mean fluorescence intensity per cell compared to that of controls that were not operated on (62 ± 10 versus 138 ± 62; P = 0.05). In contrast, no corresponding changes were seen in the surface density of CD45, a nonspecific maker for myeloid cells, on the same population of BALF cells after CLP when these cells were compared to cells from controls that were not operated on (3,808 ± 110 versus 3,370 ± 100).
DISCUSSION
In this study, we found that CD40 KO mice had improved survival and a reduction in the development of remote organ failure as manifested by pulmonary albumin leakage after CLP. Improved survival was associated with reductions in IL-6 and IL-10 induction and elimination of significant IL-12 and IFN-γ production after CLP. This alteration in inflammatory cytokine production was associated with reductions in both lung and hepatic NF-κB and STAT-3 activities. Finally, alveolar macrophages exhibited twofold upregulation of CD40 18 h after CLP.
The attenuation of the septic response in CD40 KO mice is surprising. Inhibition of most members of the TNF superfamily often results in increased mortality in polymicrobial sepsis (34). Furthermore, the early phases of septic shock and ALI examined by the protocol which we used are governed primarily by the innate immune response, an arm of the immune response not previously ascribed to CD40 in vivo (6, 7, 20). Numerous activators for the innate immune response in sepsis are being investigated, including TLR4, which is associated with LPS responsiveness and increased production of IL-6, TNF-α, and IL-12 (2, 25). However, TLR4 KO mice are not protected from the lethality of polymicrobial sepsis, suggesting that additional receptors play a role in the innate immune response (12). Since CD40-deficient macrophages respond normally to LPS, TLR4 signal transduction is likely intact. Therefore, our data suggest that CD40 is an important part of this early innate response operating in parallel with the TLR system.
CD40 is a potent activator of NF-κB (39, 48) and is induced by NF-κB, which likely leads to the observed increase in CD40 expression on alveolar macrophages after CLP (38). The improved survival in CD40 KO mice was associated with a twofold reduction in NF-κB DNA binding and nuclear translocation activity after CLP. Altered regulation of NF-κB may explain the improved survival of CD40 KO mice after CLP since NF-κB activation correlates with mortality and the degree of injury during sepsis (8, 46).
STAT-3 is another transcription factor involved in the innate immune response during polymicrobial sepsis. Like other workers, we documented increased STAT-3 activation in the lung and liver after CLP (3). In contrast, CD40 KO mice had persistently lower levels of STAT-3 activation than WT mice. This is in contrast to the findings of other workers, who observed that lethality after CLP is associated with a loss of hepatic STAT-3 activity (3). One explanation for the discrepancy is that the reduction in STAT-3 activity in CD40 KO mice correlates with an overall reduction in the host inflammatory response and specifically with reductions in both pro-and anti-inflammatory pathways.
CD40 KO mice also have reduced IL-6 production after CLP. Increased mortality in sepsis is associated with increased circulating levels of IL-6 (28, 29). There are many possible explanations for the deleterious effects of IL-6, including increased activation of resident macrophages and increasing local neutrophil influx. This may in part explain the reduction in the amount of hepatic MPO observed in CD40 KO mice. CD40 is a known inducer of IL-6; however, the role of this pathway in sepsis is unknown (22). The reduction in the amount of IL-6 is not due to increased production of IL-10 as CD40 KO mice exhibited a similar reduction in the amount of IL-10.
CD40 KO mice also exhibit markedly reduced IL-12 and IFN-γ production after CLP. However, the ability of macrophages from CD40 KO mice to produce normal amounts of IL-12 in response to LPS indicates that the in vivo observations are not the result of nonspecific impairment of IL-12 generation. The role of Th1 cytokines in the innate immune response to polymicrobial sepsis is the subject of much debate. IL-12 levels increase during polymicrobial sepsis, and this stimulates IFN-γ production (36, 49). The consequences of increased production of Th1 cytokines in sepsis are unclear, as harmful and beneficial effects have been documented depending on the model and time course evaluated (36, 49). CD40 activation is a potent inducer of IL-12 in vitro in both macrophages and B cells (27). In intracellular infection models, in which Th1 cell-mediated immunity is critical, failure to activate CD40 results in submaximal induction of both IL-12 and IFN-γ (10). Therefore, our data suggest that CD40 is vital to the initiation of Th1 cytokine production during early phases of sepsis.
The mechanism of CD40 activation in our model remains unclear. There are multiple potential activators of CD40 during sepsis. The most common activator of CD40 is CD154, either in soluble form or as a cell surface molecule (18, 39, 45). Another possibility is that CD40 is an innate immune receptor which senses bacterial proteins, similar to TLR2 and TLR4. This possibility is supported by the observation that Escherichia coli and mycobacterial HSP-70 bind directly to CD40 and lead to chemokine production (41). These hypotheses are not mutually exclusive and suggest that CD40 may be a bridge between the innate and adaptive immune responses.
In conclusion, our data support the hypothesis that CD40 plays an essential role in the innate immune response during polymicrobial sepsis. Upstream control of numerous proinflammatory pathways, including NF-κB, and control of proinflammatory cytokines, especially those traditionally part of a Th1 pathway, appear to be central to this effect. These findings combined with the role of CD40 in the host response and CD40-induced cytokine expression suggest that CD40 modulation may provide a novel therapeutic opportunity for treatment of sepsis-induced ALI.
Acknowledgments
We acknowledge Elbert Ching for his technical support.
This work was supported by the Parker B. Francis Foundation, by grants NIH K08-070710 and NIH NCRR GCRC MO1 RR0096 from the National Institutes of Health, by an ALA career investigator award, by grants RO1 HL57879 and AI44729, and by the Center for AIDS Research.
Editor: A. D. O'Brien
REFERENCES
- 1.Abraham, E., A. Anzueto, G. Gutierrez, S. Tessler, G. San Pedro, R. Wunderink, A. Dal Nogare, S. Nasraway, S. Berman, R. Cooney, H. Levy, R. Baughman, M. Rumbak, R. B. Light, L. Poole, R. Allred, J. Constant, J. Pennington, and S. Porter. 1998. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet 351:929-933. [PubMed] [Google Scholar]
- 2.Adachi, K., H. Tsutsui, S. Kashiwamura, E. Seki, H. Nakano, O. Takeuchi, K. Takeda, K. Okumura, L. Van Kaer, H. Okamura, S. Akira, and K. Nakanishi. 2001. Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J. Immunol. 167:5928-5934. [DOI] [PubMed] [Google Scholar]
- 3.Andrejko, K. M., J. Chen, and C. S. Deutschman. 1998. Intrahepatic STAT-3 activation and acute phase gene expression predict outcome after CLP sepsis in the rat. Am. J. Physiol. 275:G1423-G1429. [DOI] [PubMed]
- 4.Arinobu, Y., R. Sugimoto, M. Akaiwa, K. Arima, T. Otsuka, N. Hamasaki, and K. Izuhara. 2000. Augmentation of signal transducer and activation of transcription (STAT)6 and STAT3 expression in stimulated B and T cells. Biochem. Biophys. Res. Commun. 277:317-324. [DOI] [PubMed] [Google Scholar]
- 5.Bernard, G. R., J. L. Vincent, P. F. Laterre, S. P. LaRosa, J. F. Dhainaut, A. Lopez-Rodriguez, J. S. Steingrub, G. E. Garber, J. D. Helterbrand, E. W. Ely, and C. J. Fisher, Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699-709. [DOI] [PubMed] [Google Scholar]
- 6.Beutler, B. 2000. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr. Opin. Microbiol. 3:23-28. [DOI] [PubMed] [Google Scholar]
- 7.Beutler, B., and A. Poltorak. 2001. Sepsis and evolution of the innate immune response. Crit. Care Med. 29:S2-S6. (Discussion, 29:S6-S7.) [DOI] [PubMed]
- 8.Bohrer, H., F. Qiu, T. Zimmermann, Y. Zhang, T. Jllmer, D. Mannel, B. W. Bottiger, D. M. Stern, R. Waldherr, H. D. Saeger, R. Ziegler, A. Bierhaus, E. Martin, and P. P. Nawroth. 1997. Role of NFkappaB in the mortality of sepsis. J. Clin. Investig. 100:972-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calandra, T., B. Echtenacher, D. L. Roy, J. Pugin, C. N. Metz, L. Hultner, D. Heumann, D. Mannel, R. Bucala, and M. P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164-170. [DOI] [PubMed] [Google Scholar]
- 10.Chaussabel, D., F. Jacobs, J. de Jonge, M. de Veerman, Y. Carlier, K. Thielemans, M. Goldman, and B. Vray. 1999. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation. Infect. Immun. 67:1929-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark, E. A., and G. Shu. 1990. Association between IL-6 and CD40 signaling. IL-6 induces phosphorylation of CD40 receptors. J. Immunol. 145:1400-1406. [PubMed] [Google Scholar]
- 12.Echtenacher, B., M. A. Freudenberg, R. S. Jack, and D. N. Mannel. 2001. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect. Immun. 69:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foey, A. D., M. Feldmann, and F. M. Brennan. 2000. Route of monocyte differentiation determines their cytokine production profile: CD40 ligation induces interleukin 10 expression. Cytokine 12:1496-1505. [DOI] [PubMed] [Google Scholar]
- 14.Francis, D. A., J. G. Karras, X. Y. Ke, R. Sen, and T. L. Rothstein. 1995. Induction of the transcription factors NF-kappa B, AP-1 and NF-AT during B cell stimulation through the CD40 receptor. Int. Immunol. 7:151-161. [DOI] [PubMed] [Google Scholar]
- 15.Gawaz, M., S. Fateh-Moghadam, G. Pilz, H. J. Gurland, and K. Werdan. 1995. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur. J. Clin. Investig. 25:843-851. [DOI] [PubMed] [Google Scholar]
- 16.Green, T. P., D. E. Johnson, R. P. Marchessault, and C. W. Gatto. 1988. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J. Lab. Clin. Med. 111:173-183. [PubMed] [Google Scholar]
- 17.Henderson, R. A., S. C. Watkins, and J. L. Flynn. 1997. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J. Immunol. 159:635-643. [PubMed] [Google Scholar]
- 18.Henn, V., J. R. Slupsky, M. Grafe, I. Anagnostopoulos, R. Forster, G. Muller-Berghaus, and R. A. Kroczek. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391:591-594. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino, Y., S. Hoshino, K. Nakata, Y. Honda, D. Tse, T. Shioda, W. N. Rom, and M. Weiden. 2002. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J. Exp. Med. 195:495-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang, Y., M. H. Nahm, D. E. Briles, D. Thomas, and J. M. Purkerson. 2000. Acquired, but not innate, immune responses to Streptococcus pneumoniae are compromised by neutralization of CD40L. Infect. Immun. 68:511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jyothi, M. D., and A. Khar. 2000. Regulation of CD40L expression on natural killer cells by interleukin-12 and interferon gamma: its role in the elicitation of an effective antitumor immune response. Cancer Immunol. Immunother. 49:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiener, P. A., P. Moran-Davis, B. M. Rankin, A. F. Wahl, A. Aruffo, and D. Hollenbaugh. 1995. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J. Immunol. 155:4917-4925. [PubMed] [Google Scholar]
- 23.Larsen, C. P., and T. C. Pearson. 1997. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr. Opin. Immunol. 9:641-647. [DOI] [PubMed] [Google Scholar]
- 24.Levy, D. E. 1999. Physiological significance of STAT proteins: investigations through gene disruption in vivo. Cell. Mol. Life Sci. 55:1559-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsukawa, A., M. H. Kaplan, C. M. Hogaboam, N. W. Lukacs, and S. L. Kunkel. 2001. Pivotal role of signal transducer and activator of transcription (Stat)4 and Stat6 in the innate immune response during sepsis. J. Exp. Med. 193:679-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDyer, J. F., T. J. Goletz, E. Thomas, C. H. June, and R. A. Seder. 1998. CD40 ligand/CD40 stimulation regulates the production of IFN-gamma from human peripheral blood mononuclear cells in an IL-12- and/or CD28- dependent manner. J. Immunol. 160:1701-1707. [PubMed] [Google Scholar]
- 28.Meduri, G. U., S. Headley, G. Kohler, F. Stentz, E. Tolley, R. Umberger, and K. Leeper. 1995. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107:1062-1073. [DOI] [PubMed] [Google Scholar]
- 29.Meduri, G. U., G. Kohler, S. Headley, E. Tolley, F. Stentz, and A. Postlethwaite. 1995. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest 108:1303-1314. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen, V. T., and E. N. Benveniste. 2000. Involvement of STAT-1 and ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. J. Biol. Chem. 275:23674-23684. [DOI] [PubMed] [Google Scholar]
- 31.Opal, S. M., C. J. Fisher, Jr., J. F. Dhainaut, J. L. Vincent, R. Brase, S. F. Lowry, J. C. Sadoff, G. J. Slotman, H. Levy, R. A. Balk, M. P. Shelly, J. P. Pribble, J. F. LaBrecque, J. Lookabaugh, H. Donovan, H. Dubin, R. Baughman, J. Norman, E. DeMaria, K. Matzel, E. Abraham, and M. Seneff. 1997. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 25:1115-1124. [DOI] [PubMed] [Google Scholar]
- 32.Parsey, M. V., R. M. Tuder, and E. Abraham. 1998. Neutrophils are major contributors to intraparenchymal lung IL-1 beta expression after hemorrhage and endotoxemia. J. Immunol. 160:1007-1013. [PubMed] [Google Scholar]
- 33.Piguet, P. F., C. Da Kan, C. Vesin, A. Rochat, Y. Donati, and C. Barazzone. 2001. Role of cd40-cd40l in mouse severe malaria. Am. J. Pathol. 159:733-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remick, D., P. Manohar, G. Bolgos, J. Rodriguez, L. Moldawer, and G. Wollenberg. 1995. Blockade of tumor necrosis factor reduces lipopolysaccharide lethality, but not the lethality of cecal ligation and puncture. Shock 4:89-95. [DOI] [PubMed] [Google Scholar]
- 35.Standiford, T. J., S. L. Kunkel, N. W. Lukacs, M. J. Greenberger, J. M. Danforth, R. G. Kunkel, and R. M. Strieter. 1995. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J. Immunol. 155:1515-1524. [PubMed] [Google Scholar]
- 36.Steinhauser, M. L., C. M. Hogaboam, N. W. Lukacs, R. M. Strieter, and S. L. Kunkel. 1999. Multiple roles for IL-12 in a model of acute septic peritonitis. J. Immunol. 162:5437-5443. [PubMed] [Google Scholar]
- 37.Takeda, K., B. E. Clausen, T. Kaisho, T. Tsujimura, N. Terada, I. Forster, and S. Akira. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10:39-49. [DOI] [PubMed] [Google Scholar]
- 38.Tone, M., Y. Tone, J. M. Babik, C. Y. Lin, and H. Waldmann. 2001. The role of Sp1 and NF-kB in regulating CD40 gene expression. J. Biol. Chem. 20:20.. [DOI] [PubMed] [Google Scholar]
- 39.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]
- 40.Walley, K. R., N. W. Lukacs, T. J. Standiford, R. M. Strieter, and S. L. Kunkel. 1996. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect. Immun. 64:4733-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang, Y., C. G. Kelly, J. T. Karttunen, T. Whittall, P. J. Lehner, L. Duncan, P. MacAry, J. S. Younson, M. Singh, W. Oehlmann, G. Cheng, L. Bergmeier, and T. Lehner. 2001. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity 15:971-983. [DOI] [PubMed] [Google Scholar]
- 42.Ware, L. B., and M. A. Matthay. 2000. The acute respiratory distress syndrome. N. Engl. J. Med. 342:1334-1349. [DOI] [PubMed] [Google Scholar]
- 43.Wheeler, A. P., and G. R. Bernard. 1999. Treating patients with severe sepsis. N. Engl J. Med. 340:207-214. [DOI] [PubMed] [Google Scholar]
- 44.Wichterman, K. A., A. E. Baue, and I. H. Chaudry. 1980. Sepsis and septic shock—a review of laboratory models and a proposal. J. Surg. Res. 29:189-201. [DOI] [PubMed] [Google Scholar]
- 45.Wiley, J. A., R. Geha, and A. G. Harmsen. 1997. Exogenous CD40 ligand induces a pulmonary inflammation response. J. Immunol. 158:2932-2938. [PubMed] [Google Scholar]
- 46.Williams, D. L., T. Ha, C. Li, J. H. Kalbfleisch, and D. A. Ferguson, Jr. 1999. Early activation of hepatic NFkappaB and NF-IL6 in polymicrobial sepsis correlates with bacteremia, cytokine expression, and mortality. Ann. Surg. 230:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yellin, M. J., V. D'Agati, G. Parkinson, A. S. Han, A. Szema, D. Baum, D. Estes, M. Szabolcs, and L. Chess. 1997. Immunohistologic analysis of renal CD40 and CD40L expression in lupus nephritis and other glomerulonephritides. Arthritis Rheum. 40:124-134. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimoto, T., H. Nagase, T. Ishida, J. Inoue, and H. Nariuchi. 1997. Induction of interleukin-12 p40 transcript by CD40 ligation via activation of nuclear factor-kappaB. Eur. J. Immunol. 27:3461-3470. [DOI] [PubMed] [Google Scholar]
- 49.Zisman, D. A., S. L. Kunkel, R. M. Strieter, J. Gauldie, W. C. Tsai, J. Bramson, J. M. Wilkowski, K. A. Bucknell, and T. J. Standiford. 1997. Anti-interleukin-12 therapy protects mice in lethal endotoxemia but impairs bacterial clearance in murine Escherichia coli peritoneal sepsis. Shock 8:349-356. [DOI] [PubMed] [Google Scholar]