Abstract
β-Endorphin blocks release of luteinizing hormone (LH)-releasing hormone (LHRH) into the hypophyseal portal vessels by stimulating μ-opiate receptors, thereby inhibiting secretion of LH. LHRH release is controlled by release of nitric oxide from nitricoxidergic (NOergic) neurons in the basal tuberal hypothalamus. To determine whether β-endorphin exerts its inhibitory action on this NOergic pathway, medial basal hypothalami (MBH) from male rats were incubated with β-endorphin (10−8 M). β-Endorphin decreased basal secretion of LHRH, and significantly inhibited the release of prostaglandin E2 (PGE2), a known stimulant of LHRH release. Incubation of MBH with β-endorphin at various concentrations (10−9–10−6 M) in vitro decreased the activity of NO synthase (NOS) (measured by the conversion of [14C]arginine to labeled citrulline). Conversely, the activity of NOS was increased by the μ-receptor antagonist, naltrexone (10−8 M). Not only was the inhibitory action of β-endorphin on LHRH and PGE2 release blocked by naltrexone (10−8 M), but it increased NOS activity and LHRH and PGE2 release. β-Endorphin also stimulated γ-aminobutyric acid (GABA) release. Because GABA inhibits both nitroprusside (NP-induced PGE2 and LHRH release by blocking the activation of cyclooxygenase by NO, this is another mechanism by which β-endorphin inhibits NP-induced PGE2 and LHRH release. The results indicate that β-endorphin stimulates μ-opioid receptors on NOergic neurons to inhibit the activation and consequent synthesis of NOS in the MBH. β-Endorphin also blocks the action of NO on PGE2 release and, consequently, on LHRH release, by stimulating GABAergic inhibitory input to LHRH terminals that blocks NO-induced activation of cyclooxygenase and consequent PGE2 secretion.
Keywords: μ-opiate receptor, nitric oxide synthase, cyclooxygenase, prostaglandin E2, naltrexone
The endogenous opioid peptide, β-endorphin, plays an important role in inhibiting the release of luteinizing hormone (LH)-releasing hormone (LHRH) (1–3). Changes in release of β-endorphin within the hypothalamus are important in controlling the cyclic release of LHRH that induces the preovulatory surge of LH (3–5). Inhibitory opioid tone mediated by β-endorphin is removed in response to the increased plasma estrogen concentrations resulting from estrogen secretion by the preovulatory follicles, thereby facilitating the preovulatory release of LHRH. This tone maintains LHRH secretion at low levels during the rest of the estrous cycle of the rat and the menstrual cycle of monkeys (4). β-Endorphin acts mainly by activating μ-opioid receptors because μ-opioid receptor blockers such as naltrexone prevent its action (5).
The mechanism by which β-endorphin inhibits LHRH release is not well understood. Recent evidence indicates that LHRH release is controlled primarily by nitricoxidergic (NOergic) neurons in the arcuate-median eminence region (6–9). The NO released from these neurons diffuses into the terminals of the LHRH neurons and activates not only guanylyl cyclase, leading to the release of cGMP, but also cyclooxygenase, leading to release of prostaglandins, in particular prostaglandin E2 (PGE2) (10). The combined action of these mediators causes exocytosis of LHRH secretory granules from the terminals of the LHRH neurons that terminate in the median eminence in juxtaposition to the hypophyseal portal vessels. The released LHRH is delivered to the anterior pituitary by these vessels to cause release of LH from the gonadotropes.
During the time of the preovulatory surge of gonadotropins in the rat, there is increased noradrenergic input from the brainstem to the hypothalamus that results in release of norepinephrine from the neurons that terminate on the NOergic neurons (11). The norepinephrine acts on α1 receptors that increase intracellular Ca2+ concentrations within the NOergic neurons (12). The Ca2+ combines with calmodulin to activate neural NOS. This then converts arginine plus equimolar quantities of oxygen in the presence of various cofactors into NO. The NO diffuses into the LHRH neuron to activate cyclooxygenase and guanylyl cyclase. A number of other neurons including glutamergic neurons impinge on the noradrenergic terminals (13). These glutamergic neurons appear to play a physiological role in the preovulatory release of LHRH by stimulating the noradrenergic neurons. Oxytocin similarly stimulates these neurons; therefore, there is a multisynaptic input to the noradenergic terminals that brings about a large increase in NO release that is responsible for the preovulatory surge of LHRH (14). We hypothesize that the inhibitory β-endorphinergic input into the system uses this NOergic pathway. The present experiments were designed to test at what site in this complicated pathway β-endorphin acts.
MATERIALS AND METHODS
Male rats of the Wistar strain (200–250 g) from our colony were used. All rats were kept in group cages in a light-controlled (0500–1900 h) and temperature-controlled (23–25°C) room with free access to laboratory chow and water.
In Vitro Studies.
After the rat was decapitated and the brain was removed, the medial basal hypothalami (MBH) were dissected by making frontal cuts just behind the optic chiasm, extending dorsally 1.0 mm. A horizontal cut extended from this point caudally to just behind the pituitary stalk, where another frontal cut was made. Longitudinal cuts were made 1 mm lateral to the midline bilaterally. The hypothalami were preincubated individually in Krebs–Ringer bicarbonate-buffered medium (pH 7.4) containing 0.1% glucose for 15 min before replacement with fresh medium or medium containing the compounds to be tested. The incubation was continued for 30 min, after which samples were removed and stored at −20°C before the assay for LHRH, β-endorphin, or PGE2. When NOS activity was to be measured the incubation time was only 15 min. All incubations were carried out in a Dubnoff metabolic shaker (50 cycles per min; 95% 02/5% CO2) at 37°C.
LHRH was measured by RIA with highly specific LHRH antiserum kindly provided by Ayala Barnea (University of Texas Southwestern Medical Center, Dallas). The sensitivity of the assay was 0.2 pg per tube and the curve was linear up to 100 pg of LHRH. PGE2 was quantified by RIA as described (15) by using rabbit antiserum from Sigma. The sensitivity of the assay was 12.5 pg per tube. The crossreactivity with PGE1 was 100% and less than 0.1% with other prostaglandins. β-Endorphin also was measured by RIA and a highly specific antiserum kindly provided by George Chroussos (National Institute of Child Health and Human Development, Bethesda).
For the determination of γ-aminobutyric acid (GABA) release, two MBH were used for each determination. They were incubated in Krebs–Ringer bicarbonate-buffered medium (pH 7.4) containing 0.1% glucose with addition of 20 mM Hepes, 1 mM ascorbic acid, and 0.1% BSA. The preincubation and incubation times were the same as for LHRH, as described above. At the end of the incubation period, the media were heated for 10 min at 100°C and centrifuged at 10,000 × g for 10 min. The supernatant was frozen at −70°C until determination of GABA by the [3H]muscimol radioreceptor assay as described by Bernasconi et al. (16) (sensitivity range 12.5–200 pmol). This radioreceptor method measures the concentration of endogenous GABA and other GABA receptor ligands metabolically related to GABA (17). Aliquots of homogenates of tissue were used in determining protein concentration by method of Lowry et al. (18).
Determination of NOS activity was performed by a modification (19) of the [14C]citrulline method of Bredt and Snyder (20). Briefly, after preincubation of the MBH (one per tube) for 15 min in Krebs–Ringer bicarbonate-buffered medium (pH 7.4) containing 0.1% glucose medium as before, they were exposed to different compounds in Krebs–Ringer bicarbonate-buffered medium (pH 7.4) containing 0.1% glucose for 15 min. At the end of the incubation period the medium was discarded, and the MBH were homogenized in 20 mM Hepes (pH 7.4) with addition of 1.25 mM CaCL2 and 1 mM DTT. After the sample was homogenized, 120 μM NADPH and 200,000 dpm of [14C]arginine (292 mCi/mmol, Amersham, Buckinghamshire, U.K.) were added to each tube and incubated for an additional 10 min under the same conditions as described above. At the end of this 10 min the tubes were transferred immediately to a refrigerated centrifuge and spun at 10,000 × g for 10 min, and the supernatants were immediately applied to individual columns of Dowex AG 50W-X8 200- to 400-mesh sodium form (Bio-Rad) and washed with 2.5 ml of double-distilled water. All collected fluid from each column was counted for [14C]citrulline activity in a scintillation counter. Because NOS converts arginine into equimolar quantities of NO and citrulline, the data were expressed as NO produced per MBH per min.
Chemicals.
LHRH and β-endorphin for iodination and standards were purchased from Penninsula Laboratories. Iodine-125 for iodination was purchased from New England Nuclear. Naltrexone, GABA, ethanol, and sodium nitroprusside (NP) were from Sigma.
Statistics.
All data are expressed as means ± SEM. Comparisons between groups were performed by using a one-way ANOVA followed by the Student–Newman–Keuls multiple comparison test for unequal replicates. Dunnett’s test was used when comparisons were made versus the control group. Student’s t test was used when comparing two groups. Differences with P values <0.05 were considered significant.
RESULTS
Effect of β-Endorphin on Basal Release of LHRH and PGE2 from MBH Explants.
Incubation of MBH explants for 30 min with β-endorphin (10−8 M) slightly (P nonsignificant; Pns) reduced the release of LHRH (Fig. 1), but significantly decreased the release of PGE2 into the medium (P < 0.05; Fig. 2).
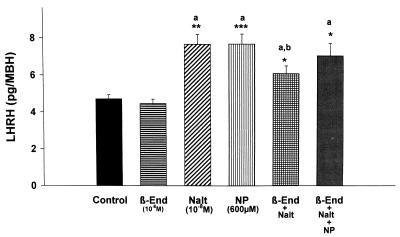
Figure 1.
The effect of β-endorphin (10−8 M; β-End), naltrexone (10−8 M; Nalt), NP (600 μM), and their combinations on LHRH release from MBH incubated in vitro. In this and subsequent figures, the height of the column represents the mean and the vertical line represents 1 SEM. Significantly different from control: ∗, P < 0.05, ∗∗, P < 0.01; ∗∗∗, P < 0.001. a, Not significantly different from other columns labeled with a. b, Significantly different from columns labeled with a. n = 8 for each column.
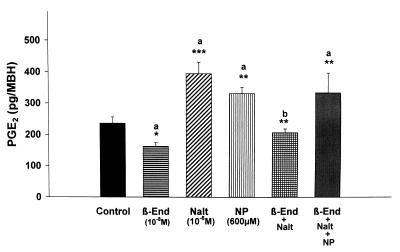
Figure 2.
The effect of β-endorphin (β-End), naltrexone (Nalt), NP, and their combinations on PGE2 release from MBH incubated in vitro.
Effect of β-Endorphin on the Secretion of LHRH and PGE2 Stimulated by NO.
Previous experiments demonstrated that incubation of MBH explants with NP (10−3 M), a releaser of NO, increased LHRH release (6). This was confirmed in the current experiments (Fig. 1), and β-endorphin (10−8 M) that did not significantly decrease basal release of LHRH completely and significantly blocked the response to NP (data not shown). NP nearly doubled PGE2 release (Fig. 2).
Effect of the μ-Opioid Receptor Blocker Naltrexone.
To determine whether there was any basal μ-opioid receptor tone affecting LHRH and PGE2 release, the tissue was incubated with naltrexone (10−8 M). Naltrexone induced a significant increase in basal LHRH (Fig. 1) and PGE2 release (Fig. 2).
Effect of Naltrexone on the Inhibitory Action of β-Endorphin on the Secretion of LHRH.
Because β-endorphin is thought to act mainly by μ receptors, we hypothesized that the μ-receptor blocker, naltrexone, would reverse the action of β-endorphin and also the inhibitory action of β-endorphin on the response to NP. Indeed, naltrexone markedly increased the release of LHRH (Fig. 1). Naltrexone reversed the slight blockade of LHRH release induced by β-endorphin, but the LHRH release was significantly less than in the case of naltrexone alone. The NP-induced stimulation of LHRH release was unaffected by incubation with both β-endorphin and naltrexone (Fig. 1).
Effect of Naltrexone on the Inhibitory Action of β-Endorphin on PGE2 Release.
Naltrexone produced a marked stimulation of PGE2 release that was blocked by coincubation with β-endorphin (Fig. 2), but NP-stimulated PGE2 release was not altered by incubation with β-endorphin plus naltrexone (Fig. 2). Thus, incubation with both β-endorphin and naltrexone resulted in a highly significant reversal of the β-endorphin-induced block of both PGE2 and LHRH release, such that there was no difference between the results with naltrexone alone or with NP alone.
Effect of Various Concentrations of β-Endorphin on NOS Activity.
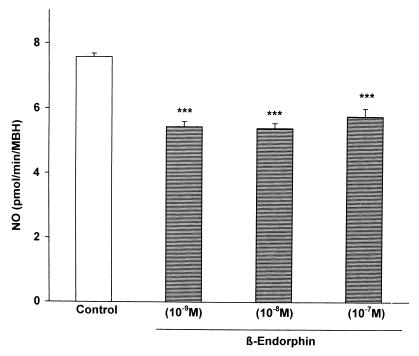
To determine whether the effect of β-endorphin might be related to an alteration in the activity of neural NOS, MBH were incubated with various concentrations of β-endorphin (10−9–10−6 M), and the activity of the enzyme in the tissue homogenate was determined by the citrulline method. All concentrations of β-endorphin produced a highly significant, approximately 20% decrease in enzyme activity (Fig. 3).
Figure 3.
The effect of β-endorphin on NOS activity measured by the citrulline method. Because activation of the enzyme yields equimolar release of NO and citrulline, the results are plotted as NO release. β-Endorphin highly significantly (P < 0.001) reduced the activity of NOS, but there was no significant dose–response relationship.
Effect of Naltrexone on NOS Activity.
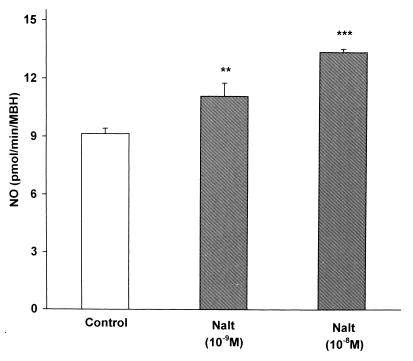
To determine whether the action of naltrexone might be related to a change in the activity of NOS, MBH were incubated with naltrexone (10−9 or 10−8 M), and the homogenate was assayed for NOS activity by the citrulline method. The NOS activity was highly significantly increased in a dose-related manner by naltrexone (Fig. 4).
Figure 4.
The effect of naltrexone (Nalt) at two concentrations on NOS activity. Naltrexone (10−9 M) highly significantly increased NOS activity, which was significantly further increased by the higher concentration (10−8 M).
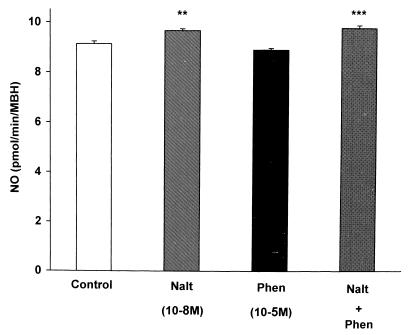
Effect of α-Adrenergic Receptor Blockade on the Naltrexone-Induced Increase in NOS Activity.
As before, naltrexone (10−8 M) increased NOS activity (Fig. 5). The increase was not affected by the α-receptor blocker, phentolamine (10−5 M), which by itself had no affect on NOS activity.
Figure 5.
The effect of phentolamine (Phen) on the activity of NOS. Phentolamine did not alter the activity of NOS or the increase induced by naltrexone(Nalt) (P < 0.001).
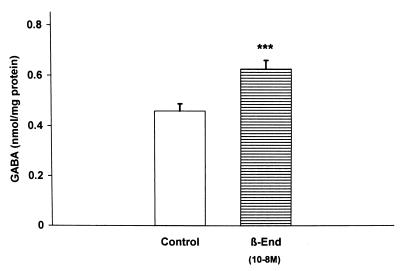
β-Endorphin GABA Interactions.
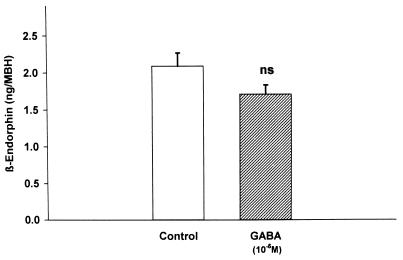
Because GABA inhibits the response of the LHRH terminals to NO (20), it was of interest to determine whether β-endorphin would increase GABA release. Indeed, β-endorphin (10−8 M) increased GABA release into the medium (P < 0.001; Fig. 6). To evaluate a possible negative feedback of GABA on β-endorphin release, GABA (10−5 M) was incubated with MBH explants. It did not significantly reduce β-endorphin efflux from the MBH explants (Fig. 7).
Figure 6.
The effect of β-endorphin (10−8 M) on the release of GABA from MBH explants.
Figure 7.
The effect of GABA on the release of β-endorphin from MBH explants. The decrease observed was not significant in this experiment or in any of the four replicates of the experiment.
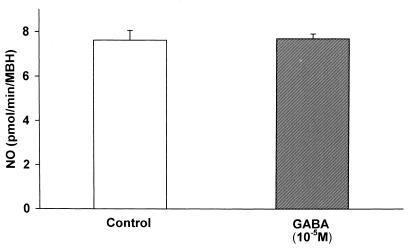
Furthermore, the possible inhibitory action of GABA on NOergic neurons was evaluated by incubating the explants for 30 min and measuring NOS activity. GABA (10−5 M) had no significant effect on NOS activity in four consecutive experiments (Fig. 8).
Figure 8.
The lack of effect of GABA on NOS activity.
DISCUSSION
The results presented here show that β-endorphin has an inhibitory effect on not only LHRH but also prostaglandin release from MBH explants in vitro. The results with LHRH agree with those obtained in vivo. Furthermore, the effects are mediated largely by μ receptors, the major receptor activated by β-endorphin because naltrexone, a relatively specific μ-receptor blocker, not only increased basal release of LHRH and PGE2 but also blocked the action of β-endorphin on both LHRH and prostaglandin release. These results appear to be mediated at least in part through the ability of β-endorphin to block the action of NO to stimulate the release of LHRH because the peptide blocked the release of LHRH induced by NP. The mechanism of the blockade is apparently by blocking the activation of cyclooxygenase because β-endorphin completely blocked the release of PGE2 induced by NP, a releaser of NO.
There appeared to be a basal opioidergic tone in the incubated hypothalami because naltrexone elevated LHRH secretion above basal. That this might be mediated by activation of cyclooxygenase was indicated by the increase in PGE2 release that accompanied the LHRH release. Because the LHRH and PGE2 release induced by naltrexone was blunted by β-endorphin, it would appear that there is an action of β-endorphin that indirectly or directly blocks the response of the LHRH neuron to NO. Because β-endorphin stimulated GABA release from neurons that have previously been shown to synapse with the LHRH terminals and cause a blockade of the LHRH release induced by NO (20), thereby providing a feedforward inhibitory influence on LHRH release, we hypothesize that part of the inhibitory action of β-endorphin on LHRH release is mediated by β-endorphin stimulation of GABA release that acts on GABA a receptors, demonstrated on LHRH neurons, to block the action of NO to stimulate cyclooxygenase, thereby blocking PGE2 followed by LHRH release.
A very interesting finding was the ability of β-endorphin at very low concentrations to reduce the activity of NOS. We previously found that this is increased by incubating the tissue with norepinephrine, which causes the activation of NOS associated with increased release of NO and LHRH (7). Removal of inhibitory opioid tone with naltrexone also increased the activity of NOS. These results indicate that β-endorphin directly or indirectly inhibits activity in the NOergic neurons resulting in decreased synthesis of NOS, which coupled with rapid inactivation of the enzyme resulted in decreased NOS content at the end of the 15-min incubation period. We believe these are effects on synthesis of the enzyme because N-methyl-d-aspartate or norepinephrine-induced increases in NOS activity were blocked by actinomycin D, an inhibitor of DNA-directed RNA synthesis (V.R., G. Canteros, and S.M.M., unpublished data). Also, the increased NOS activity induced by norepinephrine was measurable even when the enzyme was highly purified (7). Furthermore, in vitro incubation of the tissue results in a rapid decline in enzyme activity (V.R., G. Canteros, and S.M.M., unpublished data). Therefore, the results indicate that β-endorphin inhibits the activity of the NOergic neurons either directly via receptors on these neurons or indirectly by activating another inhibitory pathway. Because the opiodergic actions were not blocked by phentolamine, it appears that they are not mediated by removal of stimulatory noradrenergic drive.
It occurred to us that the β-endorphin stimulation of GABA release might activate a negative feedback mechanism by which GABA would inhibit β-endorphin release, but we found that GABA at a concentration that inhibited LHRH release (20) had no significant inhibitory action on β-endorphin release. Therefore, we believe that the stimulatory effect of naltrexone on NOS activity was not related to removal of inhibitory GABAergic tone mediated by GABA receptors on the NOergic neurons. Furthermore, GABA repeatedly failed to alter NOS activity. It is probable that the action of β-endorphin may be explained by the presence of μ receptors on both GABA and NOergic neurons. Indeed, Wang and Nakai (21) reported the presence of μ receptors on lateral raphé NOergic neurons. Recent dual labeling in situ hybridization studies provided no evidence for the existence of mRNA for μ-, κ-, or γ-opiate receptors on LHRH neurons (22).
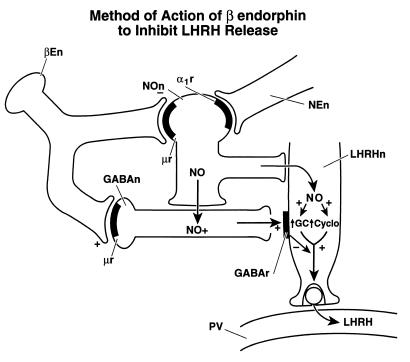
The postulated mechanisms of action of β-endorphin to inhibit LHRH release are diagrammed in Fig. 9. This pathway may be of profound importance in mediation of inhibitory influences on LHRH release. The inhibitory effect of alcohol on LHRH release is mediated by stimulation of μ receptors in all probability by β-endorphin (23). This pathway may also be activated by other drugs of abuse, such as Δ-9-tetrahydrocanabinol (24), and by cytokines (25). As in the case of β-endorphin, all of these agents inhibit LHRH release by inhibiting the NOergic neurons and blocking the response of the LHRH neuronal terminals to NO, primarily by inhibiting NO activation of cyclooxygenase. β-Endorphin may also block the activation of guanylyl cyclase by NO as shown recently in vivo (26).
Figure 9.
Schematic diagram of the proposed mechanism of action of β-endorphin to inhibit LHRH release by suppressing the activity of NOS and activating the release of GABA that by GABAA receptors blocks the stimulatory action of NO on the LHRH terminals. For further explanation see text. βEn, β-endorphin neuron; NOn, NOergic neuron; NEn, noradrenergic neuron; GABAn, GABAergic neuron; LHRHn, LHRH neuron; GC, guanylyl cyclase; Cyclo, cyclooxygenase; PV, hypophyseal portal vessel, + or ↑, stimulates; −, inhibits.
Acknowledgments
We thank Judy Scott and Jill Lucius for their excellent secretarial assistance. This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina (PIA 6649) and National Institutes of Health (MH51853).
ABBREVIATIONS
- NP
nitroprusside
- LH
luteinizing hormone
- LHRH
LH-releasing hormone
- NOergic
nitricoxidergic
- NOS
NO synthase
- PGE2
prostaglandin E2
- MBH
medial basal hypothalami
- GABA
γ-aminobutyric acid
References
- 1.Ferin M, Van Vugt D, Wardlaw S. Recent Prog Horm Res. 1984;40:441–485. doi: 10.1016/b978-0-12-571140-1.50015-3. [DOI] [PubMed] [Google Scholar]
- 2.Kesner J S, Kaufman J-M, Wilson R C, Kuroda G, Knobil E. Neuroendocrinology. 1986;43:686–688. doi: 10.1159/000124605. [DOI] [PubMed] [Google Scholar]
- 3.Kalra S P. Endocr Rev. 1993;14:507–538. doi: 10.1210/edrv-14-5-507. [DOI] [PubMed] [Google Scholar]
- 4.Sarkar D K, Minami S. Biol Reprod. 1995;53:38–45. doi: 10.1095/biolreprod53.1.38. [DOI] [PubMed] [Google Scholar]
- 5.Evans W S, Weltman J Y, Johnson M L, Weltman A, Veldhuis J D, Rogol A D. J Endocrinol Invest. 1992;15:525–531. doi: 10.1007/BF03348799. [DOI] [PubMed] [Google Scholar]
- 6.Rettori V, Belova N, Dees W L, Nyberg C L, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canteros G, Rettori V, Franchi A, Genaro A, Cebral E, Faletti A, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1995;92:3416–3420. doi: 10.1073/pnas.92.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moretto M, Lopez F J, Negro-Vilar A. Endocrinology. 1993;133:2399–2402. doi: 10.1210/endo.133.5.8104781. [DOI] [PubMed] [Google Scholar]
- 9.Bhat G, Mahesh V B, Aguan K, Brann D W. Neuroendocrinology. 1996;64:93–102. doi: 10.1159/000127104. [DOI] [PubMed] [Google Scholar]
- 10.Rettori V, Gimeno M, Lyson K, McCann S M. Proc Natl Acad Sci USA. 1992;89:11543–11546. doi: 10.1073/pnas.89.23.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franci J A, Franci C, Krulich L, Antunes-Rodrigues J, McCann S M. Brain Res. 1997;767:289–296. doi: 10.1016/s0006-8993(97)00613-6. [DOI] [PubMed] [Google Scholar]
- 12.Canteros G, Rettori V, Genaro A, Suburo A, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1996;93:4246–4250. doi: 10.1073/pnas.93.9.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.McCann S M, Rettori V. Proc Soc Exp Biol Med. 1996;211:7–15. doi: 10.3181/00379727-211-43950b. [DOI] [PubMed] [Google Scholar]
- 13.Kamat A, Yu W H, Rettori V, McCann S M. Brain Res Bull. 1995;37:233–235. doi: 10.1016/0361-9230(94)00280-e. [DOI] [PubMed] [Google Scholar]
- 14.Rettori V, Canteros G, Renoso R, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1997;94:2741–2744. doi: 10.1073/pnas.94.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faletti A, Viggiano J M, Gimeno M A F. Prostaglandins. 1995;9:93–103. doi: 10.1016/0090-6980(94)00006-i. [DOI] [PubMed] [Google Scholar]
- 16.Bernasconi R, Bittinger H, Hied Y, Martin P. Neurochemistry. 1980;34:614–618. doi: 10.1111/j.1471-4159.1980.tb11188.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell R, Grieve G, Dow R, Fink G. Neuroendocrinology. 1983;37:169–176. doi: 10.1159/000123539. [DOI] [PubMed] [Google Scholar]
- 18.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1989;8:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seilicovich A, Duvilanski B H, Pisera D, Thies S, Gimeno M, Rettori V, McCann S M. Proc Natl Acad Sci USA. 1995;92:3421–3424. doi: 10.1073/pnas.92.8.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q P, Nakai Y. Brain Res. 1995;684:185–193. doi: 10.1016/0006-8993(95)00418-p. [DOI] [PubMed] [Google Scholar]
- 22.Sannella M I, Petersen S L. Endocrinology. 1997;138:1667–1672. doi: 10.1210/endo.138.4.5091. [DOI] [PubMed] [Google Scholar]
- 23.Rettori V, McCann S M. Mol Psychiatry. 1997;2:350–354. doi: 10.1038/sj.mp.4000306. [DOI] [PubMed] [Google Scholar]
- 24.Rettori V, Aguila M C, Gimeno M F, Franchi A M, McCann S M. Proc Natl Acad Sci USA. 1990;87:10063–10066. doi: 10.1073/pnas.87.24.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura M, Yu W H, Rettori V, McCann S M. Neuroimmunomodulation. 1997;4:237–243. doi: 10.1159/000097342. [DOI] [PubMed] [Google Scholar]
- 26.Pu S, Horvath T L, Diano S, Naftolin F, Kalra P S, Kalra S P. Endocrinology. 1997;138:1537–1543. doi: 10.1210/endo.138.4.5086. [DOI] [PubMed] [Google Scholar]