Abstract
We showed that Borrelia burgdorferi-vaccinated interferon gamma-deficient (IFN-γ0) mice challenged with the Lyme spirochete developed a prominent chronic severe destructive osteoarthropathy. The immune response underlying the development of the severe destructive arthritis involves interleukin-17 (IL-17). Treatment of vaccinated IFN-γ0 mice challenged with B. burgdorferi with anti-IL-17 antibody delayed the onset of swelling of the hind paws but, more importantly, inhibited the development of arthritis. Histopathologic examination confirmed that treatment with anti-IL-17 antibody prevented the destructive arthropathy seen in vaccinated and challenged IFN-γ0 mice. Similar preventive results were obtained when vaccinated and challenged IFN-γ0 mice were treated with anti-IL-17 receptor antibody or sequentially with anti-IL-17 antibody followed by anti-IL-17 receptor antibody. By contrast, treatment of vaccinated and challenged IFN-γ0 mice with recombinant IL-17 (rIL-17) did not alter the development and progression of arthritis found in vaccinated and challenged IFN-γ0 mice without treatment with rIL-17. Therapeutic intervention may be a realistic approach to prevent arthritis, especially if IL-17 is involved in the perpetuation of chronic or intermittent arthritis.
Arthritis is a frequent and major complication of natural and experimental infection with Borrelia burgdorferi (1, 5, 30, 34). Moreover, arthritis is induced following vaccination, with (14, 25) or without (28) challenge with B. burgdorferi. The immunologic events responsible for these B. burgdorferi-associated arthritides are poorly understood. We demonstrated that induction of chronic severe destructive arthritis following vaccination and challenge was dependent on B. burgdorferi-specific T lymphocytes (23). Treatment of vaccinated animals with anti-CD4 antibody prevented the development of severe destructive arthritis when the animals were challenged with the Lyme spirochete (24). Activated or primed CD4+ T cells participated in the development of arthritis that included cartilage and bone erosion (24).
Recently, we also showed that B. burgdorferi-vaccinated interferon gamma-deficient (IFN-γ0) mice challenged with B. burgdorferi developed a prominent chronic severe destructive osteoarthropathy (12). These results along with those of Brown and Reiner (6) present compelling evidence that interferon gamma (IFN-γ) does not play a major role in the induction or propagation of arthritis in the infection (6) or vaccination-challenge (12) model of B. burgdorferi-associated arthritis. This suggests that other cytokines, chemokines, or other immune regulators are responsible for the induction of arthritis.
Interleukin-17 (IL-17) is a recently discovered cytokine that is secreted by activated or primed CD4+ T cells (1). IL-17 induces the production of proinflammatory cytokines by stromal cells, synoviocytes, chondrocytes, and macrophages (11, 16, 31). Neutralization of IL-17 causes substantial reduction of collagenase activity (9), osteoclast formation (20), and production of proinflammatory cytokines (1, 10, 19). Therefore, it is likely that IL-17 is a major contributor to the pathogenesis of arthritis (1). Its role, however, in the arthritides associated with B. burgdorferi has not been defined.
In the present report, we determined whether administration of anti-IL-17 antibody, anti-IL-17 receptor antibody, or recombinant IL-17 (rIL-17) to vaccinated IFN-γ0 mice challenged with B. burgdorferi altered the development and progression of severe destructive arthritis.
MATERIALS AND METHODS
Mice.
IFN-γ gene-deficient mice (parental strain C57BL/6) were obtained from W. P. Weidanz (University of Wisconsin) with permission from Genetech, Inc. (South San Francisco, Calif.). We showed that B. burgdorferi-vaccinated IFN-γ0 mice challenged with B. burgdorferi developed a prominent chronic severe destructive osteoarthropathy (12). The parental strain also developed arthritis. We use IFN-γ0 mice to determine the role that other proinflammatory cytokines play in the generation of arthritis in the absence of IFN-γ.
The mice were bred at the animal facility located at the Wisconsin State Laboratory of Hygiene, Madison. Six- to 10-week-old inbred male and female IFN-γ0 mice weighing 20 to 30 g were housed at an ambient temperature of 21°C. Food and acidified water were provided ad libitum during a light and dark cycle of 12 h. Experimental protocols were reviewed and approved by the Animal Care and Use Committee for the University of Wisconsin Medical School, Madison.
Organisms and preparation.
Low-passage (<10) B. burgdorferi isolates of strains 297 (from human spinal fluid) and C-1-11 (from Ixodes scapularis) were grown at 32°C in modified Barbour-Stoenner-Kelly (BSK) medium until they reached a concentration of approximately 107 spirochetes/ml. Samples (500 μl) were then dispensed into 1.5-ml screw cap tubes (Sarstedt, Newton, N.C.) containing 500 μl of BSK medium supplemented with 10% glycerol (Sigma Chemical Co., St. Louis, Mo.). The tubes were sealed and stored at −70°C. Six days prior to infection of the mice, a frozen suspension of spirochetes was thawed, added to 9 ml of fresh BSK medium, and incubated at 32°C. On the day of infection, the organisms were visualized by dark-field microscopy and enumerated by using a Petroff-Hausser counting chamber.
Vaccine preparation.
B. burgdorferi 297 isolates were grown in 1 liter of BSK medium for 6 days, pelleted by centrifugation (10,000 × g, 15°C, 10 min), and washed three times with phosphate-buffered saline (PBS; pH 7.4). The washed pellet was resuspended in 1% formalin, incubated at 32°C with periodic mixing for 30 min, washed three times by centrifugation with PBS (10,000 × g, 10°C, 15 min), and resuspended in PBS. Subsequently, the formalin-inactivated spirochetes were mixed with a sufficient volume of 1% aluminum hydroxide (Reheis, Berkeley Heights, N.J.) to yield 4 × 106 spirochetes/ml.
Vaccination of mice.
Mice were anesthetized with ether and injected subcutaneously in the inguinal regions with 0.25 ml of the formalin-inactivated whole-cell vaccine preparation. Sham-vaccinated mice received either BSK medium or 1% aluminum hydroxide. Whole B. burgdorferi cells are not recommended for vaccination of humans. However, the ability of whole cells to consistently induce arthritis in mice allows for the evaluation of the immunological mechanisms responsible for the arthritis.
Infection of mice.
Twenty-two days after vaccination with B. burgdorferi 297 in alum, mice were anesthetized with ether contained in a nose-and-mouth cup and they were injected subcutaneously in the right rear paws with 50 μl of BSK medium containing 106 viable B. burgdorferi C-1-11 organisms. It was necessary to infect with B. burgdorferi C-1-11 because vaccination with B. burgdorferi 297 induces protective antibodies that prevent the homologous infection from eliciting arthritis (14, 25). Other infectious isolates of B. burgdorferi, besides C-1-11, are also effective in eliciting arthritis (14). Controls included vaccinated and nonvaccinated mice injected with BSK medium or B. burgdorferi C-1-11.
Administration of anti-IL-17 antibody, anti-IL-17 receptor antibody, or rIL-17.
Lyophilized rat anti-mouse IL-17 antibodies (200 μg) and goat anti-mouse IL-17 receptor antibodies (200 μg) along with mouse rIL-17 (50 μg) were obtained from R & D Systems (Minneapolis, Minn.). The antibodies and rIL-17 were resuspended in filter-sterilized (0.2 μm-pore-size Acrodisk filter; Gelman Sciences, Ann Arbor, Mich.) PBS (pH 7.2) to yield concentrations of 50 and 12.5 μg/ml, respectively. Twenty-two days after vaccination, three groups of four mice each were infected with 106 viable B. burgdorferi organisms in the right rear paws. Less than 1 h after infection with B. burgdorferi, the mice were injected in the right rear paws with 50 μl of the antibodies or rIL-17. Anti-IL-17 antibody, anti-IL-17 receptor antibody, and rIL-17 were injected daily for 11 days. In other experiments, anti-IL-17 was administered for 6 days, followed by the administration of anti-IL-17 receptor for 6 days.
Assessment of arthritis.
Hind paw swelling was used to determine the level of the inflammatory response in vaccinated mice challenged with B. burgdorferi. Prior to experimentation, the right rear paws of randomly selected and age-matched mice were measured to determine the baseline of paw size. After infection, the mice were measured every other day for 20 days with a dial-type Vernifer caliper (Fisher Scientific, Pittsburgh, Pa.) graduated in 0.1-cm increments. Measurements were obtained by anesthetizing each mouse with ether and carefully measuring the width and thickness of each right hind paw. The daily mean value for each group was obtained by dividing the sum of the caliper values for all hind paws in the group by the number of hind paws measured in each group. This average value represented the severity of hind paw swelling.
Preparation of tissues for histologic examination.
At 8 and 20 days after infection, mice were euthanized with ether and their hind paws were amputated at midfemur. The amputated paws were then bisected longitudinally and fixed in 10% neutral buffered zinc formalin for 24 h. Subsequently, the legs were placed in decalcifying solution (Lerner Laboratories, Pittsburgh, Pa.) for 24 h, followed by the addition of fresh decalcifying solution for an additional 48 h. Following decalcification, the legs were placed in tissue-embedding cassettes (Fisher Scientific), embedded in paraffin, and cut into 6-μm-thick sections. The sections were placed on glass slides and stained with hematoxylin and eosin. Sections were cryptically coded, and unbiased histopathologic examination was performed by a board-certified pathologist (D. M. England).
Statistical analysis.
The mean caliper values among groups were tested by analysis of variance (33). The standard error for the experiment was then determined. The Fisher least significant difference test was used to examine pairs of means when a significant F ratio indicated significant mean differences. The alpha level was set at 0.05 before the experiments were started.
RESULTS
Effects of anti-IL-17 treatment on development and progression of destructive arthritis.
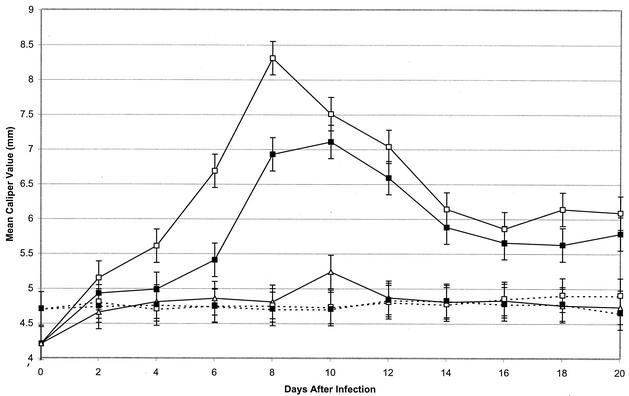
Two groups of four vaccinated mice each were challenged with 106 viable B. burgdorferi organisms 22 days after vaccination. Concomitantly, one of the two groups of vaccinated and challenged mice was treated with anti-IL-17 antibody on the day of challenge and daily thereafter for 11 days. Significant (P < 0.05) swelling of the hind paws was detected in vaccinated and challenged mice 4 days after challenge. It peaked on day 8 and then decreased (Fig. 1). By contrast, treatment of vaccinated and challenged mice with anti-IL-17 antibody delayed the onset of swelling of the hind paws by 2 days and decreased its severity. No swelling of the hind paws was detected in vaccinated, nonchallenged mice treated with anti-IL-17 antibody or in untreated vaccinated mice. Moreover, nonvaccinated mice challenged with B. burgdorferi failed to develop swelling of the hind paws at all intervals, except on day 10 after challenge. When these studies were repeated three times with four mice per group, similar results were obtained.
FIG. 1.
Development of swelling of the hind paws of vaccinated mice with (—) and without (- - -) challenge with B. burgdorferi and with (▪) and without (□) treatment with anti-IL-17 antibody. The remaining nonvaccinated, challenged group (▵) did not receive treatment with anti-IL-17 antibody. Data are the means ± standard errors for the experiment.
Confirmation that anti-IL-17 treatment inhibited development of arthritis.
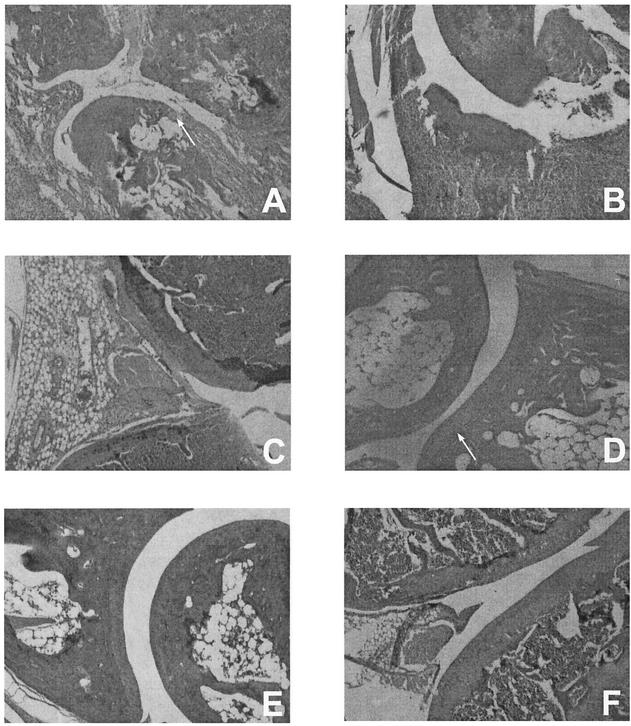
Vaccinated mice challenged with B. burgdorferi showed severe inflammation and edematous changes throughout the paws, including the muscles and subsynovial tissues surrounding the ankle joints, 8 days after infection (Fig. 2A). The inflammation extended to the tissues surrounding the long bones of the legs. Furthermore, the joints of the ankles exhibited destruction of the articular cartilage, synovial hyperplasia, and pannus formation (Fig. 2A). The small bones of the paws also showed inflammation and periosteal erosion. However, no evidence of histopathologic changes was detected in the knee joints of these animals 8 days after infection. Twenty days after infection of vaccinated mice with B. burgdorferi, prominent destructive inflammation was observed in the ankle joints (Fig. 2B), the small bones of the feet, and the tissues along the long bones. The knee joints (Fig. 2C) of these animals on day 20 postinfection also exhibited extensive inflammation as well as significant synovial hyperplasia.
FIG. 2.
Histopathology of the ankles (A, B, D, and E) and knees (C and F) of vaccinated mice challenged with B. burgdorferi with (D, E, and F) or without (A, B, and C) treatment with anti-IL-17 antibody at day 8 (A and D) and day 20 (B, C, E, and F) after infection. Controls included vaccinated, nonchallenged mice with or without treatment with anti-IL-17 antibody and nonvaccinated but challenged mice. No histopathologic changes were detected in vaccinated mice or in vaccinated, nonchallenged mice after treatment with anti-IL-17 antibody. Only minor inflammation (day 10) was observed in nonvaccinated, challenged mice. Arrows indicate areas of pannus formation (A) and no pannus formation (D).
By contrast, vaccinated mice challenged with B. burgdorferi and treated with anti-IL-17 antibody developed only minimal inflammatory changes. Although these animals showed mild edematous changes in the paws and periarticular soft tissues, they were free of inflammatory changes in the joints and bones (Fig. 2D). Moreover, the ankle (Fig. 2E) and knee (Fig. 2F) joints remained free of inflammation, even 20 days after infection. When nonvaccinated mice were challenged with B. burgdorferi, only mild localized inflammation was present in the muscles and subsynovial tissues. Furthermore, no significant changes were detected in vaccinated mice treated with anti-IL-17. In addition, vaccinated mice and nonvaccinated, nonchallenged mice were free of pathology.
Effects of anti-IL-17 receptor antibody and rIL-17 on arthritis.
Three groups of four vaccinated mice each were challenged with 106 B. burgdorferi organisms. Two of the three groups challenged with B. burgdorferi were treated with either anti-IL-17 receptor antibody or rIL-17. Treatment began on the day of challenge and continued for the following 11 days. The remaining group did not receive any treatment. Controls included groups of five vaccinated mice each injected with anti-IL-17 receptor antibody or with rIL-17 but not challenged.
Hind paw swelling was detected in vaccinated mice challenged with B. burgdorferi on day 4 of infection. It peaked on day 8 and gradually decreased thereafter. A similar response was obtained in vaccinated, challenged mice treated with rIL-17. However, treatment of vaccinated and challenged mice with anti-IL-17 receptor delayed the onset of swelling in the hind paws and significantly (P < 0.05) decreased its severity at all intervals except day 4 after infection. No swelling of the hind paws was detected in vaccinated, but not challenged, mice treated with anti-IL-17 receptor antibody or rIL-17. These studies were repeated three times with four mice per group with similar results.
In other experiments, four vaccinated, challenged mice were treated with anti-IL-17 antibody for 6 days, followed by treatment with anti-IL-17 receptor antibody. The sequential dual treatment did not enhance the protective effect obtained when four vaccinated and challenged mice were treated with anti-IL-17 receptor antibody or anti-IL-17 antibody alone.
Histopathology of anti-IL-17 receptor antibody- and rIL-17-treated vaccinated and challenged mice.
Vaccinated and challenged mice showed severe inflammation of the hind paws. The response included destruction of articular cartilage, synovial hyperplasia, and pannus formation within the ankle joints. In addition, periosteal erosion of the small bones occurred. A similar histopathologic response was detected in vaccinated and challenged mice treated with rIL-17. However, treatment of vaccinated and challenged mice with anti-IL-17 receptor ameliorated the destructive arthritis.
DISCUSSION
We have shown that severe destructive arthritis can be consistently elicited when vaccinated IFN-γ0 mice are challenged with an infectious isolate of B. burgdorferi. These results confirm and extend the findings of Christopherson et al. (12). Because these mice lack the gene that encodes IFN-γ and its receptor, the role of IFN-γ in the development of B. burgdorferi vaccination- and challenge-associated arthritis is less controversial than the role of IFN-γ in the induction of arthritis in other Lyme animal models (3, 22). In support of our findings (12), Brown and Reiner (6) also showed that IFN-γ is not required for increased susceptibility of mice to arthritis following infection with B. burgdorferi. Therefore, other cytokines, chemokines, and degradative enzymes are involved in the induction and maintenance of severe destructive arthritis.
We focused on IL-17. Considerable evidence has accumulated that IL-17 plays a central role in the induction of arthritis. IL-17 is found at high levels in the synovial fluid of patients with rheumatoid arthritis (10, 36). IL-17 is also secreted by CD4-activated memory T cells (1), which can adoptively transfer susceptibility to Lyme arthritis (23). Moreover, IL-17 shows synergy with other cytokines (1, 19, 21) for induction of bone resorption (20, 26) and stimulation of osteoclast differentiation (20). These properties of IL-17 make it a primary promoter of cartilage and bone damage in the development of arthritis.
We showed that treatment with anti-IL-17 antibody of vaccinated IFN-γ0 mice challenged with B. burgdorferi delayed the onset of swelling of the hind paws, but more importantly, it consistently inhibited the development of arthritis. Histopathologic examination confirmed that treatment with anti-IL-17 antibody prevented the extensive destructive arthropathy detected in non-IL-17-treated, vaccinated and challenged IFN-γ0 mice. Treatment of vaccinated and challenged IFN-γ0 mice with anti-IL-17 receptor antibody also delayed the onset of swelling of the hind paws and prevented cartilage and bone destruction. Similar ameliorative results were obtained when vaccinated and challenged IFN-γ0 mice were treated sequentially with anti-IL-17 antibody and then anti-IL-17 receptor antibody. By contrast, rIL-17 treatment of vaccinated and challenged IFN-γ0 mice did not alter the course of hind paw swelling or the development and progression of arthritis. No histopathologic differences were detected between vaccinated and challenged IFN-γ0 mice treated with rIL-17 and those not treated with rIL-17.
The immune response underlying the development of severe destructive arthritis involves IL-17. Vaccinated and challenged IFN-γ0 mice treated with antibodies to IL-17 or its receptor and those without treatment developed different levels of edema (swollen hind paws). More importantly, histologic examination clearly separated the two groups. Animals treated with anti-IL-17 antibody or with anti-IL-17 receptor antibody developed only minimal inflammatory changes. No destruction of cartilage, of the small bones of the feet, or of the ankle joints, long bones, or knee joints was found. Moreover, blockage of IL-17 completely alleviated damage to articular cartilage within joint spaces and prevented periarticular inflammation. By contrast, non-IL-17 antibody-treated vaccinated mice challenged with B. burgdorferi showed thickened synovial membranes in the joints and severe destruction of bone. Clearly, therapy with anti-IL-17 antibody or with anti-IL-17 receptor antibody benefits vaccinated IFN-γ0 mice challenged with B. burgdorferi.
What is the mechanism by which IL-17 induces arthritis? IL-17 may drive the arthritis by stimulating the production of IL-1 and tumor necrosis factor alpha (TNF-α) (1, 19, 21), key mediators in the pathogenesis of other experimental models of arthritis (7, 27, 29). We showed, however, that blockage of TNF-α by treatment with anti-TNF-α antibody augmented the severity of the destructive arthritis in vaccinated IFN-γ0 mice challenged with B. burgdorferi (12). This suggests that other immune regulators may be responsible for the severe destructive arthritis in B. burgdorferi-vaccinated and -challenged IFN-γ0 mice. Several reports have shown that IL-1 plays a critical role in synovial inflammation and joint destruction (15, 18, 32). However, blocking IL-1 with neutralizing antibodies had no effect on IL-17-induced inflammation and joint damage (26). IL-17 may contribute to synovial inflammation and joint destruction independently of IL-1 (26). Therefore, IL-17 may be a novel upstream target for treatment of severe destructive arthritis.
We found the results obtained with rIL-17 surprising. We expected rIL-17 to enhance the severity of the destructive arthritis detected in vaccinated IFN-γ0 mice challenged with B. burgdorferi. Infante-Duarte et al. (17) also suggested that chronic expression of IL-17 induced by lipoproteins of B. burgdorferi could be an important mediator of infection-induced immunopathology. Local overexpression of IL-17 can also promote destructive arthritis (8) and enhance pathology observed with psoriasis, rheumatoid arthritis, and other connective tissue diseases (2, 26). By contrast, we found the courses of hind paw swelling and destructive arthritis in vaccinated IFN-γ0 mice challenged with B. burgdorferi to be similar for mice treated with rIL-17 and those without treatment. An explanation is that excess IL-17 does not promote destructive arthritis unless the spirochete burden is elevated to interact with activated memory T cells. It is known that the number of B. burgdorferi organisms rapidly decreases after infection of the host (13). The decrease in the number of spirochetes also correlates with the resolution of severe destructive arthritis (35). Only high infectious challenges can again induce the destructive response (25, 30).
This study demonstrates that IL-17 contributes to joint and bone destruction along with inflammation of subsynovial, subchondrial, and connective tissues. This may be due to a compensatory mechanism that is dominant in IFN-γ0 mice. Whether IL-17 is involved in induction and pathogenesis of the other arthritides associated with B. burgdorferi infection (4, 5) and vaccination (28) needs to be determined. It seems likely that the B. burgdorferi-associated arthritides, regardless of method of induction or elicitation (7, 27, 29), share some pathways of pathogenesis. Since IL-17 is an upstream promoter of proinflammatory cytokines, chemokines, and other immune regulators, therapeutic intervention may be a realistic approach to prevent the Lyme arthritides. We are presently evaluating the role of IL-17 in models of infection-induced and -reactivated arthritis.
In conclusion, we showed that treatment with anti-IL-17 antibody or anti-IL-17 receptor antibody can prevent the development of severe destructive arthritis. Additional studies are needed to define whether anti-IL-17 therapy can cure not only severe destructive arthritis but also intermittent and chronic arthritis associated with natural infection of humans with B. burgdorferi.
Acknowledgments
This study was supported by the Wisconsin State Laboratory of Hygiene; the public health laboratory for the state of Wisconsin, Madison, Wis.; and the Gundersen Medical Foundation, La Crosse, Wis.
Editor: V. J. DiRita
REFERENCES
- 1.Aggarwal, S., and A. L. Gurney. 2001. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 71:1-8. [PubMed] [Google Scholar]
- 2.Albanesi, C., C. Scarpo, N. I. A. Carvani, M. Federici, F. Nasorri, and G. Girolomoni. 2000. Interleukin-17 is produced by both Th1 and Th2 lymphocytes, and modulates interferon-gamma and interleukin-4-induced activation of human keratinocytes. J. Investig. Dermatol. 115:81-87. [DOI] [PubMed] [Google Scholar]
- 3.Anguita, J., S. W. Barthold, R. Persinski, M. N. Hedrick, C. A. Huy, R. J. Davis, R. A. Flavell, and E. Fikrig. 2002. Murine Lyme arthritis development mediated by p38 mitogen-activated protein kinase activity. J. Immunol. 168:6352-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W., K. D. Moody, G. A. Terwilliger, P. H. Duray, R. O. Jacoby, and A. C. Steere. 1988. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J. Infect. Dis. 157:842-846. [DOI] [PubMed] [Google Scholar]
- 5.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 6.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush, K. A., J. S. Walker, C. S. Lee, and B. W. Kirkham. 2001. Cytokine expression and synovial pathology in the initiation and spontaneous resolution phases of adjuvant arthritis: interleukin-17 expression is upregulated in early disease. Clin. Exp. Immunol. 123:487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai, L., J. Yin, M. A. Starovasnik, D. A. Hogue, K. J. Hillan, J. S. Mort, and E. H. Filvaroff. 2001. Pathways by which interleukin 17 induces articular cartilage breakdown in vitro and in vivo. Cytokine 16:10-21. [DOI] [PubMed] [Google Scholar]
- 9.Chabaud, M., P. Garnero, J. M. Dayer, P. A. Guerne, F. Fossiez, and P. Miossec. 2000. Contribution of interleukin-17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 12:1092-1099. [DOI] [PubMed] [Google Scholar]
- 10.Chabaud, M., J. M. Durand, N. Bush, F. Fossiez, G. Page, L. Frapport, and P. Miossec. 1999. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42:963-970. [DOI] [PubMed] [Google Scholar]
- 11.Chabaud, M., F. Fossiez., J. L. Taupin, and P. Miossec. 1998. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation of Th2 cytokines. J. Immunol. 161:409-414. [PubMed] [Google Scholar]
- 12.Christopherson, J. A., E. L. Munson, D. M. England, C. L. Croke, M. C. Remington, M. L. Molitor, D. J. DeCoster, S. M. Callister, and R. F. Schell. 2003. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin. Diagn. Lab. Immunol. 10:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creson, J. R., L. C. L. Lim, N. J. Glowacki, S. M. Callister, and R. F. Schell. 1996. Detection of anti-Borrelia burgdorferi antibody responses with the borreliacidal antibody test, indirect fluorescent-antibody assay performed by flow cytometry, and Western immunoblotting. Clin. Diagn. Lab. Immunol. 3:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deon, D., S. Ahmed, D. Tai, N. Scaletta, C. Herrero, I. Lee, A. Krause, and L. B. Ivashkiv. 2001. Cross-talk between IL-1 and IL-6 signaling pathways in rheumatoid arthritis synovial fibroblasts. J. Immunol. 167:5395-5403. [DOI] [PubMed] [Google Scholar]
- 16.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J. J. Pin, P. Garrone, E. Garcia, S. Saeland, D. Blanchard, C. Gaillard, B. Das Mahapatra, E. Rouveir, P. Golstein, J. Banchereau, and S. Lebecque. 1996. T-cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante-Duarte, C., H. F. Horton, M. C. Byrne, and T. Kamradt. 2000. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 165:6107-6115. [DOI] [PubMed] [Google Scholar]
- 18.Ivashkiv, L. B. 1996. Cytokine expression and cell activation in inflammatory arthritis. Adv. Immunol. 63:337-376. [DOI] [PubMed] [Google Scholar]
- 19.Jovanovic, D. V., J. A. Di Battista, J. Martel-Pelletier, F. C. Jolicoeur, Y. He., M. Zhang, F. Mineau, and J. P. Pelletier. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J. Immunol. 160:3513-3521. [PubMed] [Google Scholar]
- 20.Katake, S., N. Udagawa, N. Takahashi, K. Matsuzaki, K. Itoh, S. Ishiyama, S. Saito, K. Inoue, N. Kamatani, M. T. Gillespie, T. J. Martin, and T. Suda. 1999. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 103:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz, Y., O. Nadiv, and Y. Beer. 2001. Interleukin-17 enhances tumor necrosis factor α-induced synthesis of interleukin 1, 6 and 8 in skin and synovial fibroblasts. Arthritis Rheum. 44:2176-2184. [DOI] [PubMed] [Google Scholar]
- 22.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020-2028. [PubMed] [Google Scholar]
- 23.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, and R. F. Schell. 1995. Borrelia burgdorferi-specific T lymphocytes induce severe destructive Lyme arthritis. Infect. Immun. 63:1400-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim, L. C. L., D. M. England, N. J. Glowacki, B. K. DuChateau, and R. F. Schell. 1995. Involvement of CD4+ T lymphocytes in induction of severe destructive Lyme arthritis in inbred LSH hamsters. Infect. Immun. 63:4818-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubberts, E., L. A. B. Joosten, B. Oppers, L Van den Bersselaar, C. J. J. Coenen-de-Roo, J. D. Kolles, P. Schwarzenberger, F. A. J. Van de Loo, and W. B. Van den Berg. 2001. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J. Immunol. 167:1004-1013. [DOI] [PubMed] [Google Scholar]
- 27.Piquet, P. F., G. E. Grau, G. Vesin, H. Loetscher, R. Gentz, and W. Leslauer. 1992. Evolution of collagen arthritis in mice is arrested by treatment with anti-tumor necrosis factor (TNF) antibody or a recombinant soluble TNF receptor. Immunology 77:510-514. [PMC free article] [PubMed] [Google Scholar]
- 28.Rose, C. D., P. T. Fawcett, and K. M. Gibney. 2001. Arthritis following recombinant osp A vaccination for Lyme disease. J. Rheumatol. 28:1400-1408. [PubMed] [Google Scholar]
- 29.Saez-Llorens, X., H. S. Jafari, D. D. Olsen, H. Naruichi, E. J. Hansen, and G. H. McCraken, Jr. 1991. Induction of suppurative arthritis in rabbits by Haemophilus endotoxin, tumor necrosis factor-α and interleukin-1β. J. Infect. Dis. 163:1267-1272. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz, J. L., R. F. Schell, A. Hejka, D. M. England, and L. Konick. 1988. Induction of Lyme arthritis in LSH hamsters. Infect. Immun. 56:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shalom-Barak, T., J. Quach, and M. Lotz. 1998. Interleukin-17 induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-κB. J. Biol. Chem. 273:27467-27473. [DOI] [PubMed] [Google Scholar]
- 32.Shingu, M., T. Isayama, C. Yasutake, T. Naono, M. Nobunaga, K. Tomari, K. Horie, and Y. Goto. 1994. Role of oxygen radicals and IL-6 in IL-1-dependent cartilage matrix degradation. Inflammation 18:613-623. [DOI] [PubMed] [Google Scholar]
- 33.Steel, R. G. D., and J. H. Torrie. 1960. Principles and procedures of statistics with special references to the biological sciences, p. 90-160. McGraw-Hill Book Co., New York, N.Y.
- 34.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 35.Yang, L., J. Weiss, E. Eichwald, C. Kolbert, D. Persing, and J. Weiss. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziolkowska, M., A. Koc, G. Luszczykiewicz, K. Ksiezopolska-Pietrzak, E. Klimczak, H. Chwalinska-Sadowska, and W. Maslinski. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 164:2832-2838. [DOI] [PubMed] [Google Scholar]