In order to successfully colonize or invade a host, microorganisms need mechanisms to escape recognition by the immune system or to modulate the responses directed against the infecting agent. Streptococcus pyogenes produces a number of extracellular enzymes, several of which interact with the host immune system. These interactions might be of importance for the host-parasite interplay and the development of disease. Of specific interest are enzymatic activities that are targeted toward components of the host immune system (summarized in Table 1). These enzymes either directly or indirectly modulate the activity of immune defense molecules such as immunoglobulins, complement factors, or other inflammatory mediators. Our review presents an update on immunomodulating enzymes from S. pyogenes and discusses their influence on the development of both acute and secondary diseases. These enzymatic activities are also discussed in a wider perspective by comparisons with similar systems of other human pathogens.
TABLE 1.
Immunomodulating enzymatic activities expressed by S. pyogenes
| Enzyme | Type of enzyme | Activities | Reference(s) |
|---|---|---|---|
| C5a peptidase | Serine proteinase | Cleaves the complement factor C5a | 114 |
| EndoS | Endo-β-N-acetylglucosaminidase | Hydrolyzes the N-linked glycan on IgG | 16 |
| IdeS/Mac | Cysteine proteinase | Cleaves IgG and inhibits opsonophagocytosis | 61, 111 |
| NADase | NAD+-glycohydrolase | Alters neutrophil migration | 55, 71, 103 |
| ADP-ribosyltransferase | Translocates into cells mediated by SLO | ||
| SAGP | Arginine deiminase | Antitumor activity; inhibition of T-cell proliferation | 23, 24, 52 |
| SOD | SOD | Detoxification of oxygen radicals | 35 |
| SpeB | Cysteine proteinase | See Table 2 | See Table 2 |
S. pyogenes is one of the most common human pathogens and the causative agent of pharyngitis accounting for 15 to 30% of all cases in children and 5 to 10% in adults (5). S. pyogenes also infects skin and soft tissue, especially among people living in warm and humid climates. Most of these infections, such as impetigo, erysipelas, and cellulitis, are localized to the skin (6). However, in a significant proportion of these infections bacteria disseminate into deeper tissue, which subsequently leads to necrotizing fasciitis with substantial destruction of fascia and adipose tissue. Further dissemination of the bacteria can ultimately lead to sepsis and a toxic shock syndrome (TSS) with high mortality. The incidence of these types of serious infection has increased lately, with an overrepresentation of isolates of the M1 and M3 serotypes (82, 102).
In addition to acute infections, there are a number of aseptic sequelae affecting different organ systems. For example, acute poststreptococcal glomerulonephritis (APSGN) that can lead to renal failure, and acute rheumatic fever (ARF) presenting with joint inflammation, carditis, symptoms from the central nervous system, and skin manifestations (reference 21 and references therein).
MICROBIAL IMMUNOGLOBULIN PROTEASES
Immunoglobulins (antibodies) produced by B lymphocytes in response to foreign material are crucial molecules in the humoral and mucosal defense against infectious agents. Antibodies that are directed toward microorganisms recruit complement factors and direct leukocytes to the site of infection, which ultimately leads to phagocytosis and killing of the microorganism. In order to combat an attack from the immune system many microbial pathogens produces enzymes that cleave or inactivate immunoglobulins, which have been suggested to contribute to pathogenesis. For instance, microbial proteases capable of cleaving the hinge region of human mucosal antibodies, e.g., immunoglobulin A (IgA), have been extensively studied. Even though the flexible hinge region of IgA1 is protected from proteolysis by multiple O-linked glycans (75), several pathogens have evolved specific IgA-proteases that cleave at specific sites in the hinge region of IgA and thus overcome the protective ability of the glycans (for a review, see reference 84). The first examples of IgA-proteases were described in Streptococcus sanguis and Neisseria spp. in the mid-1970s (85). Subsequently, IgA-proteases have been described for a number of bacterial species that colonize or infect the mucosal membranes of humans, such as oral streptococci (57), Haemophilus influenzae, and Streptococcus pneumoniae (58, 72). As a result of this specific IgA-protease activity, the IgA molecule is cleaved into a stable Fc fragment and two monomeric Fab fragments that retain their antigen-binding capacity (73, 74). IgA2 is more resistant to proteolysis due to the lack of a specific peptide stretch that can be found in the hinge region of IgA1 (87). These IgA-proteases have been shown to inactivate IgA by cleaving in the hinge region (86), but their importance as virulence determinants has been debated. Early studies suggested that IgA-protease activity distinguishes pathogenic from nonpathogenic Neisseria spp. (80), and recent studies indicate that invasive Neisseria meningitidis isolates are enhanced in the IgA-protease activity compared to colonizing strains (110). Furthermore, IgA-proteases have been identified as virulence factors in nontypeable H. influenzae infections (109).
Interestingly, no specific IgA-protease that cleaves the hinge region has been described in S. pyogenes. However, it was recently shown that the activity from the streptococcal cysteine proteinase SpeB is capable of degrading the COOH-terminal part of IgA (15). The importance of this IgA-degrading activity needs to be further investigated (see below).
Microbial IgG-proteases have not been as extensively studied as the IgA-proteases. However, there are accumulating data describing microbial IgG-degrading proteases. These include the oral pathogens Prevotella intermedia and Prevotella nigrescens isolated from periodontal pockets and oral abscesses. Inhibition experiments suggest that these pathogens degrade IgG due to cysteine proteinase activity (47). In addition, a Pseudomonas aeruginosa elastase implicated as a virulence factor degrades human IgG in vitro, and its activity could be inhibited by local treatment with the protease inhibitor α2-macroglobulin (43). Furthermore, a secreted cysteine proteinase from the helminth parasite Paragonimus westermani attenuated the effector functions of human eosinophils stimulated with IgG (100), and proteases from Serratia marcescens cleave both IgG and IgA around the hinge region (78). Thus, modulations of IgG by cysteine proteinases seem to be a common theme among pathogenic microorganisms.
Streptococcal cysteine proteinase SpeB.
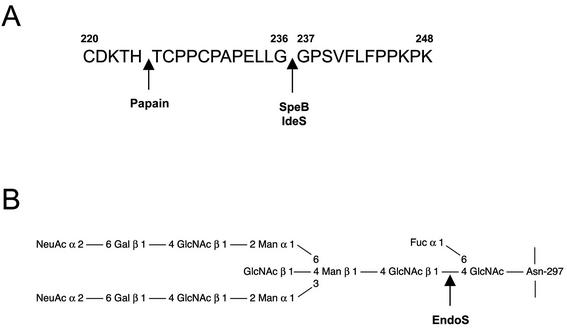
S. pyogenes expresses a cysteine proteinase, SpeB, shown to be identical to the streptococcal pyrogenic-erytrogenic exotoxin B (33, 38). This proteinase is one of the most extensively studied secreted proteins from S. pyogenes. Its activity on the streptococcal M protein and human fibrin was discovered by Stuart Elliott already in the 1940s (28). The gene encoding SpeB is highly conserved and is present in essentially all S. pyogenes isolates (117). The three-dimensional structure of the zymogen form of SpeB has been determined, revealing a fold similar to papain despite negligible sequence identity (51). Papain is a cysteine proteinase that cleaves IgG in the hinge region and is commonly used to obtain Fc and monomeric Fab fragments from IgG (see Fig. 1A) (8).
FIG. 1.
SpeB, IdeS, and EndoS activity on human IgG. Cleavage sites in human IgG of S. pyogenes IgG-proteases and IgG glycan-hydrolases. (A) Residues 220 to 248 of the flexible hinge region of IgG1 heavy chain showing where SpeB and IdeS cleave between glycine residues 236 and 237. The papain cleavage site is shown for comparison. (B) Diagram showing one of the two identical conserved N-linked glycans attached to asparagine 297 in the heavy chain of IgG and also where EndoS hydrolyzes the chitibiose core of the glycan, leaving the innermost GlcNAc with a core fucose.
Interestingly, it was recently shown that SpeB cleaves IgG in a manner similar to IgA-proteases; IgG is cleaved at a defined site in the hinge region into two stable monomeric Fab fragments and one Fc fragment (16). The degradation pattern of IgG resembles that of papain, but the cleavage site is different; SpeB cleaves between two glycines where the IgG heavy chain is more flexible (Fig. 1A). It is not very surprising that the IgG degradation by SpeB significantly reduces the capacity of opsonizing IgG to kill S. pyogenes in human blood (18). This was further supported when an isogenic SpeB mutant strain was shown to persist a significantly shorter time than the corresponding wild-type strain in blood containing strain-specific antibodies (29). SpeB was the first described S. pyogenes enzyme with immunoglobulin-protease activity, adding to the growing list of its activities, emphasizing its involvement in pathogenesis. In addition to the activity on IgG, SpeB degrades the COOH-terminal parts of the heavy chains of IgA, IgM, and IgD into low-molecular-weight fragments, whereas the heavy chains of IgE are completely degraded (15).
SpeB also has other immunomodulating activities such as the release of proinflammatory molecules that could be important for the symptoms seen in S. pyogenes infections (summarized in Table 2). For instance, the cytokine precursor interleukin-1β (IL-1β) is cleaved by SpeB into an active IL-1β, which is a strong inflammatory mediator (54). Furthermore, SpeB has the ability to release the potent proinflammatory and vasoactive peptide hormone bradykinin from its precursor H-kininogen (42). This release of bradykinin could be one of the explanations for the hypovolemic hypotension seen in sepsis caused by S. pyogenes. Interestingly, kinin release by cysteine proteinases has also been demonstrated in the periodontitis-causing bacterium Porphyromonas gingivalis (98), as well as in the parasitic protozoan Trypanosoma cruzi (25). In addition, SpeB can degrade proteoglycans such as decorin with the release of dermatan sulfate that inhibits the neutrophil-derived antibacterial peptide α-defensin (97). Furthermore, SpeB directly cleaves and inactivates the antibacterial peptide LL-37, which is capable of killing S. pyogenes (96). Moreover, a recent study showed that purified SpeB stimulates the release of histamine from a human mast cell line (113). Some reports have suggested that SpeB also functions as a superantigen, with stimulation of T lymphocytes without antigen presentation, and that the activity is independent of the proteolytic activity (30, 64). In contrast, evidence has been presented that SpeB does not have superantigenic properties and that the observed activity rather originates from contaminating SpeA, SpeC, or unknown mitogens (34).
TABLE 2.
Immunomodulating activities of SpeB
| Host molecule | Activity | Reference |
|---|---|---|
| H-kininogen | Release of bradykinin | 42 |
| IL-1β | Activation | 54 |
| Decorin | Release of dermatan sulfate that inhibits antibacterial peptides | 97 |
| Antibacterial peptide LL-37 | Degradation | 96 |
| IgG | Cleavage in the hinge region | 16 |
| IgA, IgM, IgD, and IgE | Degradation | 15 |
| Histamine | Release from mast cells | 113 |
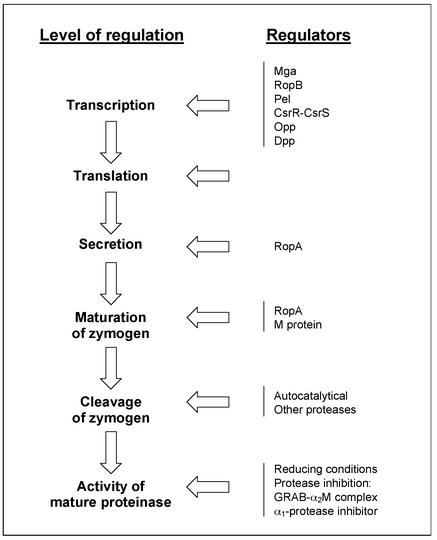
The regulation of SpeB activity is highly complex and influenced by a number of parameters during synthesis (summarized in Fig. 2). SpeB is transcribed during early stationary phase and downregulated by glucose and other nutrients in the growth medium (11). The global transcriptional regulator mga (91), the speB-specific ropB in the Rop loci (regulation of proteinase) (70), and pel (pleotropic effect locus) have been shown to be positive regulators of speB expression (65). Whether the two-component system CsrR-CsrS represses speB is still debated (31, 40). Two peptide permeases have also been suggested to regulate SpeB production (88, 89).
FIG. 2.
Schematic overview of the factors influencing SpeB activity. The column to the left outlines the synthesis and maturation of SpeB from transcription to mature active enzyme. The right column shows examples of different regulators know to affect the process and on what level it takes place.
The proregion of SpeB has a unique fold and inactivation mechanism that displaces the catalytically essential His residue from the active site (51). It is known that purified zymogen from streptococci is partly enzymatically active and can cleave itself under reducing conditions (9). Autocatalysis is an intermolecular event with sequential processing with at least six intermediates (26). This process does not occur when ropA, the second gene of the Rop loci, has been inactivated. RopA assists SpeB in translocation via the secretory pathway and functions as a molecular chaperone that aids the zymogen to its autocatalytically active state (70). Furthermore, when there is no cell wall-anchored M protein, the SpeB zymogen is secreted in a conformational state that does not allow autocatalytical processing (17).
Active SpeB is inhibited by a peptide inhibitor based on the active site of cystatin C (7). S. pyogenes binds the broad-spectrum proteinase inhibitor α2-macroglobulin via the cell wall-anchored protein GRAB, forming a complex that regulates proteolytic activity of SpeB (94). The S-nitrosylated form of α1-protease inhibitor also inhibits SpeB (76).
SpeB production does not affect bacterial viability in vitro (10), but many animal studies have suggested its importance for the balance of the host-parasite interaction. For example, isogenic speB mutant strains are significantly less lethal to mice when challenged intraperitoneally (68) and caused less mortality and tissue damage when mice were infected subcutaneously (59, 67). Furthermore, bacteria lacking SpeB are less resistant to phagocytosis and do not disseminate into internal organs as do the wild-type bacteria (66). SpeB also plays a role in host tissue tropism, since SpeB activity increased the bacterial reproduction in a mouse impetigo model (104), and SpeB acts synergistically with cell wall antigens and streptolysin O (SLO) to induce lung injury in rats (99).
The relevance of animal models can be debated, especially since S. pyogenes exclusively infects humans, but there are some studies of human infections also supporting a role for SpeB in pathogenesis, even though there are somewhat conflicting results. Patients with invasive disease caused by different serotypes of S. pyogenes seroconverted to SpeB, indicating that SpeB is expressed in vivo during infection (36). On the other hand, patients with severe invasive disease have low antibody titers against SpeB, suggesting that an inability to produce SpeB-specific antibodies contributes to the development of serious conditions (44). Furthermore, isolates of the M1 serotype from TSS patients are associated with SpeB production (105). In contrast, another study showed that there is an inverse relationship between SpeB production and disease severity, possibly due to a sparing of the M protein on the surface (53). Epidemiological evidence suggests a correlation between SpeB production and a genetic marker for preferred tissue site of infection at the skin (104). Furthermore, SpeB has been suggested to play a role in the development of APSGN (20); patients with APSGN have elevated antibody levels against SpeB, and SpeB can be detected in glomerulonephritis biopsies (19).
Taken together, SpeB is a multifunctional protease with several immunomodulating activities that could affect both mucosal and systemic immunological functions. Even though there are conflicting reports of the importance of SpeB as a virulence factor, it is clear that SpeB needs to be taken into account in any consideration of the various aspects of the interaction between S. pyogenes and the human host both during acute infection and in aseptic sequelae.
IdeS, a second cysteine proteinase.
In addition to SpeB, a second secreted cysteine proteinase, IdeS (immunoglobulin G-degrading enzyme of S. pyogenes), was recently discovered (111). Interestingly, IdeS cleaves human IgG in the hinge region at the same position as SpeB (Fig. 1A). This results in an inhibition of antibody-mediated phagocytosis of the bacteria. IdeS, which has a higher degree of specificity compared to SpeB, does not degrade the other immunoglobulin isotypes (111). Interestingly, IdeS is identical to the previously described protein Mac (group A streptococcal Mac-1-like protein) that binds Fc receptors (61). Lei et al. (61) suggested that the activity of Mac/IdeS mimics that of a leukocyte integrin, Mac-1, and thereby inhibits opsonophagocytosis of the bacteria. However, von Pawel-Rammingen et al. (111) discovered that IdeS is a cysteine proteinase that specifically cleaves IgG in the hinge region, which most likely explains the activity described for Mac. Furthermore, an enzymatically inactive IdeS generated by site-directed mutagenesis still binds to neutrophils but does not inhibit phagocytosis as the wild-type protein (112). Whether IdeS/Mac can inhibit phagocytosis independently of proteolytic activity is still debated (62), and a recent study reported that site-directed mutagenesis of the active site did not affect the ability to inhibit phagocytosis (63). The gene encoding IdeS was shown to be present in many isolates (111), and the protein is secreted by several serotypes. Moreover, patients with S. pyogenes infections have antibody titers against IdeS, indicating in vivo expression (61). Interestingly, a recent study identified a protein identical to IdeS/Mac as a surface-localized anchorless protein that binds with high affinity to immunoglobulins, thus confirming the interaction with IgG, but did not discuss the cysteine proteinase activity or similarity to IdeS/Mac (56). Taken together, these data indicate that S. pyogenes expresses two unrelated cysteine proteinases that can cleave IgG, thus emphasizing the importance of this immunomodulating mechanism.
IMMUNOGLOBULIN GLYCAN-HYDROLASES
Apart from enzymes capable of cleaving or degrading the peptide backbone of IgG, S. pyogenes expresses enzymes that hydrolyze the conserved N-linked glycans on glycoproteins. First, an extracellular neuraminidase activity was described to release sialic acid from bovine submaxillary mucins (22, 39). Another study showed that strains isolated from patients with APSGN produce a neuraminidase activity that releases terminal sialic acids from the glycans on human IgM, IgG, fibrinogen, and renal basement membranes. These alterations of the immunoglobulins were suggested to play a role in the development of APSGN, since all of the nephritogenic strains tested, but none of the rheumatogenic strains tested, expressed this activity (79). However, no neuraminidase gene has been described or found in the S. pyogenes genomes that have been sequenced, and convincing data indicate that S. pyogenes does not produce any true neuraminidase (95). The sialic acid-releasing activity previously observed is most likely due to other glycan-hydrolyzing enzymes, such as EndoS, that cleave further down in the N-linked glycans of human glycoproteins.
EndoS, a specific IgG glycan-hydrolase.
An extracellular endoglycosidase, EndoS, which has a specific activity on the conserved N-linked glycan located in the constant portion of the heavy chain of IgG, was recently identified (16). EndoS is a 108-kDa secreted enzyme with a conserved family 18-chitinase motif. Enzymes belonging to this family hydrolyze N-acetylglucosamine polymers (chitin) and some of them, e.g., EndoF and EndoH, have activity on the chitin core of N-linked glycans on glycoproteins (41, 107, 108). EndoS hydrolyzes the β1,4-di-N-acetylchitobiose core on the N-linked oligosaccharide of IgG, which leaves the innermost GlcNAc with an attached fucose on the peptide backbone (Fig. 1B).
The conserved N-linked glycan on the IgG heavy chain is crucial for several effector functions, including complement activation and binding to Fc receptors on leukocytes (69, 93, 115). Therefore, a bacterial enzyme hydrolyzing this functionally important glycan could have profound effects on IgG-mediated processes.
A classical method in streptococcal research was developed by Lancefield (60) that involves a bactericidal or antiphagocytic assay based on the notion that S. pyogenes with M protein present on its surface survives in fresh human nonimmune blood but is rapidly opsonized and killed if the blood contains M-type-specific antibodies.
An experiment was designed to investigate the importance of the conserved glycan structure on opsonizing antibodies. Thus, when purified opsonizing IgG directed against the M protein was treated with EndoS in vitro and used in a modified classical bactericidal assay, it was significantly impaired in its ability to kill S. pyogenes bacteria in human blood compared to untreated IgG (18). The main explanations for the reduced killing of bacteria was the inability of the EndoS-treated IgG to bind to Fc receptors on monocytic cells and also decreased IgG-mediated complement deposition. Our results underline the functional importance of the glycan on IgG and reveal a novel interaction between pathogenic bacteria and immunoglobulins. Thus, EndoS is an example of a bacterial strategy that interferes with the function of an important host defense molecule, IgG. The specificity of EndoS was further reinforced when native and denatured IgG was incubated with EndoS, which revealed that when IgG is denatured, EndoS is unable to hydrolyze the glycan (15). As a comparison, many other endoglycosidases such as EndoF1 and EndoF2 have enhanced activities when the substrate glycoprotein is denatured (106). It indicates that the tertiary structure of the whole IgG molecule, and not only the glycan, is important for the enzymatic activity.
EndoS was originally identified in the AP1 strain of M1 serotype by N-terminal sequencing of extracellular proteins. By using the first completed S. pyogenes genome-sequencing project of the M1 strain SF370, it was possible to identify the complete gene encoding EndoS, ndoS, which was subsequently sequenced also in the AP1 strain (16, 32) (accession numbers NP_269818 and AAK00850, respectively). PCR experiments revealed that the ndoS gene could be amplified in strains of 10 different M serotypes, suggesting that ndoS is a widely distributed in S. pyogenes (M. Collin and A. Olsén, unpublished results). In addition, ndoS homologs have been sequenced and annotated in the M18 strain MGAS8232 completed sequencing project (101) (accession number AAL98385), as well as in the recently published genome of the M3 strain MGAS315 (3) (accession number AAM80175). Furthermore, a similar open reading frame could be identified in the unfinished genome sequence of the S. pyogenes M5 strain Manfredo (http://www.sanger.ac.uk/Projects/S_pyogenes/).
Interestingly, an open reading frame similar to ndoS could also be identified in the unfinished genome sequence of a Streptococcus equi strain (http://www.sanger.ac.uk/Projects/S_equi/). S. equi belongs to group C streptococci and is closely related to group A streptococci (S. pyogenes). S. equi is primarily a horse pathogen, but group C streptococci can cause a number of suppurative mucosal infections in mammals, including humans (37). Preliminary experiments indicate that group C streptococci isolated from human infections secrete an EndoS homolog that hydrolyzes the glycan on human IgG, which cross-react with EndoS antibodies (Collin and Olsén, unpublished).
When the deduced amino acid sequences of the proteins similar to EndoS are aligned, it is clear that the proteins are highly similar and most likely true homologs (Fig. 3). The sequence data are still limited, but this might indicate a selective pressure on streptococci infecting humans to conserve these IgG glycan hydrolases.
FIG. 3.
CLUSTALW amino acid sequence alignment of EndoS homologs from different S. pyogenes serotypes and S. equi. Strain names or species are shown to the left and M serotypes are shown in parentheses. Amino acid identities are shown in dark gray, similarities are shown in light gray, and the consensus sequence is shown under the alignment. The conserved chitinase motif is boxed, and the glutamic acid essential for activity is marked with an asterisk above the alignment.
Immunoglobulin glycosylation and autoimmunity.
Abnormalities in IgG glycosylation have been discovered in a number of human autoimmune disorders such as systemic lupus erythematosus, inflammatory bowel diseases, and rheumatoid arthritis (27, 83). In rheumatoid arthritis, it has been demonstrated that isolated B lymphocytes have reduced galactosyltransferase activity, leading to higher levels of truncated, so-called agalactosyl glycans on IgG (2). Defects in IgG-glycosylation have also been shown to be important for the development of cryoglobulin-induced lupus-like glomerulonephritis in mice (77). An interesting hypothesis could thus be postulated: that IgG hydrolyzed by EndoS is important in the development of ARF and APSGN, where autoimmunity and antibody complexes are implicated in the disease processes. It should be noted that one of the major factors shown to contribute to the development of APSGN are the specific streptokinases (45, 81), but of potential interest is the finding that a neuraminidase activity against IgG is associated with glomerulonephritis induced by S. pyogenes (79). As previously discussed, EndoS might contribute to the observed neuraminidase activity, but further investigation is needed to evaluate the importance of EndoS in the development of diseases such as ARF and APSGN
COMPLEMENT-DEGRADING ENZYMES
C5a peptidase.
One of the most studied immunomodulating enzymes from S. pyogenes is the C5a peptidase, ScpA. ScpA is an excellent example of an enzyme that targets a specific component of the human immune defense. ScpA is a cell-wall-anchored 130-kDa serine endopeptidase that specifically cleaves the complement factor C5a (14, 114). By cleaving the chemotactic complement factor C5a, ScpA inhibits recruitment of phagocytic cells to the infectious site (49). C5a has also been shown to be important in activating neutrophils that phagocytize the bacteria, underlining the relevance of the activity of ScpA (50). Furthermore, intranasal immunization with C5a peptidase has been shown to prevent nasopharyngeal colonization of mice by S. pyogenes (48). Moreover, in a mouse model of long-term colonization, an S. pyogenes strain lacking the gene encoding ScpA caused pneumonia at a lower frequency than did wild-type bacteria (46). An interesting finding is that SpeB can release functional fragments of ScpA that subsequently inactivate C5a at a distance from the bacterium (4). The scpA gene has been shown to be highly similar to the group B streptococcal scpB gene, suggesting horizontal gene transfer between the species (13). Furthermore, ScpB in group B streptococci has been shown to contribute to cellular invasion in addition to the enzymatic activity (12). Taken together, these findings indicate that ScpA is important for the disease process of S. pyogenes infections.
ADDITIONAL ENZYMES WITH IMMUNOMODULATING ACTIVITIES
In addition to the immunomodulating activities such as protein hydrolysis or glycan modification, S. pyogenes produces various enzymes belonging to other classes. One such enzyme is represented by the streptococcal acid glycoprotein (SAGP) that was originally described as an antitumour protein (52). SAGP has arginine deiminase activity and inhibits T-lymphocyte proliferation (24). In addition, this SAGP is important for bacterial survival at low pH, possibly contributing to intracellular survival (23).
Another enzyme is the NAD+-glycohydrolase (NADase) that not only hydrolyzes NAD+ into adenosin-diphosphoribose and nicotinamide but also synthesizes the signaling molecule cyclic ADP-ribose (1, 55). It has also been shown that the pore-forming cytolysin SLO aids NADase to penetrate the membrane of host cells and thereby induces cytotoxicity (71). An explanation for the earlier finding could be that NADase purified from group A streptococci alters neutrophil-directed migration (103). Moreover, in their study they also found NADase to be expressed by S. pyogenes strains associated with an outburst of TSS, suggesting that NADase activity contributes to the development of severe streptococcal disease (103).
Superoxide anions are involved in the phagocytic killing of bacteria. Bacterial superoxide dismutases (SODs) detoxify superoxide anions and are a major defense mechanism against oxidative stress. Genes encoding SOD have been identified in several gram-positive bacteria, including S. pyogenes (92). S. pyogenes produces a manganese-dependent SOD (35), but no direct evidence for a role of SOD in oxidative stress resistance has been presented. However, insertional inactivation of sodA, encoding a manganese-dependent SOD homolog in S. pneumoniae, significantly reduced the pneumococcal virulence in a mouse model (116). This indirectly suggests that SOD in S. pyogenes could be of importance for resistance against oxygen radicals produced by the hosts phagocytic cells.
CONCLUDING REMARKS
The strictly human pathogen S. pyogenes has evolved a number of extracellular enzymes of various types that interact with the host immune defense. Some of them, like SpeB, are nonspecific and targeted toward many host molecules, whereas others, such as the C5a peptidase, EndoS, and IdeS, are specific for single molecules in the human immune system. Thus, S. pyogenes expresses a set of broad-spectrum detoxifying and immunomodulating enzymes, providing basal protection against, for instance, oxygen radicals and low pH, and also a number of specific immunomodulating enzymes targeted toward important host molecules, such as IgG and the complement factor C5a. This indicates that S. pyogenes has evolved several different enzymatic strategies to circumvent host defense mechanisms in order to successfully colonize and disseminate within a host. Moreover, the fact that one of the key molecules in the adaptive immune response, IgG, serves as a substrate for at least two proteases (SpeB and IdeS) and one endoglycosidase (EndoS) is unlikely to be a coincidence but rather indicates a underlying evolutionary pressure driving the development of enzymes modulating immunoglobulins and also conservation of such genes. This hypothesis is supported by the fact that the speB gene is present in most isolates and is, in addition, highly conserved. Even though there is still limited sequence data available for ndoS and ideS, these genes seem to be abundant and/or highly conserved as well.
One could argue that a hydrolyzing activity toward immunoglobulins, which circulate in high concentrations in human blood, could primarily have evolved for bacterial nutrient acquisition. However, when it comes to SpeB it has been shown that its regulation is influenced by nutrient concentrations but is not directly involved in the acquisition of the nutrients (90). Furthermore, an isogenic EndoS mutant strain was not affected in its growth rate compared to the wild type when it was grown in a plasma environment, suggesting that EndoS does not play a major role in nutrient acquisition (16).
Some of the discussed enzymatic activities inhibit inflammatory processes (immunoglobulin and complement degradation and inhibition of T-lymphocyte proliferation), whereas others are proinflammatory (kinin and interleukin release, superantigenic activity). Probably all of these activities working in different directions contribute to balancing the host-parasite relationship, leading to a more successful colonization and spread of the bacteria. Unbalanced or uncontrolled production of enzymes could contribute to severe invasive disease but also be of importance for postinfectious autoimmune reactions such as ARF and APSGN. Further studies on extracellular enzymes targeted toward components of the immune defense will broaden our understanding of how this pathogen interacts with its host and causes disease.
Acknowledgments
This work was supported by grants from the Wenner-Gren Foundation and the Swedish Research Council (project 14272).
We especially thank Vincent A. Fischetti for currently hosting M.C. in his laboratory. We are indebted to the Sanger Institute, Michael Kehoe (University of Newcastle), Duncan Maskell (The University of Cambridge), and Neil Chanter (Animal Health Trust) for making the in-progress genomic sequence of S. pyogenes Manfredo and S. equi publicly available.
Editor: D. A. Portnoy
REFERENCES
- 1.Ajdic, D., W. M. McShan, D. J. Savic, D. Gerlach, and J. J. Ferretti. 2000. The NAD-glycohydrolase (nga) gene of Streptococcus pyogenes. FEMS Microbiol. Lett. 191:235-241. [DOI] [PubMed] [Google Scholar]
- 2.Axford, J. S., L. Mackenzie, P. M. Lydyard, F. C. Hay, D. A. Isenberg, and I. M. Roitt. 1987. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet ii:1486-1488. [DOI] [PubMed]
- 3.Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M. Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berge, A., and L. Björck. 1995. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J. Biol. Chem. 270:9862-9867. [DOI] [PubMed] [Google Scholar]
- 5.Bisno, A. L. 2001. Acute pharyngitis. N. Engl. J. Med. 344:205-211. [DOI] [PubMed] [Google Scholar]
- 6.Bisno, A. L., and D. L. Stevens. 1996. Streptococcal infection of skin and soft tissue. N. Engl. J. Med. 334:240-245. [DOI] [PubMed] [Google Scholar]
- 7.Björck, L., P. Åkesson, M. Bohus, J. Trojnar, M. Abrahamson, I. Olafsson, and A. Grubb. 1989. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature 337:385-386. [DOI] [PubMed] [Google Scholar]
- 8.Burton, D. R. 1985. Immunoglobulin G: functional sites. Mol. Immunol. 22:161-206. [DOI] [PubMed] [Google Scholar]
- 9.Bustin, M., M. C. Lin, W. H. Stein, and S. Moore. 1970. Activity of the reduced zymogen of streptococcal proteinase. J. Biol. Chem. 245:846-849. [PubMed] [Google Scholar]
- 10.Chaussee, M. S., D. Gerlach, C.-E. Yu, and J. J. Ferretti. 1993. Inactivation of the streptococcal erythrogenic toxin B gene (speB) in Streptococcus pyogenes. Infect. Immun. 61:3719-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaussee, M. S., E. R. Phillips, and J. J. Ferretti. 1997. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect. Immun. 65:1956-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng, Q., D. Stafslien, S. S. Purushothaman, and P. Cleary. 2002. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect. Immun. 70:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chmouryguina, I., A. Suvurov, P. Ferrieri, and P. P. Cleary. 1996. Conservation of the C5a peptidase genes in group A and B streptococci. Infect. Immun. 64:2387-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleary, P. P., U. Prahbu, J. B. Dale, D. E. Wexler, and J. Handley. 1992. Streptococcal C5a peptidase is a highly specific endopeptidase. Infect. Immun. 60:5219-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collin, M., and A. Olsén. 2001. Effect of SpeB and EndoS from Streptococcus pyogenes on human immunoglobulins. Infect. Immun. 69:7187-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collin, M., and A. Olsén. 2001. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 20:3046-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin, M., and A. Olsén. 2000. Generation of a mature streptococcal cysteine proteinase is dependent on cell wall-anchored M1 protein. Mol. Microbiol. 36:1306-1318. [DOI] [PubMed] [Google Scholar]
- 18.Collin, M., M. D. Svensson, A. G. Sjöholm, J. C. Jensenius, U. Sjöbring, and A. Olsén. 2002. EndoS and SpeB from Streptococcus pyogenes inhibit immunoglobulin-mediated opsonophagocytosis. Infect. Immun. 12:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cu, G. A., S. Mezzano, J. D. Bannan, and J. B. Zabriskie. 1998. Immunohistochemical and serological evidence for the role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Kidney Int. 54:819-826. [DOI] [PubMed] [Google Scholar]
- 20.Cu, G. A., S. Mezzano, and J. B. Zabriskie. 1997. Role of streptococcal proteinase in acute post-streptococcal glomerulonephritis. Adv. Exp. Med. Biol. 418:137-140. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis, L., M. M. Baig, and E. M. Ayoub. 1979. Properties of extracellular neuraminidase produced by group A streptococcus. Infect. Immun. 24:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degnan, B. A., M. C. Fontaine, A. H. Doebereiner, J. J. Lee, P. Mastroeni, G. Dougan, J. A. Goodacre, and M. A. Kehoe. 2000. Characterization of an isogenic mutant of Streptococcus pyogenes Manfredo lacking the ability to make streptococcal acid glycoprotein. Infect. Immun. 68:2441-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degnan, B. A., J. M. Palmer, T. Robson, C. E. Jones, M. Fischer, M. Glanville, G. D. Mellor, A. G. Diamond, M. A. Kehoe, and J. A. Goodacre. 1998. Inhibition of human peripheral blood mononuclear cell proliferation by Streptococcus pyogenes cell extract is associated with arginine deiminase activity. Infect. Immun. 66:3050-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Nery, E., M. A. Juliano, A. P. Lima, J. Scharfstein, and L. Juliano. 1997. Kininogenase activity by the major cysteinyl proteinase (cruzipain) from Trypanosoma cruzi. J. Biol. Chem. 272:25713-25718. [DOI] [PubMed] [Google Scholar]
- 26.Doran, J. D., M. Nomizu, S. Takebe, R. Menard, D. Griffith, and E. Ziomek. 1999. Autocatalytic processing of the streptococcal cysteine protease zymogen: processing mechanism and characterization of the autoproteolytic cleavage sites. Eur. J. Biochem. 263:145-151. [DOI] [PubMed] [Google Scholar]
- 27.Dube, R., G. A. Rook, J. Steele, R. Brealey, R. Dwek, T. Rademacher, and J. Lennard-Jones. 1990. Agalactosyl IgG in inflammatory bowel disease: correlation with C-reactive protein. Gut 31:431-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott, S. D. 1945. A proteolytic enzyme produced by group A streptococci with special reference to its effect on the type-specific M antigen. J. Exp. Med. 81:573-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson, A., and M. Norgren. 2003. Cleavage of antigen-bound immunoglobulin G by SpeB contributes to streptococcal persistence in opsonizing blood. Infect. Immun. 71:211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson, A., and M. Norgren. 1999. The superantigenic activity of streptococcal pyrogenic exotoxin B is independent of the protease activity. FEMS Immunol. Med. Microbiol. 25:355-363. [DOI] [PubMed] [Google Scholar]
- 31.Federle, M. J., K. S. McIver, and J. R. Scott. 1999. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J. Bacteriol. 181:3649-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerlach, D., H. Knöll, W. Köhler, J.-H. Ozegowski, and V. Hribalova. 1983. Isolation and characterization of erythrogenic toxins. V. Communication: identity of erythrogenic toxin type B and streptococcal proteinase precursor. Zentbl. Bakteriol. Hyg. I Abt. Orig. A 225:221-233. [PubMed] [Google Scholar]
- 34.Gerlach, D., W. Reichardt, B. Fleischer, and K. H. Schmidt. 1994. Separation of mitogenic and pyrogenic activities from so-called erythrogenic toxin type B (streptococcal proteinase). Zentbl. Bakteriol. 280:507-514. [DOI] [PubMed] [Google Scholar]
- 35.Gerlach, D., W. Reichardt, and S. Vettermann. 1998. Extracellular superoxide dismutase from Streptococcus pyogenes type 12 strain is manganese-dependent. FEMS Microbiol. Lett. 160:217-224. [DOI] [PubMed] [Google Scholar]
- 36.Gubba, S., D. E. Low, and J. M. Musser. 1998. Expression and characterization of group A streptococcus extracellular cysteine protease recombinant mutant proteins and documentation of seroconversion during human invasive disease episodes. Infect. Immun. 66:765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington, D. J., I. C. Sutcliffe, and N. Chanter. 2002. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 4:501-510. [DOI] [PubMed] [Google Scholar]
- 38.Hauser, A. R., and P. M. Schlievert. 1990. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and streptococcal proteinase precursor. J. Bacteriol. 172:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayano, S., and A. Tanaka. 1969. Sialidase-like enzymes produced by group A, B, C, G, and L streptococci and by Streptococcus sanguis. J. Bacteriol. 97:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heath, A., V. J. DiRita, N. L. Barg, and N. C. Engleberg. 1999. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect. Immun. 67:5298-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henrissat, B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 280:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herwald, H., M. Collin, W. Müller-Esterl, and L. Björck. 1996. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J. Exp. Med. 184:665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holder, I. A., and R. Wheeler. 1984. Experimental studies of the pathogenesis of infections owing to Pseudomonas aeruginosa: elastase, an IgG protease. Can. J. Microbiol. 30:1118-1124. [DOI] [PubMed] [Google Scholar]
- 44.Holm, S. E., A. Norrby, A.-M. Bergholm, and M. Norgren. 1992. Aspects of pathogenesis of serious group A streptococcal infections in Sweden 1988-1989. J. Infect. Dis. 166:31-37. [DOI] [PubMed] [Google Scholar]
- 45.Huang, T. T., H. Malke, and J. J. Ferretti. 1989. The streptokinase gene of group A streptococci: cloning, expression in Escherichia coli, and sequence analysis. Mol. Microbiol. 3:197-205. [DOI] [PubMed] [Google Scholar]
- 46.Husmann, L. K., D. L. Yung, S. K. Hollingshead, and J. R. Scott. 1997. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infect. Immun. 65:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jansen, H. J., D. Grenier, and J. S. Van der Hoeven. 1995. Characterization of immunoglobulin G-degrading proteases of Prevotella intermedia and Prevotella nigrescens. Oral Microbiol. Immunol. 10:138-145. [DOI] [PubMed] [Google Scholar]
- 48.Ji, Y., B. Carlson, A. Kondagunta, and P. P. Cleary. 1997. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect. Immun. 65:2080-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji, Y., L. McLandsborough, A. Kondagunta, and P. P. Cleary. 1996. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji, Y., N. Schnitzler, E. DeMaster, and P. Cleary. 1998. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect. Immun. 66:5399-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kagawa, T. F., J. C. Cooney, H. M. Baker, S. McSweeney, M. Liu, S. Gubba, J. M. Musser, and E. N. Baker. 2000. Crystal structure of the zymogen form of the group A Streptococcus virulence factor SpeB: an integrin-binding cysteine protease. Proc. Natl. Acad. Sci. USA 97:2235-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanaoka, M., Y. Fukita, K. Taya, C. Kawanaka, T. Negoro, and H. Agui. 1987. Antitumor activity of streptococcal acid glycoprotein produced by Streptococcus pyogenes Su. Jpn. J. Cancer Res. 78:1409-1414. [PubMed] [Google Scholar]
- 53.Kansal, R. G., A. McGeer, D. E. Low, A. Norrby-Teglund, and M. Kotb. 2000. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect. Immun. 68:6362-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kapur, V., M. W. Malesky, L.-L. Li, R. A. Black, and J. M. Musser. 1993. Cleavage of interleukin 1β (IL-1β) precursor to produce active IL-1β by a conserved extracellular cysteine proteinase from Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 90:7676-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karasawa, T., S. Takasawa, K. Yamakawa, H. Yonekura, H. Okamoto, and S. Nakamura. 1995. NAD+-glycohydrolase from Streptococcus pyogenes shows cyclic ADP-ribose forming activity. FEMS Microbiol. Lett. 130:201-204. [DOI] [PubMed] [Google Scholar]
- 56.Kawabata, S., Y. Tamura, J. Murakami, Y. Terao, I. Nakagawa, and S. Hamada. 2002. A novel, anchorless streptococcal surface protein that binds to human immunoglobulins. Biochem. Biophys. Res. Commun. 296:1329-1333. [DOI] [PubMed] [Google Scholar]
- 57.Kilian, M., and K. Holmgren. 1981. Ecology and nature of immunoglobulin A1 protease-producing streptococci in the human oral cavity and pharynx. Infect. Immun. 31:868-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kilian, M., J. Mestecky, and R. E. Schrohenloher. 1979. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect. Immun. 26:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuo, C. F., J. J. Wu, K. Y. Lin, P. J. Tsai, S. C. Lee, Y. T. Jin, H. Y. Lei, and Y. S. Lin. 1998. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect. Immun. 66:3931-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lancefield, R. C. 1962. Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 89:307-313. [PubMed] [Google Scholar]
- 61.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and aquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 7:1298-1305. [DOI] [PubMed] [Google Scholar]
- 62.Lei, B., F. R. DeLeo, and J. M. Musser. 2002. Reply to “Streptococcus pyogenes and phagocytic killing.” Nat. Med. 8:1045-1046. [DOI] [PubMed] [Google Scholar]
- 63.Lei, B., F. R. DeLeo, S. D. Reid, J. M. Voyich, L. Magoun, M. Liu, K. R. Braughton, S. Ricklefs, N. P. Hoe, R. L. Cole, J. M. Leong, and J. M. Musser. 2002. Opsonophagocytosis-inhibiting mac protein of group a streptococcus: identification and characteristics of two genetic complexes. Infect. Immun. 70:6880-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leonard, B. A. B., P. K. Lee, M. K. Jenkins, and P. M. Schlievert. 1991. Cell and receptor requirements for streptococcal pyrogenic exotoxin T-cell mitogenicity. Infect. Immun. 59:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li, Z., D. D. Sledjeski, B. Kreikemeyer, A. Podbielski, and M. D. Boyle. 1999. Identification of pel, a Streptococcus pyogenes locus that affects both surface and secreted proteins. J. Bacteriol. 181:6019-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lukomski, S., E. H. Burns, Jr., P. R. Wyde, A. Podbielski, J. Rurangirwa, D. K. Moore-Poveda, and J. M. Musser. 1998. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect. Immun. 66:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lukomski, S., C. A. Montgomery, J. Rurangirwa, R. S. Geske, J. P. Barrish, G. J. Adams, and J. M. Musser. 1999. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect. Immun. 67:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lukomski, S., S. Sreevatsan, A. Amberg, W. Reichardt, M. Woischnik, A. Podbielski, and J. M. Musser. 1997. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J. Clin. Investig. 99:2574-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lund, J., N. Takahashi, J. D. Pound, M. Goodall, H. Nakagawa, and R. Jefferis. 1995. Oligosaccharide-protein interactions in IgG can modulate recognition by Fc gamma receptors. FASEB J. 9:115-119. [DOI] [PubMed] [Google Scholar]
- 70.Lyon, W. R., C. M. Gibson, and M. G. Caparon. 1998. A role for trigger factor and an Rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 17:6263-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 72.Male, C. J. 1979. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect. Immun. 26:254-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mallett, C. P., R. J. Boylan, and D. L. Everhart. 1984. Competent antigen-binding fragments (Fab) from secretory immunoglobulin A using Streptococcus sanguis immunoglobulin A protease. Caries Res. 18:201-208. [DOI] [PubMed] [Google Scholar]
- 74.Mansa, B., and M. Kilian. 1986. Retained antigen-binding activity of Fab alpha fragments of human monoclonal immunoglobulin A1 (IgA1) cleaved by IgA1 protease. Infect. Immun. 52:171-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattu, T. S., R. J. Pleass, A. C. Willis, M. Kilian, M. R. Wormald, A. C. Lellouch, P. M. Rudd, J. M. Woof, and R. A. Dwek. 1998. The glycosylation and structure of human serum IgA1, Fab, and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J. Biol. Chem. 273:2260-2272. [DOI] [PubMed] [Google Scholar]
- 76.Miyamoto, Y., T. Akaike, M. S. Alam, K. Inoue, T. Hamamoto, N. Ikebe, J. Yoshitake, T. Okamoto, and H. Maeda. 2000. Novel functions of human α1-protease inhibitor after S-nitrosylation: inhibition of cysteine protease and antibacterial activity. Biochem. Biophys. Res. Commun. 267:918-923. [DOI] [PubMed] [Google Scholar]
- 77.Mizuochi, T., Y. Pastore, K. Shikata, A. Kuroki, S. Kikuchi, T. Fulpius, M. Nakata, L. Fossati-Jimack, L. Reininger, M. Matsushita, T. Fujita, and S. Izui. 2001. Role of galactosylation in the renal pathogenicity of murine immunoglobulin G3 monoclonal cryoglobulins. Blood 97:3537-3543. [DOI] [PubMed] [Google Scholar]
- 78.Molla, A., T. Kagimoto, and H. Maeda. 1988. Cleavage of immunoglobulin G (IgG) and IgA around the hinge region by proteases from Serratia marcescens. Infect. Immun. 56:916-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mosquera, J. A., V. N. Katiyar, J. Coello, and B. Rodriguez-Iturbe. 1985. Neuraminidase production by streptococci from patients with glomerulonephritis. J. Infect. Dis. 151:259-263. [DOI] [PubMed] [Google Scholar]
- 80.Mulks, M. H., and A. G. Plaut. 1978. IgA protease production as a characteristic distinguishing pathogenic from harmless neisseriaceae. N. Engl. J. Med. 299:973-976. [DOI] [PubMed] [Google Scholar]
- 81.Nordstrand, A., W. M. McShan, J. J. Ferretti, S. E. Holm, and M. Norgren. 2000. Allele substitution of the streptokinase gene reduces the nephritogenic capacity of group A streptococcal strain NZ131. Infect. Immun. 68:1019-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nowak, R. 1994. Flesh-eating bacteria: not new, but still worrisome. Science 264:1655. [DOI] [PubMed]
- 83.Parekh, R. B., R. A. Dwek, B. J. Sutton, D. L. Fernandes, A. Leung, D. Stanworth, T. W. Rademacher, T. Mizuochi, T. Taniguchi, K. Matsuta, F. Takeuchi, Y. Nagano, T. Miyamoto, and A. Kobata. 1985. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316:452-457. [DOI] [PubMed] [Google Scholar]
- 84.Plaut, A. G. 1983. The IgA1 proteases of pathogenic bacteria. Annu. Rev. Microbiol. 37:603-622. [DOI] [PubMed] [Google Scholar]
- 85.Plaut, A. G., R. J. Genco, and T. B. Tomasi, Jr. 1974. Isolation of an enzyme from Streptococcus sanguis which specifically cleaves IgA. J. Immunol. 113:589-591. [PubMed] [Google Scholar]
- 86.Plaut, A. G., J. V. Gilbert, and R. Wistar, Jr. 1977. Loss of antibody activity in human immunoglobulin A exposed extracellular immunoglobulin A proteases of Neisseria gonorrhoeae and Streptococcus sanguis. Infect. Immun. 17:130-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plaut, A. G., R. Wistar, Jr., and J. D. Capra. 1974. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J. Clin. Investig. 54:1295-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Podbielski, A., and B. A. Leonard. 1998. The group A streptococcal dipeptide permease (Dpp) is involved in the uptake of essential amino acids and affects the expression of cysteine protease. Mol. Microbiol. 28:1323-1334. [DOI] [PubMed] [Google Scholar]
- 89.Podbielski, A., B. Pohl, M. Woischnik, C. Körner, K.-H. Schmidt, E. Rozdzinski, and B. A. B. Leonard. 1996. Molecular characterization of group A streptococcal (GAS) oligopermease (Opp) and its effect on cysteine protease production. Mol. Microbiol. 21:1087-1099. [DOI] [PubMed] [Google Scholar]
- 90.Podbielski, A., M. Woischnik, B. Kreikemeyer, K. Bettenbrock, and B. A. Buttaro. 1999. Cysteine protease SpeB expression in group A streptococci is influenced by the nutritional environment but SpeB does not contribute to obtaining essential nutrients. Med. Microbiol. Immunol. 188:99-109. [DOI] [PubMed] [Google Scholar]
- 91.Podbielski, A., M. Woischnik, B. Pohl, and K. H. Schmidt. 1996. What is the size of the group A streptococcal vir regulon? The mga regulator affects expression of secreted and surface virulence factors. Med. Microbiol. Immunol. 185:171-181. [DOI] [PubMed] [Google Scholar]
- 92.Poyart, C., P. Berche, and P. Trieu-Cuot. 1995. Characterization of superoxide dismutase genes from gram-positive bacteria by polymerase chain reaction using degenerate primers. FEMS Microbiol. Lett. 131:41-45. [DOI] [PubMed] [Google Scholar]
- 93.Radaev, S., and P. D. Sun. 2001. Recognition of IgG by Fcγ receptor: the role of Fc glycosylation and the binding of peptide inhibitors. J. Biol. Chem. 276:16478-16483. [DOI] [PubMed] [Google Scholar]
- 94.Rasmussen, M., H.-P. Müller, and L. Björck. 1999. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial surface by binding α2-macroglobulin. J. Biol. Chem. 274:15336-15344. [DOI] [PubMed] [Google Scholar]
- 95.Savic, D. J., and J. J. Ferretti. 1994. Group A streptococci do not produce neuraminidase (sialidase). Med. Microbiol. Lett. 3:358-362. [Google Scholar]
- 96.Schmidtchen, A., Frick, I., E. Andersson, H. Tapper, and L. Björck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 97.Schmidtchen, A., I.-M. Frick, and L. Björck. 2001. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial α-defensin. Mol. Microbiol. 39:708-713. [DOI] [PubMed] [Google Scholar]
- 98.Scott, C. F., E. J. Whitaker, B. F. Hammond, and R. W. Colman. 1993. Purification and characterization of a potent 70-kDa thiol lysyl-proteinase (Lys-gingivain) from Porphyromonas gingivalis that cleaves kininogens and fibrinogens. J. Biol. Chem. 268:7935-7942. [PubMed] [Google Scholar]
- 99.Shanley, T. P., D. Schrier, V. Kapur, M. Kehoe, J. M. Musser, and P. A. Ward. 1996. Streptococcal cysteine protease augments lung injury induced by products of group A streptococci. Infect. Immun. 64:870-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin, M. H., H. Kita, H. Y. Park, and J. Y. Seoh. 2001. Cysteine protease secreted by Paragonimus westermani attenuates effector functions of human eosinophils stimulated with immunoglobulin G. Infect. Immun. 69:1599-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smoot, J. C., K. D. Barbian, J. J. Van Gompel, L. M. Smoot, M. S. Chaussee, G. L. Sylva, D. E. Sturdevant, S. M. Ricklefs, S. F. Porcella, L. D. Parkins, S. B. Beres, D. S. Campbell, T. M. Smith, Q. Zhang, V. Kapur, J. A. Daly, L. G. Veasy, and J. M. Musser. 2002. Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc. Natl. Acad. Sci. USA 99:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stevens, D. L. 1992. Invasive group A streptococcus infections. Clin. Infect. Dis. 14:2-13. [DOI] [PubMed] [Google Scholar]
- 103.Stevens, D. L., D. B. Salmi, E. R. McIndoo, and A. E. Bryant. 2000. Molecular epidemiology of nga and NAD glycohydrolase/ADP-ribosyltransferase activity among Streptococcus pyogenes causing streptococcal toxic shock syndrome. J. Infect. Dis. 182:1117-1128. [DOI] [PubMed] [Google Scholar]
- 104.Svensson, M. D., D. A. Scaramuzzino, U. Sjöbring, A. Olsén, C. Frank, and D. E. Bessen. 2000. Role for a secreted cysteine proteinase in the establishment of host tissue tropism by group A streptococci. Mol. Microbiol. 38:242-253. [DOI] [PubMed] [Google Scholar]
- 105.Talkington, D. F., B. Schwartz, C. M. Black, J. K. Todd, J. Elliott, R. F. Breiman, and R. R. Facklam. 1993. Association of phenotypic and genotypic characteristics of invasive Streptococcus pyogenes isolates with clinical components of streptococcal toxic shock syndrome. Infect. Immun. 61:3369-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tarentino, A. L., and T. H. Plummer, Jr. 1994. Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 230:44-57. [DOI] [PubMed] [Google Scholar]
- 107.Trimble, R. B., and A. L. Tarentino. 1991. Identification of distinct endoglycosidase (endo) activities in Flavobacterium meningosepticum: endo F1, endo F2, and endo F3. Endo F1 and endo H hydrolyze only high mannose and hybrid glycans. J. Biol. Chem. 266:1646-1651. [PubMed] [Google Scholar]
- 108.Trumbly, R. J., P. W. Robbins, M. Belfort, F. D. Ziegler, F. Maley, and R. B. Trimble. 1985. Amplified expression of streptomyces endo-β-N-acetylglucosaminidase H in Escherichia coli and characterization of the enzyme product. J. Biol. Chem. 260:5683-5690. [PubMed] [Google Scholar]
- 109.Vitovski, S., K. T. Dunkin, A. J. Howard, and J. R. Sayers. 2002. Nontypeable Haemophilus influenzae in carriage and disease: a difference in IgA1 protease activity levels. JAMA 287:1699-1705. [DOI] [PubMed] [Google Scholar]
- 110.Vitovski, S., R. C. Read, and J. R. Sayers. 1999. Invasive isolates of Neisseria meningitidis possess enhanced immunoglobulin A1 protease activity compared to colonizing strains. FASEB J. 13:331-337. [DOI] [PubMed] [Google Scholar]
- 111.von Pawel-Rammingen, U., B. P. Johansson, and L. Björck. 2002. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 21:1607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.von Pawel-Rammingen, U., B. P. Johansson, H. Tapper, and L. Björck. 2002. Streptococcus pyogenes and phagocytic killing. Nat. Med. 8:1044-1045. [DOI] [PubMed] [Google Scholar]
- 113.Watanabe, Y., Y. Todome, H. Ohkuni, S. Sakurada, T. Ishikawa, T. Yutsudo, V. A. Fischetti, and J. B. Zabriskie. 2002. Cysteine protease activity and histamine release from the human mast cell line HMC-1 stimulated by recombinant streptococcal pyrogenic exotoxin B/streptococcal cysteine protease. Infect. Immun. 70:3944-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wexler, D. E., D. E. Chenoweth, and P. P. Cleary. 1985. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 82:8144-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wright, A., and S. L. Morrison. 1994. Effect of altered CH2-associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin G1. J. Exp. Med. 180:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yesilkaya, H., A. Kadioglu, N. Gingles, J. E. Alexander, T. J. Mitchell, and P. W. Andrew. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu, C.-N., and J. J. Ferretti. 1991. Frequency of the erythrogenic toxin B and C genes (speB and speC) among clinical isolates of group A Streptococci. Infect. Immun. 59:211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]