Abstract
Burkholderia pseudomallei is the causative agent of melioidosis, a life-threatening disease that affects both humans and animals. This bacterium is able to survive and multiply inside both phagocytic and nonphagocytic cells. We recently reported that mouse macrophages infected with B. pseudomallei fail to produce a significant level of inducible nitric oxide synthase (iNOS), a crucial enzyme needed for the cells to control the intracellular growth of this bacterium. In the present study, we extended our investigation to demonstrate that, unlike other gram-negative bacteria that have been investigated, B. pseudomallei only minimally activates beta interferon (IFN-β) production; this minimal activation leads to a low level of interferon regulating factor 1 (IRF-1) in the macrophages, in parallel with poor iNOS expression. Adding exogenous IFN-β to the system could upregulate IRF-1 production, which in turn could enhance iNOS expression in the B. pseudomallei-infected macrophages and lead to suppression of the intracellular growth of this bacterium. Taken together, these results imply that the failure of macrophages to successfully control the growth and survival of intracellular B. pseudomallei is related, at least in part, to the defective production of IFN-β, which modulates the ability of macrophages to synthesize iNOS.
Burkholderia pseudomallei is the causative agent of melioidosis, an endemic disease in tropical countries, including Southeast Asia and northern Australia (4, 11, 18, 30). This facultative intracellular bacterium is known to survive and multiply inside both phagocytic and nonphagocytic cells (13). After internalization, B. pseudomallei can escape from a membrane-bound phagosome into the cytoplasm (13). The organism can induce cell fusion, resulting in a multinucleated giant cell formation (8, 16). This phenomenon may facilitate the spread of B. pseudomallei from one cell to another.
Although the interaction of the host cell and B. pseudomallei has been extensively studied, the mechanism(s) by which this microorganism escapes macrophage killing is not clearly understood. Recently, Utaisincharoen et al. reported that B. pseudomallei is able to invade mouse macrophages without activating inducible nitric oxide synthase (iNOS) production (27). This enzyme is known to play an essential role in controlling the intracellular survival and multiplication of B. pseudomallei (21, 27). The expression of iNOS was markedly enhanced when macrophages were preactivated with gamma interferon (IFN-γ), leading to the production of NO to a concentration needed to suppress the intracellular growth of this bacterium (27). The enhancement of host defense by IFN-γ was also observed in mice infected with B. pseudomallei (23).
Besides IFN-γ, IFN-β (a type I interferon) has been implicated in playing a role in innate immunity against various microbial infections. IFN-β is largely produced by macrophages infected with microbial pathogens (e.g., Leishmania major) or exposed to microbial products (e.g., lipopolysaccharide [LPS]) (2, 3, 9, 12). The potent antimicrobial functions of IFN-β have been observed in macrophages infected with microorganisms such as L. major and Toxoplasma gondii (20, 22). Different lines of evidence suggest that iNOS and NO production could be enhanced by IFN-β (12, 28). For example, simultaneous exposure of macrophages to IFN-α/β and L. major enhanced iNOS expression, resulting in inhibition of the intracellular survival of L. major (20). Jacobs and Ignarro recently demonstrated that IFN-β served in an autocrine fashion, mediating interferon regulatory factor 1 (IRF-1) production (12). The expression of this transcriptional activator and the presence of a crucial IRF-1 binding site within the promoter of the iNOS gene are necessary for the induction of iNOS in murine macrophages (14, 19). In the present study, we demonstrated that B. pseudomallei failed to stimulate IFN-β production in mouse macrophages. The inability of B. pseudomallei-infected macrophages to produce IFN-β resulted in depressed iNOS production, thus allowing the bacteria to survive inside the cells.
MATERIALS AND METHODS
Cell line and culture condition.
Mouse macrophage cell line RAW 264.7 was obtained from the American Type Culture Collection (ATCC, Manassas, Va.). If not indicated otherwise, the cells were cultured in Dulbecco's modified Eagle's medium (Gibco Laboratories, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah) at 37°C under a 5% CO2 atmosphere.
Bacterial isolation.
B. pseudomallei strain 844 (arabinose-negative strain) was originally isolated from a patient admitted to Srinagarind Hospital in the Khon Kaen province of Thailand, in which melioidosis is endemic. The bacterium was originally identified as B. pseudomallei based on its biochemical characteristics, colonial morphology on selective media, antibiotic sensitivity profiles, and reaction with polyclonal antibody (1, 15, 29) and was used in previous studies (16, 27). Salmonella enterica serovar Typhi, used for comparison throughout these experiments, was maintained at Ramathibodi Hospital (Mahidol University, Bangkok, Thailand) and kept as a stock culture in our laboratory.
Infection of mouse macrophages (RAW 264.7).
Mouse macrophages (106 cells) were cultured in a six-well plate overnight before being exposed to bacteria at a multiplicity of infection (MOI) of 2:1 for 1 h. To remove extracellular bacteria, the cells were washed three times with 2 ml of phosphate-buffered saline (PBS) before Dulbecco's modified Eagle's medium containing 250 μg of kanamycin (Gibco)/ml was added. At various times, the cells were lysed before being subjected to immunoblotting, and the supernatant was used for IFN-β analysis.
Immunoblotting.
Various mouse macrophage preparations were lysed in a buffer containing 20 mM Tris, 100 mM NaCl, and 1% NP-40. Lysates containing 30 μg of protein were electrophoresed by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis and then electrotransferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The membranes were blocked with 5% milk for 1 h before being incubated overnight with polyclonal antibody to mouse iNOS, IRF-1, or actin (Santa Cruz, Santa Cruz, Calif.). Blots were then reacted with horseradish peroxidase-conjugated swine anti-rabbit immunoglobulin G (Dako, Glostrup, Denmark). Protein bands were detected by use of an enhanced chemiluminescence kit as recommended by the manufacturer (Roche Diagnostic, Mannheim, Germany).
ELISA.
The concentrations of IFN-β in the supernatants of infected macrophages were measured by using an enzyme-linked immunosorbent assay (ELISA) kit (R&D, Minneapolis, Minn.). The sensitivity of the assay system was 50 U/ml.
Standard antibiotic protection assay.
Mouse macrophages (5 × 105 cells per well) were simultaneously exposed to IFN-β (100 U/ml) (R&D) and B. pseudomallei at an MOI of 2:1 at 37°C for 1 h and then washed thoroughly with PBS. The cells were incubated for an additional 7 h in medium containing 250 μg of kanamycin/ml to eliminate residual extracellular bacteria; intracellular bacteria were liberated by using 0.1% Triton X-100 and then were plated on tryptic soy agar. To inhibit NO production, 500 μM L-NAME (Sigma, St. Louis, Mo.) was added to the culture medium and maintained throughout the infection period. The viability of macrophages cultured in the presence of L-NAME was judged by trypan blue dye staining to be more than 90%. This concentration of the iNOS inhibitor did not interfere with bacterial growth, as judged by viable counting with the pour plate technique.
RESULTS
Production of IFN-β by macrophages infected with B. pseudomallei.
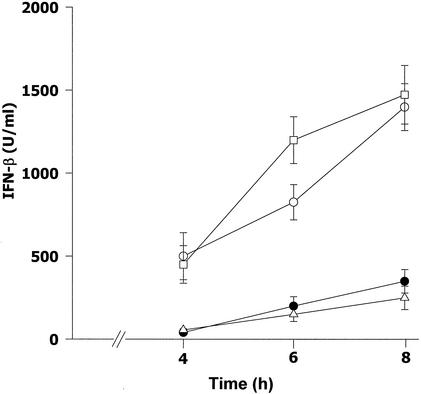
Mouse macrophages were infected with B. pseudomallei at an MOI of 2:1 for 1 h, washed with PBS, and cultured in a medium containing 250 μg of kanamycin/ml. At 4, 6, and 8 h after infection, the supernatants were analyzed for IFN-β production by an ELISA. As shown in Fig. 1, the supernatants from the cells infected with B. pseudomallei for as long as 8 h contained a negligible concentration of IFN-β. In fact, the level of IFN-β produced by the B. pseudomallei-infected macrophages was only slightly higher than that produced by the uninfected macrophages. On the other hand, the levels of IFN-β in cells infected with either Salmonella serovar Typhi or Escherichia coli, used for comparison, were significantly higher than those in B. pseudomallei-infected cells throughout the period of observation. These results clearly demonstrated that B. pseudomallei behaved in a manner different from that of other bacteria with regard to its ability to stimulate macrophages for IFN-β production.
FIG. 1.
Production of IFN-β by infected mouse macrophages. Mouse macrophages were exposed to B. pseudomallei (•), Salmonella serovar Typhi (□), or E. coli (○) at an MOI of 2:1. After 1 h of incubation, the infected cells were washed with PBS before being cultured in medium containing 250 μg of kanamycin/ml to kill residual extracellular bacteria. At 4, 6, and 8 h after infection, the supernatants were analyzed for IFN-β by an ELISA. Unstimulated macrophages were used as a control (▵). Data represent the means and standard deviations from two separate experiments with triplicate samples.
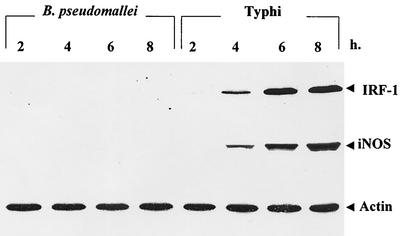
Production of IRF-1 and iNOS by macrophages infected with B. pseudomallei.
Macrophages were infected with B. pseudomallei (or Salmonella serovar Typhi, used for comparison) at an MOI of 2:1. At various times after infection, the cells were lysed and subjected to immunoblotting with monoclonal antibodies (MAbs) against IRF-1 and iNOS. The results shown in Fig. 2 demonstrated that macrophages infected with B. pseudomallei for as long as 8 h produced neither IRF-1 nor iNOS protein. However, both IRF-1 and iNOS could be readily detected in macrophages infected with Salmonella serovar Typhi even after only 4 h of infection, and the levels gradually increased with prolonged infection. The production of both IRF-1 and iNOS by Salmonella serovar Typhi-infected macrophages paralleled that of IFN-β. These results indicated that B. pseudomallei also failed to activate IRF-1 and iNOS expression in mouse macrophages.
FIG. 2.
Kinetics of iNOS and IRF-1 expression by infected macrophages. Mouse macrophages were exposed to B. pseudomallei or Salmonella serovar Typhi at an MOI of 2:1 as described in the legend to Fig. 1. At 2, 4, 6, and 8 h after infection, the cells were lysed with lysis buffer and then subjected to immunoblotting with anti-iNOS, anti-IRF-1, and antiactin (as a control).
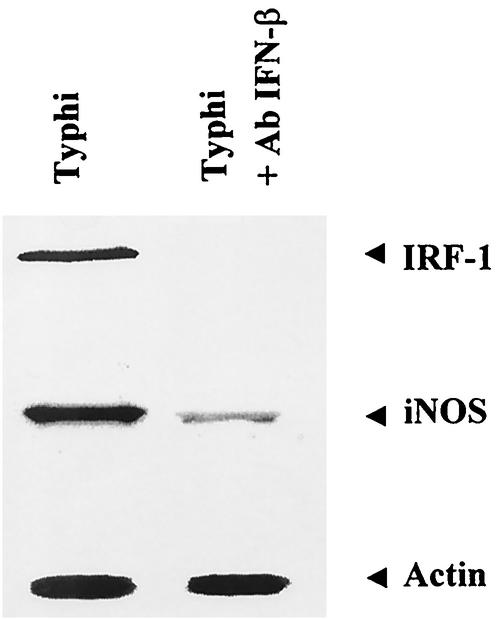
Effect of IFN-β on iNOS expression in infected macrophages.
In order to investigate the possible relationship between IFN-β produced by bacterium-infected macrophages and iNOS expression, mouse macrophages were exposed to Salmonella serovar Typhi at an MOI of 2:1 for 1 h in the presence or absence of neutralizing MAb against IFN-β (10 μg/ml). In this experiment, only Salmonella serovar Typhi was used because under the experimental conditions, no iNOS was detected in B. pseudomallei-infected macrophages. The cells were washed with PBS before being cultured in medium containing 250 μg of kanamycin/ml and MAb against IFN-β. At 6 h after infection, the cells were lysed with lysis buffer and analyzed for iNOS and IRF-1 production by immunoblotting. As shown in Fig. 3, the MAb against IFN-β totally abolished IRF-1 expression from macrophages infected with Salmonella serovar Typhi, indicating that the expression of IRF-1 was mediated by IFN-β. However, under these conditions, a very low level of iNOS could still be observed. These results implied that IFN-β endogenously produced by the bacterium-infected macrophages could enhance iNOS production in an autocrine and/or paracrine fashion.
FIG. 3.
IFN-β-neutralizing antibody attenuates the expression of iNOS and IRF-1 in macrophages infected with Salmonella serovar Typhi. Mouse macrophages were infected with Salmonella serovar Typhi at an MOI of 2:1 in the presence or absence of anti-IFN-β (10 μg/ml). At 1 h after infection, the cells were washed with PBS before being cultured in medium containing 250 μg of kanamycin/ml. The antibody (Ab) was kept in the culture medium throughout the experiment. At 8 h after infection, iNOS, IRF-1, and actin levels produced by the infected cells were determined by immunoblotting.
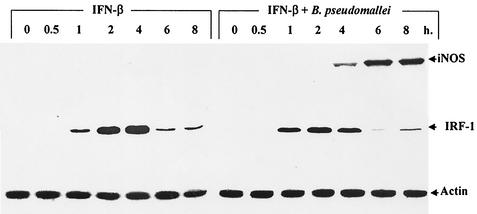
Exogenous IFN-β enhances the iNOS expression of macrophages infected with B. pseudomallei.
In this experiment, mouse macrophages were infected with B. pseudomallei at an MOI of 2:1 in the presence or absence of exogenous IFN-β (100 U/ml). At different times, iNOS and IRF-1 levels were determined by immunoblotting. IFN-β alone did not stimulate iNOS production at concentrations that could be detected by immunoblotting (Fig. 4). However, macrophages activated with IFN-β expressed IRF-1 after only 1 h of exposure, and expression peaked at 4 h before being significantly degraded. Recall that macrophages infected with B. pseudomallei expressed neither iNOS nor IRF-1 even after 8 h of infection (Fig. 2). However, in this experiment, when macrophages were simultaneously exposed to IFN-β and B. pseudomallei, a high level of iNOS could be detected within 4 h of stimulation (Fig. 4). After 8 h of infection, the level of NO produced by macrophages infected with B. pseudomallei was under the limit for analysis by the Griess reaction (7). These results indicated that exogenous IFN-β could enhance the expression of iNOS in B. pseudomallei-infected macrophages.
FIG. 4.
Exogenous IFN-β enhances the expression of iNOS in B. pseudomallei-infected mouse macrophages. Macrophages were activated with IFN-β (100 U/ml) in the absence or presence of B. pseudomallei (MOI, 2:1) for 1 h, washed with PBS, and then cultured in medium containing 250 μg of kanamycin/ml. At different time intervals, the cells were lysed and subjected to immunoblotting with anti-iNOS, anti IRF-1, and antiactin.
Exogenous IFN-β decreases the intracellular survival and multiplication of B. pseudomallei in macrophages.
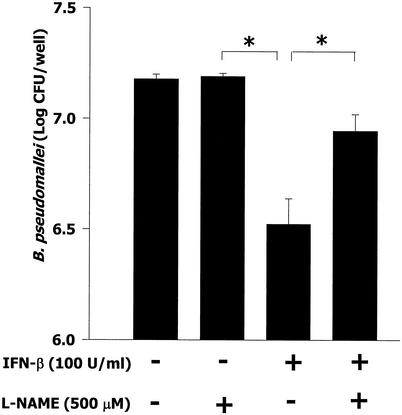
Having established that IFN-β could enhance iNOS expression in macrophages infected with B. pseudomallei, we next tested whether this phenomenon could control the intracellular growth and survival of B. pseudomallei. In this experiment, macrophages were simultaneously exposed to IFN-β and B. pseudomallei for 8 h, and the number of live B. pseudomallei organisms inside the macrophages was determined by a standard antibiotic protection assay as described in Materials and Methods. In the absence of exogenous IFN-β, a high number of intracellular bacteria was observed (Fig. 5). However, when the cells were simultaneously exposed to exogenous IFN-β and B. pseudomallei, the number of intracellular bacteria was significantly reduced. Consistent with this observation, enhancement of the antimicrobial activity of macrophages stimulated with IFN-β was reduced in the presence of L-NAME, indicating that iNOS is responsible for the intracellular killing of B. pseudomallei inside macrophages.
FIG. 5.
Exogenous IFN-β suppresses the intracellular survival and multiplication of B. pseudomallei in mouse macrophages. Macrophages were simultaneously exposed to B. pseudomallei (MOI, 2:1) and IFN-β (100 U/ml) in the presence or absence of L-NAME (500 μM) for 1 h, washed with PBS, and cultured in medium containing 250 μg of kanamycin/ml. The iNOS inhibitor L-NAME was kept in the culture medium throughout the experiment. At 8 h after infection, the number of intracellular B. pseudomallei organisms was determined by a standard antibiotic assay as described in Materials and Methods. Error bars indicate standard deviations. Asterisks indicate significant differences.
DISCUSSION
Macrophages are known to be one of the major cellular sources of IFN-β produced by the immune system. The production of IFN-β by macrophages is upregulated by microbial infection (e.g., E. coli or L. major) or by exposure to microbial products (e.g., LPS) (2, 3, 24). Among bacteria, only gram-negative ones can stimulate IFN-β production in mouse macrophages. Listeria monocytogenes, Staphylococcus aureus, or Lactobacillus bulgaricus and other gram-positive bacteria failed to activate IFN-β production (24). These results strongly suggested that LPS is the only bacterial component capable of inducing IFN-β production in mouse macrophages. Recent evidence indicated that LPS induced the expression of the gene encoding IFN-β through a Toll-like receptor 4 (TLR4) agonist but not a TLR2 agonist (25). However, LPS isolated from Porphyromonas gingivalis, which differs from enterobacterial LPS structurally and functionally, activated proinflammatory cytokines via TLR2 instead of TLR4 (10). P. gingivalis LPS did not stimulate IFN-β production by murine macrophages (25). In the present study, we demonstrated that B. pseudomallei also failed to stimulate IFN-β production in mouse macrophages (Fig. 1). The inability of this gram-negative bacterium to stimulate IFN-β may be due to the unique structure of B. pseudomallei LPS. It was reported that LPS isolated from this bacterium exhibited an unusual chemical structure in the acid-stable inner core region attached to the lipid A moiety which might lead to its weak macrophage activation activity (15, 26). Whether B. pseudomallei LPS is an agonist for TLR2 or TLR4 remains to be investigated.
The roles of IFN-β in innate immunity against microbial infections have been reported (2). Recombinant IFN-β was shown to protect mice against infection with L. monocytogenes (6). A similar protective effect of IFN-β was also observed in mice infected with Trypanosoma cruzi or T. gondii (17, 22). In mice infected with Mycobacterium avium, the continuous administration of IFN-β led to a reduction of the bacterial burden in the liver and spleen (5). The possible mechanism(s) by which IFN-β enhances the antimicrobial activity of macrophages may be related to the fact that it enhances iNOS and NO production by macrophages infected with the microorganism. In an in vitro study, costimulation of cells with IFN-β and L. major activated iNOS expression and NO production, leading to inhibition of the intracellular survival of parasites (20). In the present study, we demonstrated that a MAb against IFN-β significantly reduced iNOS expression by macrophages infected with Salmonella serovar Typhi (Fig. 3). This result implies that endogenous IFN-β, acting in an autocrine fashion, acted synergistically with the bacteria to increase the expression of iNOS. The enzyme iNOS is known to play an important role in the intracellular killing of macrophages (3). Recently, Utaisincharoen et al. reported that mouse macrophages infected with B. pseudomallei failed to trigger iNOS expression (27). However, preactivation with IFN-γ increased iNOS expression in B. pseudomallei-infected cells, resulting in a reduction in intracellular survival (27). The inability of B. pseudomallei-infected cells to produce iNOS may be due, at least in part, to the failure of B. pseudomallei-infected macrophages to produce IFN-β. However, in the presence of exogenous IFN-β, macrophages infected with B. pseudomallei were able to express iNOS at a high level (Fig. 4) and at the same time suppress the intracellular survival of B. pseudomallei (Fig. 5). However, in the presence of an iNOS inhibitor, L-NAME, the number of intracellular B. pseudomallei organisms in IFN-β-activated macrophages was significantly increased, indirectly indicating that the upregulation of iNOS in IFN-β-treated infected macrophages was associated with enhanced microbicidal activity leading to a reduction in the intracellular survival of B. pseudomallei (Fig. 5).
IRF-1 is a transcriptional activator which binds to sites within the promoters of a number of genes, including the iNOS gene (14, 19). Typically, IRF-1 is not expressed at a detectable level in unstimulated murine macrophages. However, IRF-1 expression can be induced by a variety of activators, including IFN-β (6). The expression of IRF-1 has been reported to be an important factor for the induction of the iNOS gene (14, 19). Moreover, IRF-1−/− mice were more susceptible to M. bovis than wild-type mice (14). This observation could be interpreted as indicating that the IRF-1−/− mice were unable to produce NO. In the present study, we demonstrated that a gram-negative bacterium like Salmonella serovar Typhi can stimulate IRF-1 expression and that this expression correlates with iNOS production (Fig. 2). However, when the MAb against IFN-β was added to Salmonella serovar Typhi-infected macrophages, IRF-1 was undetectable (Fig. 3). This result indicated that IFN-β acted in a paracrine fashion to autoregulate the expression of IRF-1 in macrophages infected with Salmonella serovar Typhi, leading to enhanced iNOS expression. In contrast, macrophages infected with B. pseudomallei did not produce IRF-1 at a level detectable by the immunoblotting procedure used in the present study (Fig. 2). The lack of IRF-1 production was most likely associated with the inability of B. pseudomallei-infected cells to produce IFN-β, because the addition of exogenous IFN-β to the system not only induced IRF-1 expression but also stimulated iNOS expression in these B. pseudomallei-infected macrophages (Fig. 4).
The pathogenesis of B. pseudomallei has been extensively investigated in a murine system, but very limited information on the mechanism(s) of intracellular bacterial survival is currently available. Previously, Utaisincharoen et al. demonstrated that B. pseudomallei could invade macrophages without significantly stimulating iNOS expression but that this expression could be enhanced by IFN-γ (27). In the present study, we showed that the inability of this bacterium to stimulate iNOS was related to the failure of B. pseudomallei-infected macrophages to produce IFN-β and IRF-1 (Fig. 1 and 2). However, exogenous IFN-β could reverse this defect and reinstall the antimicrobial activity of B. pseudomallei-infected macrophages (Fig. 5). It is possible that in an in vivo animal model, IFN-β in conjunction with IFN-γ produced from other sources can synergistically enhance resistance to and reduce the severity of B. pseudomallei infection. These points should be investigated to provide a clear understanding of the pathogenesis of melioidosis.
Acknowledgments
This work was supported by research grants from the Thailand Research Fund (TRF) and Chulabhorn Research Institute.
Editor: J. D. Clements
REFERENCES
- 1.Anuntagool, N., P. Intachote, V. Wuthiekanun, N. J. White, and S. Sirisinha. 1998. Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from lipopolysaccharide from virulent Ara− clinical isolate. Clin. Diagn. Lab. Immunol. 5:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdan, C. 2000. The function of type I interferon in antimicrobial immunity. Curr. Opin. Immunol. 12:419-424. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C., M. Rollinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul, W., N. J. White, D. A. B. Dance, Y. Wattanagoon, and N. Pitawatchara. 1989. Melioidosis: a major cause of community acquired septicemia in northeastern Thailand. J. Infect. Dis. 159:890-899. [DOI] [PubMed] [Google Scholar]
- 5.Denis, M. 1991. Recombinant murine beta interferon enhances resistance of mice to systemic Mycobacterium avium infection. Infect. Immun. 59:1857-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiki, T., and A. Tanaka. 1988. Antibacterial activity of recombinant mouse beta interferon. Infect. Immun. 56:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 8.Harley, V. S., D. A. B. Dance, B. J. Drasar, and G. Tovey. 1998. Effects of Burkholderia pseudomallei and other Burkholderia species on eukaryotic cells in tissue culture. Microbios 96:71-93. [PubMed] [Google Scholar]
- 9.Havell, E. A. 1986. Augmented induction of interferon during Listeria monocytogenes infection. J. Infect. Dis. 153:960-969. [DOI] [PubMed] [Google Scholar]
- 10.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe, L. C., D. A. Sampath, and M. Spotnitz. 1971. The pseudomallei group: a review. J. Infect. Dis. 124:598-604. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, A. T., and L. J. Ignarro. 2001. LPS-induced expression of IFN-β mediates the timing of iNOS induction in RAW 264.7 macrophages. J. Biol. Chem. 51:47950-47957. [DOI] [PubMed] [Google Scholar]
- 13.Jones, A. L., T. J. Beveridge, and D. E. Woods. 1996. Intracellular survival of Burkholderia pseudomallei. Infect. Immun. 64:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamijo, R., H. Harda, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, S. J. Green, T. W. Mak, T. Taniguchi, and J. Vilcek. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 15.Kawahara, K., S. Dejsirilert, H. Danbara, and T. Ezaki. 1992. Extraction and characterization of lipopolysaccharide from Pseudomonas pseudomallei. FEMS Microbiol. Lett. 96:129-134. [DOI] [PubMed] [Google Scholar]
- 16.Kespichayawattana, W., S. Rattanachetkul, T. Wanun, P. Utaisincharoen, and S. Sirisinha. 2000. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect. Immun. 68:5377-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kierszenbaum, F., and G. Sonnenfeld. 1982. Characterization of the antiviral activity produced during Trypanosoma cruzi infection and protective effects of exogenous interferon against experimental “Chagas”disease. J. Parasitol. 68:194-198. [PubMed] [Google Scholar]
- 18.Leelarasame, S., and S. Bovonkitti. 1989. Melioidosis: review and update. Rev. Infect. Dis. 11:413-425. [DOI] [PubMed] [Google Scholar]
- 19.Martin, E., C. Nathan, and Q. W. Xie. 1994. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 180:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattner, J., H. Schindler, A. Diefenbach, M. Rollinghoff, I. Gresser, and C. Bogdan. 2000. Regulation of type 2 nitric oxide synthase by type 1 interferon in macrophages infected with Leishmania major. Eur. J. Immunol. 30:2257-2267. [DOI] [PubMed] [Google Scholar]
- 21.Miyaki, K., K. Kawakami, and A. Saito. 1997. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect. Immun. 67:4108-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orellana, M. A., Y. Suzuki, F. Araujo, and J. S. Remington. 1991. Role of beta interferon in resistance to Toxoplasma gondii infection. Infect. Immun. 59:3287-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santanirand, P., V. S. Harley, D. A. B. Dance, B. S. Drasar, and G. J. Bancroft. 1999. Obligatory role of gamma interferon for host survival in murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sign, A., T. Merlin, H.-P. Knopf, P. J. Nielsen, H. Loppnow, C. Galanos, and M. A. Freudenberg. 2000. Bacterial induction of beta interferon in mice is a function of the lipopolysaccharide component. Infect. Immun. 68:1600-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toshchakov, V., B. W. Jones, P. Perera, K. Thomas, M. J. Cody, S. Zhang, B. R. G. Williams, J. Major, T. A. Hamilton, M. J. Feton, and S. N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-β-dependent gene expression in macrophages. Nat. Immunol. 3:392-398. [DOI] [PubMed] [Google Scholar]
- 26.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, N. Anuntagool, P. Chaisuriya, and S. Sirisinha. 2000. Kinetic studies of the production of nitric oxide (NO) and tumor necrosis factor alpha (TNF-α) in macrophages stimulated with Burkholderia pseudomallei endotoxin. Clin. Exp. Immunol. 122:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, P. Chaisuriya, and S. Sirisinha. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol. Immunol. 45:307-313. [DOI] [PubMed] [Google Scholar]
- 28.Vadiveloo, P. K., G. Vario, P. Hertzog, I. Kola, and J. A. Hamilton. 2000. Role of type I interferons during macrophages activation by lipopolysaccharide. Cytokine 12:1639-1646. [DOI] [PubMed] [Google Scholar]
- 29.Wuthiekanun, V., M. D. Smith, D. A. B. Dance, A. L. Walsh, T. L. Pitt, and N. J. White. 1996. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J. Med. Microbiol. 45:408-412. [DOI] [PubMed] [Google Scholar]
- 30.Yabuuchi, E., and M. Arakawa. 1993. Burkholderia pseudomallei and melioidosis: be aware in temperate area. Microbiol. Immunol. 37:823-836. [DOI] [PubMed] [Google Scholar]