Abstract
Yersinia enterocolitica is an invasive enteric pathogen that causes significant inflammatory disease. Recently, we identified and characterized a global regulator of virulence (rovA). When mice are infected orally with the rovA mutant they are attenuated by 50% lethal dose (LD50) analysis and have altered kinetics of infection. Most significantly, mice orally infected with the rovA mutant have greatly reduced inflammation in the Peyer's patches compared to those infected with wild-type Y. enterocolitica. However, we present data here indicating that when the rovA mutant bacteria are delivered intraperitoneally (i.p.), they are significantly more virulent than when delivered orally. The i.p. LD50 for the rovA mutant is only 10-fold higher than that of the wild-type Y. enterocolitica, and there are significant inflammatory responses to the rovA mutant that are evident in the liver and spleen. Altogether, these data suggest that the RovA regulon may be required for the early events of the infection that occur in the Peyer's patches. Furthermore, these data suggest that the RovA regulon may be dispensable for Y. enterocolitica systemic disease and inflammatory responses if the Peyer's patches are bypassed.
Yersinia enterocolitica is one of three species of Yersinia that are pathogenic for humans (7). The other two pathogenic species of Yersinia are Yersinia pseudotuberculosis and Yersinia pestis. Both Y. enterocolitica and Y. pseudotuberculosis are enteric pathogens, whereas Y. pestis is the causative agent of plague. Y. enterocolitica infection is usually the result of consuming contaminated food or water and usually results in a self-limiting infection that presents as gastroenteritis and/or lymphadenitis (26).
The initial site of Y. enterocolitica infection is the secondary lymphoid tissues of the small intestine called Peyer's patches (PP). To gain entry to the PP, the bacteria must first bind to and then cross the specialized intestinal epithelium (M cells) that overlay the PP. The binding and translocation event is mediated by the bacterial invasin protein (Inv) (14, 18). Inv is an outer membrane protein that binds to β1-integrins expressed on the apical surface of the M cell (9, 13, 15). Inv is required for the efficient translocation of the bacteria across the intestinal epithelium (22). In fact, when an inv mutant is examined in the mouse model of infection it has an altered pathogenesis phenotype compared to the wild-type (WT) bacteria (22). Interestingly, even though there is a delay in the colonization of the PP of mice infected with the inv mutant, there is no difference in the 50% lethal dose (LD50) (22).
When the expression characteristics of the Inv protein were examined in vitro it was found that expression was modulated by environmental factors such as temperature, pH, and growth phase (21). These data suggested that there was a regulator of Inv expression. Recently, we identified the Inv regulator and named it RovA (24). A homologue of RovA has been identified in Y. pseudotuberculosis and was shown to regulate the expression of inv in that organism as well (20). RovA is a member of the MarR family of transcriptional regulators and is responsible for the regulation of Inv expression both in vitro and in vivo (24). Subsequent analysis of the rovA mutant in the mouse provided several lines of evidence that suggested RovA plays a key role in the pathogenesis of a Y. enterocolitica infection. First, a rovA mutant is attenuated by LD50 analysis in BALB/cj mice and shows altered kinetics of infection in this mouse (24). Second, a rovA mutant is deficient in the initiation of inflammation in the PP, a phenotype that is not a consequence of the decreased levels of Inv observed in the rovA mutant. Further analysis of the rovA mutant in vivo suggested that the defect in the initiation of PP inflammation was linked to a defect in the ability of the host to produce interleukin-1α (IL-1α) in response to bacterial infection (12). Altogether, these data suggested that RovA was playing a central role in the infectious process that included the regulation of Inv and other as-yet-undetermined gene products. One or more of these unidentified genes are most likely responsible for the inflammatory pathologies observed during a Y. enterocolitica infection.
When the phenotypes of both the inv and the rovA mutant were considered together, it was reasonable to assume that RovA was important for the regulation of genes that influence the early stages of infection that occur in the PP. Therefore, it was of interest to reexamine the phenotype of the rovA mutant in vivo when the natural route of infection is bypassed. In this report we provide data suggesting that RovA is less critical for virulence in the mouse model when the bacteria are delivered by the intraperitoneal (i.p.) route of infection. Furthermore, when mice are infected i.p. with the rovA mutant they are capable of mounting a significant inflammatory response that is not observed during oral infection.
MATERIALS AND METHODS
Mice.
Female C57BL/6J mice 6 to 8 weeks of age were purchased from Jackson Laboratory and maintained in the barrier facility at Washington University School of Medicine. Mice were given free access to food and water throughout all experiments. Animals were sacrificed by carbon dioxide asphyxiation. The Washington University committee on animal studies approved all animal experiments.
Bacteria.
Y. enterocolitica strains used in the present study are WT strain (JB580v) (16) and the rovA mutant (YVM641) (22). Both strains are derivatives of the serogroup O8 strain 8081 and carry the pYV virulence plasmid. For inoculation of the mice, bacteria were grown overnight in Luria-Bertani (LB) broth at 26°C. Actual numbers of CFU were determined by serial dilutions of the overnight culture, followed by plating on LB agar containing 20 μg of nalidixic acid/ml; both JB580v and YVM641 are resistant to nalidixic acid.
LD50 and kinetics analysis.
Five groups of five mice were infected orally by gavage with successive 10-fold dilutions of the bacterial suspension (104 to 109 bacteria) or i.p. with 10 to 106 bacteria for WT or 102 to 107 bacteria for the rovA mutant. The mice were monitored twice daily for 14 days. This analysis was done in duplicate in two independent experiments. The LD50 values were determined according to the method of Reed and Muench (23). Kinetic analysis was done by infecting mice i.p. with the indicated dose of the WT Y. enterocolitica strain JB580v (16) or the rovA mutant YVM641. At various times postinfection (1, 3, or 5 days), mice from each group were sacrificed and tissues (PP, mesenteric lymph nodes, spleen, and liver) were removed. The bacterial load recovered from the infected organs was determined by plating dilutions of the macerated tissues onto LB plates containing 20 μg of nalidixic acid/ml to select for Yersinia and was reported as the CFU per gram of tissue. This kinetics analysis was done in duplicate in two independent experiments.
Histopathology.
Mice of the specified group were infected i.p. with indicated amounts of WT Y. enterocolitica strain JB580v or rovA mutant YVM641. On days 1, 3, 5, 14, and 24 postinfection the mice were sacrificed and the small intestines, mesenteric lymph nodes, spleen, liver, kidney, and lungs were removed. The lumen of the intestine was flushed with phosphate-buffered saline (PBS), and then tissues were fixed in either 10% neutral buffered formaldehyde or Bouin's fixative prior to being embedded in paraffin and stained with hematoxylin and eosin. Mice infected orally by gavage for 14 or 24 days were treated in a similar manner. Slides were investigated in a blind fashion by two independent investigators. Tissue sections were given an arbitrary score based on the frequency and severity of the lesions. Tissues were scored on a scale of 0 to 4, with 0 representing no histopathologic abnormality (consistent with the mock-infected control tissues) and 4 being the most severe pathology observed. Sections were scored for increased numbers of macrophages and neutrophils, granuloma formation, inflammatory infiltrates, necrosis, fibrin thrombi, bacterial colonization, and adhesions. A minimum of five mice per time point were examined. Tissues from dead mice were excluded from analysis.
Immunohistochemistry of paraffin-embedded sections.
Detection of Y. enterocolitica within the tissues of infected mice was performed by using the avidin-biotin complex procedure as described previously (19). Y. enterocolitica was detected by using purified rabbit polyclonal antibodies directed against membranes from the WT strain (JB580v) at a dilution of (1:500). Antibodies were prepared by the immunization of a New Zealand White rabbit with Y. enterocolitica membrane preparations. After exsanguination, serum was separated from whole blood. Serum was absorbed with acetone powders derived from E. coli strains DH5α and HB101 and Salmonella enterica serovar Typhimurium strain 14028s. Immunoglobulin G was then purified from the absorbed serum by affinity chromatography by using protein A-Sepharose. Positive staining was detected by using diaminobenzidine, and sections were counterstained with Mayer's hematoxylin.
Immunohistochemistry of frozen sections.
Mice were infected i.p. with 103 CFU of WT or rovA mutant bacteria and then sacrificed after 14 or 24 days, and the indicated tissues were removed, embedded in OCT compound, and flash frozen. Sections were cut on a cryostat and fixed in acetone or methanol prior to staining. Tissues were rehydrated in PBS. Peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide and then blocked with bovine serum albumin and milk, as well as avidin-biotin block (Vector Laboratories). Sections were incubated overnight with biotinylated antibody at the indicated concentrations. The antibodies used were anti-murine macrophage antigen F4/80 (1:250; Serotec), anti-TCRβ (1:1,000; Pharmingen), and anti-Y. enterocolitica (1:1,000). Granulocytes were detected with anti-GR-1 antigen (1:250; Pharmingen). Sections were washed in PBS and then incubated with 1 μg of streptavidin-horseradish peroxidase and goat anti-rabbit-fluorescein isothiocyanate (1:1,000)/ml for 1 h. Sections were washed in PBS, and then positive staining was detected by using tyramide signal amplification 3′ cyanine (Dupont-NEN) counterstained with Hoechst stain and visualized with fluorescence microscopy.
Statistical analysis.
All statistical analysis was done by using GraphPad Prism v.3.03 (Graphpad Software). Kinetic data was log10 transformed and then subjected to analysis of variance by using a nonparametric Mann-Whitney test to generate a two-tailed P value. Comparisons were made between a given tissue within a given time point. Statistically significant comparisons are denoted with an asterisk.
RESULTS
The rovA mutant is more virulent by the i.p. route of infection than by the oral route.
The normal route of infection for Y. enterocolitica is via the oral route. Previously, we reported that when BALB/cj mice were orally infected with the rovA mutant they were found to be 70-fold attenuated by LD50 analysis compared to the WT bacteria (24). When the oral LD50 was determined for the rovA mutant in C57BL/6j mice, the bacteria were even less virulent in this mouse background. In the C57BL/6j background the rovA mutant was 500-fold attenuated compared to the WT bacteria in an oral LD50 analysis (Fig. 1A). Interestingly, when this analysis was extended to include the i.p. route of infection the rovA mutant was almost as virulent as the WT bacteria, with only an 11-fold change in LD50, (Fig. 1A). These data suggest that rovA may play a more important role in the establishment of colonization of the PP and/or dissemination from the PP than in the elaboration of a systemic infection.
FIG. 1.
Analysis of the rovA mutant by the i.p. route of infection. (A) Five C57BL/6j mice per group were infected orally or i.p. with 10-fold serial dilutions of WT virulent Y. enterocolitica (JB580v) or rovA mutant (YVM641) and then monitored for death over a 14-day period. The LD50 was calculated by the method of Reed and Muench (21). The results are the average of two independent experiments. (B) Kinetics of infection. C57BL/6j mice were infected i.p. with 105 CFU of WT Y. enterocolitica or rovA mutant. The infection was allowed to proceed for the indicated amount of time. Mice were then sacrificed, and the indicated organs were harvested. Bacterial load was determined by enumerating the number of viable bacteria in each organ (P, PP; M, mesenteric lymph nodes; S, spleen; L, liver). Each individual symbol on the histogram represents an individual mouse. Symbols on the x axis represent tissues that did not have recoverable CFU. The results are the composite of two independent experiments. Asterisks indicate statistically significant comparisons (P = 0.0055). Eight mice were analyzed for days 1 and 3. Thirteen mice were analyzed on day 5. Solid symbols represent the WT Y. enterocolitica-infected animals, and the open symbols represent mice infected with the rovA mutant. At day 5 (the bottom panel), three of the animals for the WT day 5 time point died prior to analysis.
When the kinetics of oral infection were examined for the rovA mutant in the BALB/cj mouse model, it was apparent that there was a defect in the ability of the rovA mutant to disseminate (24). The BALB/cj mice infected orally with the rovA mutant showed a defect in the dissemination to the mesenteric lymph nodes and, to a lesser extent, the spleen compared to mice infected with WT bacteria. C57BL/6j mice infected orally with the WT bacteria or the rovA mutant showed a similar but more pronounced dissemination defect than that observed with oral infection of BALB/cj mice (data not shown). Analysis of an i.p. rovA infection of C57BL/6j mice displayed a different kinetic profile than that for the oral infection (Fig. 1B). There was significant colonization of all of the tissues by day 1 when the mice were infected with the WT bacteria. However, when the mice were infected i.p. with the rovA mutant there were fewer bacteria colonizing the deeper tissues at earlier time points (especially days 1 and 3) compared to the WT. Unlike the data from the oral infection studies, there appears to be no bias in the colonization of the deeper tissues by the rovA mutant (i.e., all tissues are equally affected) nor does there appear to be a significant virulence defect once infection is established. There only appears to be a delay in efficient colonization. Statistical analysis suggests that there were no significant differences between mice infected i.p. with WT bacteria and mice infected with the rovA mutant. When subjected to statistical analysis, only the data from spleens infected for 5 days were statistically significant (P = 0.0055). Altogether, these data, considered along with the LD50 data, suggest that RovA may be required early in i.p. infection but then, as the infectious process proceeds, the rovA mutant behaves more like the WT bacteria.
Histopathology of i.p. infection with either WT Y. enterocolitica or rovA mutant reveals inflammatory changes.
The most striking phenotype of an oral infection with the rovA mutant is a lack of PP inflammation due to decreased levels of IL-1α (12); this is in contrast to infection with WT Y. enterocolitica, which shows extensive inflammation as early as 2 days postinfection. The initial studies only examined a 7-day time course of infection. To further study the inflammatory response to the rovA mutant, an analysis of the tissues after an extended oral infection was conducted. C57BL/6j mice were infected orally with either rovA mutant or WT Y. enterocolitica for 14 or 24 days. Tissues (small intestine, spleen, liver, kidney, and mesenteric lymph nodes) were removed and prepared for hematoxylin and eosin staining, followed by histological examination. Histopathology data are summarized in Tables 1 and 2. The histopathology of earlier time points of Y. enterocolitica infection has been described (2, 6, 12). Interestingly, as previously reported for earlier time points, mice infected orally with the rovA mutant did not have a strong inflammatory response even at the 24-day time point in any of the tissues. However, an acute inflammatory response to infection with WT Y. enterocolitica was apparent by day 3 in the PP and mesenteric lymph nodes and in all tissues examined by day 7 (12). The mice infected orally with the WT Y. enterocolitica for 14 days had more severe inflammatory pathologies than the mice infected orally for 24 days. This is probably due to the mice with the most severe pathologies dying prior to the 24-day time point.
TABLE 1.
Histopathology of rovA mutant-infected mice
| Source | Avg pathological score (frequency [%]) of mice infected with rovA mutant:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orally at (day, dose [CFU], no. of mice):
|
i.p. at (day, dose [CFU], no. of mice):
|
|||||||||||
| 14, 109, 5 | 24, 109, 10 | 1, 105, 5 | 3, 105, 5 | 5, 105, 5 | 14, 104, 8 | 24
|
||||||
| 103, 3 | 104, 3 | 105, 1 | 106, 4 | 107, 2 | 108, 2 | |||||||
| Liver | ||||||||||||
| Granuloma | 0 | 0 | 0 | 0 | 0 | 4 (0.5) | 4 (1) | 3 (1) | *e | 4 (0.5) | 0 | 4 (0.5) |
| Microcolonya | 0 | 0 | 3 (0.2) | 3 (0.4) | 4 (0.4) | 3.6 (0.75) | 4 (1) | 0 | * | 4 (0.5) | 0 | 3.5 (1) |
| Microabscessb | 0 | 0 | 3 (0.2) | 3 (0.4) | 4 (0.4) | 3.6 (0.75) | 3.6 (1) | 0 | * | 4 (0.5) | 0 | 3.5 (1) |
| Necrosis | 3 (0.2) | 0 | 0 | 0 | 0 | 3.6 (0.75) | 3.3 (1) | 3 (1) | * | 4 (0.5) | 0 | 3.5 (1) |
| Infiltratec | 3 (0.2) | 0 | 2 (0.2) | 2.5 (0.4) | 4 (0.4) | 3.5 (0.1) | 4 (0.7) | 4 (1) | * | 2.8 (1) | 1 (1) | 4 (0.5) |
| Fibrin thrombi | 3 (0.2) | 0 | 0 | 3 (0.4) | 0 | 0 | 3 (0.7) | 0 | * | 4 (0.5) | 0 | 3 (0.5) |
| Adhesion | 0 | 0 | 0 | 0 | 0 | 4 (0.5) | 3.5 (0.7) | 0 | * | 4 (0.5) | 0 | 0 |
| Spleen | ||||||||||||
| Splenomegaly | 0 | 0 | 0 | 0 | 0 | 2.8 (0.1) | 2.5 (1) | 0 | * | 3 (0.5) | 0 | 3.5 (1) |
| Granuloma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Microcolony | 0 | 0 | 3 (0.2) | 3.5 (0.4) | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Microabscess | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Necrosis | 0 | 0 | 0 | 2 (0.4) | 0 | 3 (0.3) | 2 (0.3) | 0 | * | 0 | 0 | 0 |
| Infiltrate | 0 | 0 | 2 (0.2) | 2 (0.2) | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Fibrin thrombi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Adhesion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Inflammationd | 0 | 0 | 0 | 2.5 (0.6) | 0 | 2.8 (0.75) | 0 | 0 | * | 3 (0.5) | 0 | 4 (1) |
| Mesenteric lymph nodes | ||||||||||||
| Granuloma | 0 | 0 | 0 | 0 | 0 | 3.8 (1) | 4 (0.3) | 0 | * | 4 (1) | 0 | 0 |
| Microcolony | 0 | 0 | 0 | 2.5 (0.6) | 0 | 3.8 (1) | 4 (0.3) | 0 | * | 3.5 (1) | 0 | 0 |
| Microabscess | 0 | 0 | 0 | 3 (0.2) | 0 | 3.8 (1) | 4 (0.3) | 0 | * | 4 (1) | 0 | 0 |
| Necrosis | 0 | 0 | 0 | 0 | 0 | 4 (0.75) | 4 (0.3) | 0 | * | 0 | 0 | 0 |
| Infiltrate | 0 | 2 (0.1) | 2 (0.1) | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Adhesion | 0 | 0 | 0 | 0 | 0 | 4 (0.2) | 0 | 0 | * | 0 | 0 | 0 |
| Inflammation | 0 | 0 | 0 | 2.5 (0.6) | 0 | 4 (0.75) | 3.5 (0.7) | 0 | * | 3 (1) | 0 | 0 |
| PP | ||||||||||||
| Microcolony | 0 | 0 | 0 | 3 (0.2) | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Microabscess | 0 | 0 | 1.5 (0.5) | 0 | 0 | 0 | 0 | 0 | * | 0 | 0 | 0 |
| Inflammation | 2.5 (0.5) | 2 (0.2) | 0 | 0 | 0 | 2.8 (1) | 2.5 (1) | 0 | * | 2 (0.5) | 0 | 0 |
| Edema | 0 | 0 | 2 (0.2) | 0 | 2 (0.2) | 3.8 (1) | 2.5 (1) | 0 | * | 2 (0.3) | 0 | 0 |
| Infiltrate | 0 | 0 | 0 | 0 | 0 | 3 (0.75) | 2 (1) | 0 | * | 0 | 0 | 0 |
Microcolony refers to a defined infiltrate with microscopic evidence of bacterial colonization.
Microabscess refers to a defined infiltrate without evidence of bacterial colonization.
Infiltrate refers to a small defined infiltration of PMNs that is distinct from a microabscess or microcolony based upon size and organization of the lesion.
Inflammation is scored as an undefined congestion of PMNs and macrophages in the tissues; this is distinct from the defined lesions that constitute a microabscess, microcolony, or infiltrate.
An asterisk denotes that an insufficient number of animals survived to evaluate. Examples are shown in Fig. 2. Average pathological scores are reported from tissues that had pathology and the frequency that the finding was observed relative to the total number of animals in the experimental group is reported as a fraction in the parentheses (i.e., 1 = 100%).
TABLE 2.
Histopathology of WT Y. enterocolitica-infected mice
| Source | Avg pathological score (frequency [%]) of mice infected with WT Y. enteracolitica:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orally at (day, dose [CFU], no. of mice):
|
i.p. at (day, dose [CFU], no. of mice):
|
|||||||||||
| 14, 107, 5 | 24, 107, 5 | 1, 105, 5 | 3, 105, 5 | 5, 105, 5 | 14, 103, 5 | 24
|
||||||
| 102, 4 | 103, 3 | 104, 4 | 105, 4 | 106, 1 | 107, 1 | |||||||
| Liver | ||||||||||||
| Granuloma | 4 (0.2) | 0 | 0 | 0 | 4 (0.2) | 3.5 (0.8) | 0 | 0 | 0 | 0 | *e | * |
| Microcolonya | 3 (0.2) | 0 | 0 | 4 (0.2) | 3.5 (0.6) | 4 (0.8) | 0 | 0 | 0 | 0 | * | * |
| Microabscessb | 3 (0.2) | 4 (0.8) | 0 | 4 (0.2) | 3.8 (0.8) | 4 (0.8) | 3 (0.3) | 0 | 0 | 0 | * | * |
| Necrosis | 2.8 (0.8) | 3.5 (0.8) | 0 | 0 | 3.2 (0.8) | 3 (0.8) | 3 (0.3) | 0 | 0 | 2 (0.5) | * | * |
| Infiltratec | 4 (0.4) | 3 (0.8) | 2 (0.2) | 3 (0.6) | 0 | 3.5 (1) | 4 (0.3) | 0 | 3 (0.5) | 2.5 (1) | * | * |
| Fibrin thrombi | 3 (0.4) | 3.2 (0.6) | 0 | 3 (0.2) | 2 (0.2) | 3 (0.4) | 3 (0.3) | 0 | 0 | 0 | * | * |
| Adhesion | 0 | 0 | 0 | 0 | 0 | 4 (0.8) | 0 | 0 | 0 | 0 | * | * |
| Spleen | ||||||||||||
| Splenomegaly | 3.2 (1) | 4 (1) | 0 | 0 | 0 | 3.8 (1) | 4 (1) | 4 (1) | 4 (1) | 4 (1) | * | * |
| Granuloma | 4 (0.2) | 0 | 0 | 3 (0.6) | 4 (0.6) | 3 (0.2) | 0 | 0 | 0 | 0 | * | * |
| Microcolony | 4 (0.2) | 0 | 0 | 4 (0.6) | 4 (0.6) | 3.2 (1) | 0 | 0 | 0 | 0 | * | * |
| Microabscess | 4 (0.2) | 0 | 2 (0.2) | 4 (0.6) | 4 (0.6) | 3.2 (1) | 3 (0.3) | 0 | 3 (0.3) | 0 | * | * |
| Necrosis | 4 (0.2) | 0 | 0 | 0 | 0 | 0 | 3 (0.3) | 0 | 3 (0.3) | 0 | * | * |
| Infiltrate | 4 (0.2) | 0 | 2 (0.2) | 3 (0.6) | 4 (0.6) | 4 (1) | 0 | 0 | 3 (0.3) | 0 | * | * |
| Fibrin thrombi | 4 (0.2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | * | * |
| Adhesion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | * | * |
| Inflammationd | 2.5 (0.5) | 3.8 (0.8) | 2 (0.2) | 3 (0.6) | 4 (0.6) | 3.5 (1) | 3 (0.3) | 0 | 3 (0.3) | 4 (0.3) | * | * |
| Mesenteric lymph nodes | ||||||||||||
| Granuloma | 3 (0.8) | 4 (0.4) | 0 | 0 | 3 (0.2) | 3.8 (1) | 4 (0.5) | 0 | 0 | 4 (1) | * | * |
| Microcolony | 3.5 (0.8) | 4 (0.4) | 0 | 3 (0.2) | 3 (0.6) | 4 (1) | 4 (0.5) | 0 | 0 | 4 (1) | * | * |
| Microabscess | 3 (0.8) | 4 (0.4) | 0 | 3 (0.2) | 3 (0.2) | 4 (0.6) | 4 (0.5) | 0 | 0 | 4 (1) | * | * |
| Necrosis | 3 (0.8) | 4 (0.4) | 0 | 0 | 0 | 4 (0.2) | 3.2 (0.8) | 0 | 0 | 4 (0.5) | * | * |
| Infiltrate | 2.5 (0.2) | 0 | 0 | 3 (0.6) | 3 (0.6) | 4 (0.2) | 3 (0.8) | 0 | 0 | 4 (1) | * | * |
| Adhesion | 0 | 0 | 0 | 0 | 0 | 3 (0.6) | 0 | 0 | 0 | 4 (0.5) | * | * |
| Inflammation | 3.5 (1) | 3.8 (1) | 2 (0.2) | 3 (0.6) | 3 (0.6) | 3.8 (1) | 4 (1) | 0 | 0 | 3.5 (1) | * | * |
| PP | ||||||||||||
| Microcolony | 4 (0.2) | 0 | 0 | 3.8 (0.8) | 3.8 (0.8) | 4 (0.2) | 0 | 0 | 0 | 0 | * | * |
| Microabscess | 4 (0.2) | 0 | 0 | 3 (0.6) | 0 | 0 | 0 | 0 | 0 | 0 | * | * |
| Inflammation | 3 (0.6) | 3 (1) | 0 | 3 (0.6) | 3 (0.8) | 2 (1) | 0 | 0 | 0 | 0 | * | * |
| Edema | 3.8 (0.8) | 3 (0.8) | 0 | 0 | 0 | 2 (1) | 0 | 0 | 0 | 0 | * | * |
| Infiltrate | 3 (0.6) | 3 (1) | 0 | 3 (0.8) | 3.8 (0.8) | 0 | 0 | 0 | 0 | 0 | * | * |
To further examine the inflammatory response to the rovA mutant, an analysis of the inflammatory response to an i.p. infection was conducted. C57BL/6j mice were infected i.p. with either the rovA mutant or WT Y. enterocolitica for 1, 3, 5, 14, or 24 days, and then tissues (small intestine, spleen, liver, lung, kidney, and mesenteric lymph nodes) were removed and prepared for hematoxylin and eosin staining, followed by histological examination. Consistent with the kinetic data, there appeared to be a slight delay in histopathological evidence of infection by the rovA mutant compared to WT Y. enterocolitica (Tables 1 and 2). This was readily apparent during gross examination of the animals during necroscopy. The animals infected with WT Y. enterocolitica developed colonization of the serosal surfaces of the peritoneum as early as day 1, whereas the rovA mutant did not show this phenotype until day 3 or later (data not shown). This same phenotype was evident during histological examination of intestinal sections (Fig. 2). Distinct microcolonies were evident on the serosal surface of the small intestine (Fig. 2B); this was not observed during an oral infection. The presence of Y. enterocolitica in the tissues was confirmed by using a Y. enterocolitica specific antibody (Fig. 2C).
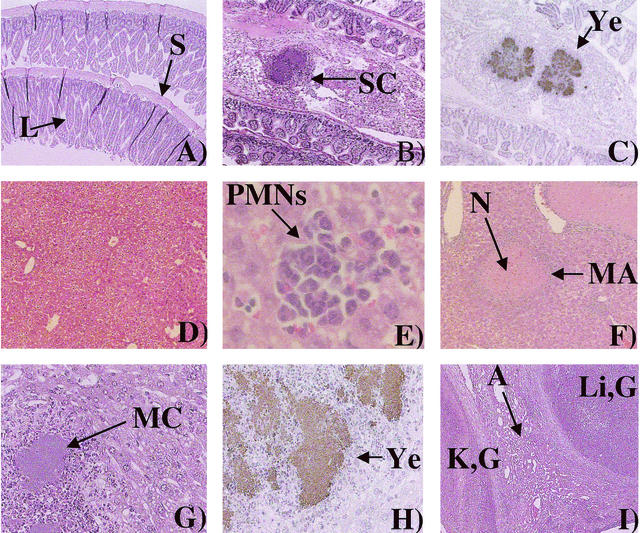
FIG. 2.
Inflammatory histopathology of mice infected i.p. with the rovA mutant. (A) Normal mouse small intestine at ×38 magnification. “L” indicates the luminal surface, and “S” indicates the serosal surface. (B) Small intestine of a mouse infected for 5 days with 103 CFU of the rovA mutant at ×38 magnification. SC, serosal colony (a colony on the serosal surface of the small intestine). (C) Immunohistochemistry of the same tissue shown in panel B). Ye, positive Y. enterocolitica immunoreactivity. (D) Normal mouse liver at ×38 magnification. (E) Small infiltrate of the liver of a mouse infected i.p. for 14 days with 102 CFU of the rovA mutant at ×380 magnification. (F) Liver of a mouse infected i.p. for 14 days with 102 CFU of the rovA mutant at ×190 magnification. “N” indicates necrosis, and “MA” indicates microabscess (a large infiltrate without microscopic evidence of bacterial colonization). (G) Liver of a mouse infected i.p. for 24 days with 102 CFU of the rovA mutant at ×190 magnification. MC, microcolony (a large infiltrate with microscopic evidence of bacterial colonization). (H) Immunohistochemistry of the same tissue shown in panel G at ×380 magnification. Ye, positive Y. enterocolitica immunoreactivity. (I) Liver and kidney of a mouse infected for 24 days with 103 CFU of the rovA mutant at ×38 magnification. K,G, kidney granuloma; Li,G, liver granuloma; A, adhesion.
Although there appeared to be an initial delay in the colonization of the peritoneal organs and the onset of inflammatory responses when mice were infected i.p. with the rovA mutant, severe inflammatory pathologies were evident at the 14- and 24-day time points. This finding is in contrast to an oral infection with the rovA mutant that showed no significant inflammatory response even at 24 days (data not shown). The most severe pathologies involved the liver. There were multiple liver abscesses in the 24-day rovA mutant i.p.-infected mouse. Within the liver, lesions included well-defined granulomas (Fig. 2I and Fig. 3), areas of necrosis (Fig. 2F), and infiltrates of polymorphonuclear leukocytes (PMNs) (Fig. 2E). The liver lesions often had microabscesses (defined infiltrates without evidence of bacterial colonization) (Fig. 2F), as well as microcolonies (defined infiltrates with evidence of bacterial colonization) (Fig. 2G and H). Adhesions between the liver and the kidney or the liver and the lung were frequently observed (Fig. 2I). In both cases, normal tissue architecture was severely disrupted. Inflammatory pathologies were also evident in other tissues as well, including granulomas of the inguinal lymph nodes, fimbrin thrombi, ischemic necrosis in the kidneys and liver, and consolidation of the lungs, as well as edema and increased macrophages observed in the PP (data not shown).
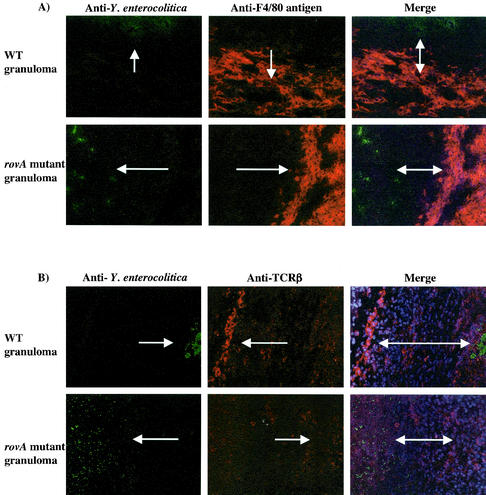
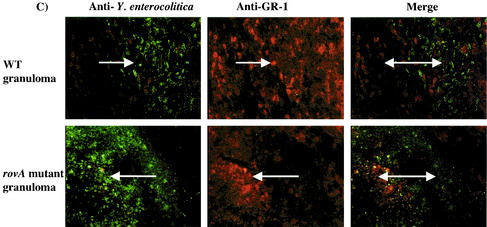
FIG. 3.
i.p. infection with the rovA mutant induces an inflammatory response. C57BL/6j mice were infected i.p. for 14 days with 103 CFU of either the rovA mutant or WT Y. enterocolitica. Livers were removed and prepared for immunohistochemistry of fresh frozen sections. All images are either from the edge of the same large granuloma (A and B) or from the center of the granuloma (C) from the respective experimental group. (A) Y. enterocolitica immunoreactivity is in green, and the macrophage-specific F4/80 antigen is in red; merged images are counterstained with Hoechst dye. (B) Y. enterocolitica immunoreactivity is in green, and the β chain of the T-cell receptor is in red; merged images are counterstained with Hoechst dye. (C) Y. enterocolitica immunoreactivity is in green, and the granulocyte-specific GR-1 antigen is in red; merged images are not counterstained with Hoechst dye in this image to facilitate visualization of both markers. In all panels arrows indicate positive immunoreactivity. All images are a magnification of ×190.
Interestingly, when the localization of the Yersinia was examined at later time points (14 or 24 days) by immunohistochemistry, the bacteria were almost exclusively contained within the granulomas (Fig. 3). This suggests that granuloma formation may provide the host partial protection during a Yersinia infection. Furthermore, these pathologies were often present in mice that received relatively low inocula (103 CFU i.p.) of either WT bacteria or rovA mutant. The architecture of granulomas formed in response to Y. enterocolitica infection is consistent with other types of granulomas. The center of the granuloma contains bacteria, PMNs (confirmed by positive GR-1 staining), and cellular debris (Fig. 3C). A concentric layer of epithelioid macrophages detected by F4/80 antigen staining and T cells detected by staining of the β chain of the T-cell receptor surrounds the center of the granuloma (Fig. 3A and B). Formation of granulomas in the mice infected i.p. with the rovA mutant was especially significant because it is indicative of a strong inflammatory response. This finding was in contrast to the pathologies observed in mice infected orally with the rovA mutant for 24 days. Mice infected orally for 24 days even with 109 CFU of the rovA mutant showed very little evidence of inflammatory pathologies (data not shown).
Not only were there significant inflammatory pathologies apparent in the tissues of the mice infected i.p. for 24 days with the rovA mutant, but there was evidence of disseminated bacterial infection. These findings range from minor findings such as splenomeglia and small inflammatory infiltrates to very severe findings such as fibrin thrombi, necrosis, and eschars (data not shown and Fig. 2 and 3). Taken together, the data presented here suggest that the rovA mutant is capable of causing severe disease in the mouse model of infection if the natural route of infection is bypassed.
DISCUSSION
Y. enterocolitica has been an invaluable tool for understanding the intricate interactions that occur between a bacterial pathogen and the mammalian host. The reason that the system is so powerful is multifactorial: (i) the bacteria and the mouse are genetically tractable; (ii) the disease that is caused in the mouse is similar to that caused in humans; and (iii) the pathogen can be delivered to the host by the natural route of infection (orally). By using this system, significant progress has been made in the understanding of the host-pathogen interaction (for reviews, see references 7, 10, 11, and 25). We now have a greater understanding of bacterial factors important for the invasion of host tissues, as well as of the molecules responsible for the regulation of these factors. Examination of the mouse as a model host also has provided insight into the T-cell, cytokine, and inflammatory responses to Y. enterocolitica infection (1, 3-5, 12).
Recently, we took advantage of this system to identify the regulator (rovA) of the principal invasion factor (inv) (14, 18, 24). Analysis of the rovA mutant in the mouse revealed an attenuated phenotype, as well as a defect in the ability to cause inflammation of the PP (12, 24). The defect in the ability to cause PP inflammation was linked to a defect in the ability of the host to induce IL-1α upon infection with the rovA mutant (12, 24). The phenotypes observed during the initial characterization of the rovA mutant in vivo utilized the natural route of infection (oral). Because RovA was identified as a regulator of inv and infection with the rovA mutant also affected the inflammatory responses in the PP (which are not linked to inv), it was reasonable to assume that RovA regulates gene products that are important for host-pathogen interactions that occur in the gut.
To further evaluate the role of rovA in the host-pathogen interaction, we bypassed the PP and delivered the bacteria directly into the peritoneum. Surprisingly, when mice are infected i.p. with the rovA mutant, the bacteria are significantly more virulent than when they are delivered by the oral route of infection. During an oral infection the rovA mutant is ∼500-fold attenuated by LD50 analysis, but during an i.p. infection the rovA mutant is 11-fold attenuated. This suggests that rovA plays a critical role in the part of the bacterial life cycle that occurs in the PP. Consistent with the data obtained from the analysis of the kinetics of an oral rovA infection, there appears to be a slight delay in the ability of the rovA mutant to colonize the tissues of the peritoneum efficiently. This may be due to a survival defect. The closely related rovA homolog (78% identical) from Salmonella (slyA) is sensitive to oxidative stress (8, 17). Although this has never been formally demonstrated for the rovA mutant of Y. enterocolitica, the delay in the colonization of the tissues of the peritoneum during an i.p. infection, along with the attenuation phenotype observed during LD50 analysis, is consistent with a partial survival defect.
The most striking defect of the rovA mutant during an oral infection is a lack of inflammation in the PP. The initial examination of this phenotype was limited to a 7-day time course. In the present study we extended the histological analysis of the oral rovA infection to 24 days. Consistent with our previous results there appears to be very little inflammation of the PP even after 14 or 24 days when mice are infected orally with the rovA mutant. Interestingly, mice infected with the WT bacteria are resolving the inflammatory pathologies of the gut at these time points but still have significant pathologies in other tissues. However, when mice are infected i.p. with the rovA mutant, inflammatory pathologies can be observed as early as day 1 and consistently by day 5. Consistent with the kinetic data there does appear to be a delay in the severity of the inflammatory pathologies observed in the rovA mutant-infected mice. At the 24-day time point very severe inflammatory pathologies can still be observed in the tissues from mice infected i.p. with the rovA mutant. The presence of granulomas and adhesions in the tissues of the mice infected i.p. with the rovA mutant for 24 days is indicative of a strong gamma interferon response. This finding suggests that when the intestine is bypassed, the rovA mutant is capable of initiating a strong inflammatory response. Furthermore, these data suggest that the host response in the gut and peritoneum may be different in significant ways that influence the outcome of the infection. Interestingly, when tissues from the day 24 rovA mutant and WT i.p. infections are stained with a Y. enterocolitica specific antibody, the bacteria are only apparent inside of the granulomas. This suggests that granuloma integrity may help protect the infected mice from the greater mortality and disease.
Altogether, the data presented in this report provides further evidence for the possible role of the virulence factor RovA during a Y. enterocolitica infection. One possible interpretation of the data, although not the only one, is that RovA regulates several sets of genes. One set of genes is involved in the immediate-early stages of infection and is responsible for the virulence phenotypes that are observed in the PP. These genes would include inv and other genes that are responsible for immediate events postinvasion of the PP, as well as genes involved in the initiation of PP inflammation. A second set of genes would be a set of genes that are expressed constitutively. These genes may be responsible for helping with survival in the host and thus play a role in both oral and i.p. infections. An example would be genes responsible for protecting the bacteria from oxidative stress. Any survival defect rovA has would only be a partial defect because viable bacteria can be recovered from the host after both an oral infection and an i.p. infection. The differential virulence that the rovA mutant displays during an oral infection versus an i.p. infection has further illustrated the importance of RovA and the RovA-regulated genes during the natural course of a Y. enterocolitica infection.
Acknowledgments
P.H.D. and S.A.H. contributed equally to this study.
This study was supported by National Institutes of Health grants AI27342 and AI52167 awarded to V.L.M. and 1 F32DK59700-01 awarded to P.H.D. Histological sections were prepared by the Washington University Digestive Diseases Research Core Facility funded by grant P30-DK52574 from the National Institutes of Health.
Editor: V. J. DiRita
REFERENCES
- 1.Autenrieth, I. B., M. Beer, E. Bohn, S. H. E. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., P. Hantschmann, B. Heymer, and J. Heesemann. 1993. Immunohistological characterization of the cellular immune response against Yersinia enterocolitica in mice: evidence for the involvement of T lymphocytes. Immunobiology 187:1-16. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., and J. Heesemann. 1992. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. 181:333-338. [DOI] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., A. Tingle, A. Reske-Kunz, and J. Heesemann. 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 60:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285-294. [DOI] [PubMed] [Google Scholar]
- 7.Bottone, E. J. 1997. Yersinia enterocolitica: The charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier, N., S. Bossie, C. Chen, F. C. Fang, D. G. Guiney, and S. J. Libby. 1997. slyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect. Immun. 66:3725-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, M. A. 1998. M cell surface β1 integrin expression and invasin mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect. Immun. 66:1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornelis, G. R. 1994. Yersinia pathogenicity factors. Curr. Top. Microbiol. Immunol. 192:243-263. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 12.Dube, P. H., P. A. Revell, D. D. Chaplin, R. G. Lorenz, and V. L. Miller. 2001. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc. Natl. Acad. Sci. USA 98:10880-10885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isberg, R. R. 1990. Pathways for the penetration of enteroinvasive Yersinia into mammalian cells. Mol. Biol. Med. 7:73-82. [PubMed] [Google Scholar]
- 14.Isberg, R. R., and S. Falkow. 1985. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 317:262-264. [DOI] [PubMed] [Google Scholar]
- 15.Isberg, R. R., and J. M. Leong. 1990. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 16.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O:8 and construction of a transformable R−M+ mutant. Gene 136:271-275. [DOI] [PubMed] [Google Scholar]
- 17.Libby, S. J., W. Goebel, A. Ludwig, N. Buchmeier, F. Bowe, F. C. Fang, D. G. Guiney, J. G. Songer, and F. Hefferon. 1994. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc. Natl. Acad. Sci. USA 91:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, V. L., and S. Falkow. 1988. Evidence for two genetic loci from Yersinia enterocolitica that can promote invasion of epithelial cells. Infect. Immun. 56:1242-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills, B. 1994. Immunohistochemistry, p. 254-255. In E. Prophet, B. Mills, J. Arrington, and L. Sobin (ed.), Laboratory methods in histotechnology. American Registry of Pathology, Washington, D.C.
- 20.Nagel, G., A. Lahrz, and P. Dersch. 2001. Environmental control of invasin expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Mol. Microbiol. 41:1249-1269. [DOI] [PubMed] [Google Scholar]
- 21.Pepe, J. C., J. L. Badger, and V. L. Miller. 1994. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol. Microbiol. 11:123-135. [DOI] [PubMed] [Google Scholar]
- 22.Pepe, J. C., and V. L. Miller. 1993. Yersinia enterocolitica invasin: a primary role in the initiation of infection. Proc. Natl. Acad. Sci. USA 90:6473-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 24.Revell, P. A., and V. L. Miller. 2000. A chromosomally encoded regulator is required for expression of the Yersinia enterocolitica inv gene and for virulence. Mol. Microbiol. 35:677-685. [DOI] [PubMed] [Google Scholar]
- 25.Revell, P. A., and V. L. Miller. 2001. Yersinia virulence: more than a plasmid. FEMS Microbiol. Lett. 205:159-164. [DOI] [PubMed] [Google Scholar]
- 26.West, A. 1997. Yersiniosis, p. 917-925. In D. Conner, F. Chandler, H. Manz, K. Baird, D. Schwartz, E. Lack, and J. Utz (ed.), Pathology of infectious diseases. Appleton and Lange, Stamford, Conn.