Abstract
OBJECTIVE
Diabetes is associated with increased urinary incontinence risk. Weight loss improves incontinence, but exercise may worsen this condition. We examined whether an intensive lifestyle intervention or metformin therapy among overweight pre-diabetic women was associated with a lower prevalence of incontinence.
RESEARCH DESIGN AND METHODS
We analyzed data from the Diabetes Prevention Program, a randomized controlled trial in 27 U.S. centers. Of the 1,957 women included in this analysis, 660(34%) were randomized to intensive lifestyle therapy, 636(32%) to metformin, and 661 (34%) to placebo with standard lifestyle advice. The main outcome measure was incontinence symptoms by frequency and type by a validated questionnaire completed at the end-of-trial visit (mean 2.9 years).
RESULTS
The prevalence of total (stress or urge) weekly incontinence was lower among women in the intensive lifestyle group (38.3%) than those randomized to metformin (48.1%) or placebo (45.7%). This difference was most apparent among women with stress incontinence (31.3% for intensive lifestyle group vs. 39.7% for metformin vs. 36.7% for placebo, P = 0.006). Changes in weight accounted for most of the protective effect of the intensive lifestyle intervention on stress incontinence.
CONCLUSIONS
Less-frequent urinary incontinence may be a powerful motivator for women to choose lifestyle modification to prevent diabetes.
Type 2 diabetes and urinary incontinence are chronic, common, and costly disorders. While ∼18 million adults in the U.S. have diabetes, estimates of adults with pre-diabetes, defined as impaired glucose tolerance and/or impaired fasting glucose (1,2), range from 17 to 43 million. Recently, large randomized controlled trials have shown that diabetes can be prevented by intensive lifestyle intervention in this high-risk pre-diabetic group (3,4)
Urinary incontinence, present in nearly 50% of middle-aged and older women, results in psychological stress and social isolation and has a profound effect on quality of life (5,6). The costs of incontinence are substantial, accounting for up to $32 billion per year in the U.S., greater than the annual direct costs for breast, ovarian, cervical, and uterine cancers combined (7,8).
Type 2 diabetes is associated with a 50–70% increased risk of incontinence in women (5,9,10). There have been no studies to determine whether nondiabetic women with abnormal glucose levels (pre-diabetes) are at increased risk for incontinence. Weight reduction and increased physical activity in individuals with pre-diabetes decreases the risk of type 2 diabetes (3,4). Weight reduction in obese women with incontinence has been shown to significantly decrease incontinence in small prospective studies (11-13). However, some studies have reported that increased physical activity worsens incontinence and that incontinence may be a barrier to exercise (14,15).
We therefore examined data from 1,987 overweight women at high risk for diabetes who were enrolled in the Diabetes Prevention Program (DPP) to determine whether an intensive lifestyle intervention with improved diet and increased physical activity or metformin therapy would be associated with lower prevalence of urinary incontinence compared with a standard lifestyle intervention alone.
RESEARCH DESIGN AND METHODS
The design, methods, baseline characteristics (16), and main findings (3) of the DPP have been published previously. Briefly, the DPP was a randomized controlled trial conducted at 27 clinical centers in the U.S. to evaluate whether intensive lifestyle intervention or treatment with metformin would prevent or delay the onset of type 2 diabetes. Eligibility criteria at baseline included age at least 25 years, BMI ≥24 kg/m2, a fasting plasma glucose level 95–125 mg/dl, and a 2-h postchallenge glucose level 140–199 mg/dl. People who were taking medications that could affect glucose tolerance or who had serious medical illness were excluded. By design, approximately half of the participants were from racial/ethnic minority groups. The three interventions included an intensive lifestyle intervention, metformin at 850 mg twice daily, or placebo twice daily. All participants received standard lifestyle recommendations, including written information and an individual meeting that emphasized a healthy diet, reduced weight, increased activity levels, and smoking cessation, at baseline and annually. The goals of the intensive lifestyle intervention were to lose and maintain at least 7% of initial body weight through a low-fat diet and to engage in moderate-intensity physical activity for at least 150 min each week. Details of the behavioral and educational curriculum have been published previously (17). The DPP was closed early after 2.9 years when lifestyle changes and metformin treatment had each reduced the incidence of diabetes (3).
Data collection
At the baseline examination, all participants completed a questionnaire reporting sex, age, self-identified race/ethnicity, smoking history (current, past, or never), and alcohol use (drinks per week). They answered questions regarding other physician-diagnosed medical conditions, self-rated overall health status, parity, menopausal status, hormone therapy use, and hysterectomy. All participants brought in medications they were currently using and a complete medication inventory was ascertained.
Body weight, height, waist circumference, and systolic and diastolic blood pressure were measured at designated clinical visits during the trial. Weight was measured in kilograms using a standard balance beam scale, height was measured in centimeters using the height rod attached to the standard balance beam scale or a stadiometer, and BMI (weight in kilograms divided the square of height in meters) was calculated. Waist circumference was measured using a flexible tape measure at the minimum circumference between the iliac crests and lower ribs. Physical activity was assessed at baseline and annually with the Modifiable Activity Questionnaire (18) and was calculated as the product of the duration and frequency of each activity, weighted by an estimate of the metabolic equivalent of that activity and summed for all activities, resulting in estimated average metabolic equivalent (MET) hours per week. Incident diabetes, the primary DPP outcome, was diagnosed by annual oral glucose tolerance test or a semiannual fasting plasma glucose test according to the 1997 American Diabetes Association criteria (19).
Outcome ascertainment
Urinary incontinence was determined at the end-of-trial visit using a self-administered questionnaire modified from validated questions used in previous studies (20-22). Frequency of incontinence was assessed by the question, “In the past 12 months, how often have you leaked even a small amount of urine?” (none, less than monthly, monthly, weekly, or daily). For participants with weekly or more frequent incontinence, type of incontinence was assessed by asking the question, “In the past 7 days, how many times, on average, did you leak urine…” “… during activities like coughing, sneezing, straining, laughing, or lifting?” (stress incontinence), “… after an urge to urinate but could not get to the bathroom fast enough?” (urge incontinence), and “… for other reasons (without an urge to urinate or without an activity)?” (other incontinence). Responses were recorded as times per week. The primary outcome of interest was incontinence that occurred at least weekly because it is clinically relevant.
Because urinary incontinence is a disorder primarily affecting women, we excluded all men from this analysis. Of the 2,191 women enrolled in the three arms of the DPP, we excluded 234 (11%) women with missing urinary incontinence data, leaving a total of 1,957 women for this analysis. Women missing data on urinary incontinence did not differ in incident diabetes, mean weight change, or mean change in physical activity overall or within treatment groups compared with women with completed urinary incontinence data.
Statistical analysis
Women in the three treatment groups who contributed information on incontinence were compared in terms of baseline variables using χ2 tests, ANOVA, and Kruskal-Wallis, as appropriate. Prevalence of incontinence, both overall and by type, was compared across these three groups at the end of the trial using χ2 tests for heterogeneity; since the overall test was statistically significant, pairwise comparisons of the lifestyle and metformin arms with placebo were conducted without adjustment of the significance level. Differences in the effects of the lifestyle intervention on incontinence across subgroups defined by baseline variables were assessed using logistic models. In these models, effects of the intensive lifestyle intervention were compared across subgroups using appropriate linear contrasts in the estimated log odds ratios (ORs) for the lifestyle versus placebo comparison; for ordinal subgroup variables including age, BMI, waist circumference, and leisure time activity, we examined trends in this parameter, while for race/ethnicity, we assessed heterogeneity across groups. The mediation of the effects of the lifestyle intervention on prevalence of weekly stress incontinence was examined using nested logistic models in which proposed mediators were added in a predetermined sequence to the basic unadjusted model comparing the lifestyle intervention to placebo. Mediation was then informally assessed by the degree of attenuation of the odds ratios for the intensive lifestyle/placebo comparison.
RESULTS
Equal proportions of the 1,957 women were randomized to each intervention group. Women were on average 50 years of age (±10 years, range 26–84), with a BMI of 35 kg/m2 and a waist circumference of 104 cm (Table 1). The treatment groups were balanced with regard to all demographic, ethnic, behavioral, body composition, reproductive, and metabolic characteristics. The only baseline variables that differed significantly by treatment assignment were prevalence of hormone therapy and self-rated general health status. More women in the metformin intervention were using hormone therapy than those in the intensive lifestyle intervention or placebo groups (P = 0.01), and a higher proportion of women assigned to intensive lifestyle therapy reported excellent or very good general health than those in the other two groups (P = 0.02).
Table 1.
Baseline characteristics of women in the DPP by treatment group
| Overall | Lifestyle | Metformin | Placebo | P* | |
|---|---|---|---|---|---|
| n | 1,957 | 660 | 636 | 661 | |
| Age (years) | 49.6 ± 10.0 | 49.3 ± 10.6 | 49.9 ± 9.6 | 49.5 ± 9.7 | 0.51 |
| Race/ethnicity | 0.71 | ||||
| White | 1,031 (52.7) | 343 (52.0) | 333 (52.4) | 355 (53.7) | |
| African American | 430 (22.0) | 138 (20.9) | 148 (23.3) | 144 (21.8) | |
| Hispanic | 294 (15.0) | 103 (15.6) | 97 (15.3) | 94 (14.2) | |
| Native American | 147 (7.5) | 51 (7.7) | 45 (7.1) | 51 (7.7) | |
| Asian | 55 (2.8) | 25 (3.8) | 13 (2.0) | 17 (2.6) | |
| Weekly alcohol use | 319 (16.3) | 105 (15.9) | 97 (15.3) | 117 (17.7) | 0.46 |
| Current smoking | 126 (6.4) | 36 (5.5) | 38 (6.0) | 52 (7.9) | 0.17 |
| General health (%) | |||||
| Excellent/very good | 1,039 (53.1) | 385 (58.3) | 324 (50.9) | 330 (49.9) | 0.02 |
| Good | 744 (38.0) | 227 (34.4) | 251 (39.5) | 266 (40.2) | |
| Fair/poor | 174 (8.9) | 48 (7.3) | 61 (9.6) | 65 (9.8) | |
| Weight (kg) | 92.0 ± 20.3 | 91.8 ± 20.5 | 92.0 ± 19.9 | 92.2 ± 20.5 | 0.92 |
| BMI (kg/m2−) | 34.9 ± 6.9 | 34.7 ± 6.9 | 34.8 ± 6.9 | 35.1 ± 7.0 | 0.66 |
| Waist circumference (cm) | 103.6 ± 14.8 | 103.5 ± 15.0 | 103.2 ± 14.9 | 103.9 ± 14.6 | 0.68 |
| Number of live births (%) | 0.97 | ||||
| None | 89 (5.3) | 30 (5.4) | 28 (5.2) | 31 (5.4) | |
| One | 292 (17.5) | 100 (18.0) | 95 (17.5) | 97 (17.0) | |
| Two | 588 (35.3) | 188 (33.8) | 190 (35.1) | 210 (36.9) | |
| Three or more | 698 (41.9) | 238 (42.8) | 229 (42.3) | 231 (40.6) | |
| Hysterectomy | 493 (25.2) | 164 (24.8) | 165 (25.9) | 164 (24.8) | 0.87 |
| Menopause | 990 (50.6) | 336 (50.9) | 309 (48.9) | 345 (52.2) | 0.78 |
| Current hormone therapy use | 489 (25.0) | 168 (25.5) | 180 (28.3) | 141 (21.3) | 0.01 |
| Years of estrogen use | 5.9 ± 7.0 | 6.3 ± 6.9 | 6.2 ± 7.8 | 5.2 ± 6.2 | 0.18 |
| Fasting glucose (mg/dl) | 105.5 ± 8.1 | 105.1 ± 7.8 | 105.7 ± 8.3 | 105.6 ± 8.2 | 0.35 |
| 2-h postchallenge glucose (mg/dl) | 164.8 ± 17.2 | 164.1 ± 16.9 | 166.1 ± 17.6 | 164.3 ± 17.2 | 0.07 |
| HbA1c (%) | 5.9 ± 0.5 | 5.9 ± 0.5 | 5.9 ± 0.5 | 5.9 ± 0.5 | 0.52 |
| Fasting insulin (μU/ml) | 26.5 ± 14.2 | 26.6 ± 14.5 | 26.9 ± 14.2 | 26.1 ± 13.9 | 0.59 |
Data are means ± SD or n (%).
P value by χ2 tests, ANOVA, and Kruskal-Wallis tests, as appropriate.
The average change in weight for women in the intensive lifestyle group was −3.4 ± 8.2 kg vs. −1.5 ± 7.6 kg in the metformin group vs. +0.5 ± 6.7 kg in the placebo group (P < 0.001). The average change in leisure-time physical activity was highest in the intensive lifestyle group (5.2 ± 19.9 MET h/week vs. 0.8 ± 18.0 in the metformin group and −0.3 ± 20.7 in the placebo group, P < 0.001). Women in the intensive lifestyle group had the lowest incidence of diabetes over the mean 2.9 years of follow-up (14.9% vs. 23.9% in the metformin group and 30.9% in the placebo group, P < 0.001 comparing three groups).
At the end-of-trial visit, the overall prevalence of weekly incontinence differed significantly by treatment. Fewer women in the intensive lifestyle intervention had weekly incontinence compared with women in the metformin or placebo groups (38.3% vs. 48.1% vs. 45.7%, respectively, P = 0.001). After adjusting for baseline hormone therapy use, general health status, and 2-h postchallenge glucose categories, women randomized to intensive lifestyle therapy had significantly lower odds of weekly urinary incontinence compared with women assigned to placebo (OR 0.76 [95% CI 0.61–0.95]). The prevalence of weekly incontinence by type (stress or urge) by treatment group is shown in Table 2. While estimates were similar for urge incontinence in each of the three groups, weekly stress incontinence was significantly lower in women assigned to intensive lifestyle therapy compared with the other two groups (P = 0.006). The apparent beneficial effect of lifestyle intervention on weekly stress incontinence did not differ by age categories, race/ethnicity, BMI, waist circumference, or physical activity at baseline (Table 3). Metformin therapy had no effect on the prevalence or odds of total weekly urinary incontinence (1.09[0.87–1.36]) or on the odds of weekly stress incontinence (1.13 [0.90–1.42]) or weekly urge incontinence (1.15 [0.90–1.48]).
Table 2.
Prevalence of weekly urinary incontinence by type at the end-of-trial visit
| Placebo | Intensive Lifestyle Intervention | P* | Metformin | P* | P† | |
|---|---|---|---|---|---|---|
| n | 660 | 659 | 635 | |||
| Stress UI | 242 (36.7) | 206 (31.3) | 0.04 | 252 (39.7) | 0.26 | 0.006 |
| Urge UI | 169 (25.6) | 156 (23.7) | 0.41 | 182 (28.7) | 0.22 | 0.12 |
Data are n (%).
P for comparison between intensive lifestyle intervention and placebo and between metformin therapy and placebo.
P for overall comparison. UI, urinary incontinence.
Table 3.
Effects of intensive lifestyle intervention versus standard lifestyle on weekly stress incontinence by baseline covariates
| Total | Intensive lifestyle group | Placebo group | OR (95% CI) | P for treatment | P for interaction* | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| 25–44 | 441 (33.4) | 67 (28.4) | 77 (37.7) | 0.65 (0.44–0.98) | 0.04 | 0.23 |
| 45–59 | 671 (50.8) | 104 (33.7) | 136 (37.7) | 0.84 (0.61–1.15) | 0.28 | |
| ≥60 | 209 (15.8) | 35 (30.7) | 29 (30.5) | 1.00 (0.56–1.82) | 0.98 | |
| Race/ethnicity | ||||||
| Caucasian | 698 (52.8) | 114 (33.2) | 146 (41.2) | 0.71 (0.52–0.97) | 0.03 | 0.66 |
| African American | 282 (21.3) | 33 (24.1) | 40 (27.8) | 0.82 (0.48–1.41) | 0.48 | |
| Hispanic | 197 (14.9) | 33 (32.0) | 29 (30.9) | 1.05 (0.58–1.93) | 0.86 | |
| Native American | 102 (7.7) | 20 (39.2) | 20 (39.2) | 1.00 (0.45–2.21) | 1.00 | |
| Asian | 42 (3.2) | 6 (24.0) | 7 (41.2) | 0.45 (0.12–1.71) | 0.24 | |
| BMI (kg/m2) | ||||||
| 22 to <25 | 36 (2.7) | 4 (20.0) | 4 (25.0) | 0.75 (0.15–3.62) | 0.72 | 0.25 |
| 25 to <30 | 323 (24.5) | 41 (25.5) | 56 (34.6) | 0.65 (0.40–1.04) | 0.07 | |
| 30 to <35 | 386 (29.2) | 55 (27.2) | 69 (37.7) | 0.62 (0.40–0.95) | 0.03 | |
| 35 to <40 | 299 (22.6) | 54 (37.0) | 59 (38.8) | 0.92 (0.58–1.48) | 0.74 | |
| ≥40 | 277 (21.0) | 52 (40.0) | 54 (36.7) | 1.15 (0.71–1.86) | 0.58 | |
| Waist circumference (cm) | ||||||
| <80 | 28 (2.1) | 5 (31.3) | 5 (41.7) | 0.64 (0.13–3.03) | 0.57 | 0.79 |
| 80 to <88 | 160 (12.1) | 19 (25.3) | 27 (31.8) | 0.73 (0.36–1.46) | 0.37 | |
| ≥88 | 1131 (85.7) | 181 (31.9) | 209 (37.2) | 0.79 (0.62–1.01) | 0.06 | |
| Leisure-time activity (MET) | ||||||
| <3.12 | 348 (26.4) | 59 (35.3) | 57 (31.8) | 1.17 (0.75–1.83) | 0.49 | 0.24 |
| 3.12 to <8.08 | 327 (24.8) | 51 (30.0) | 65 (41.4) | 0.61 (0.38–0.96) | 0.03 | |
| 8.08 to <16.88 | 320 (24.2) | 50 (29.2) | 54 (36.2) | 0.73 (0.45–1.16) | 0.18 | |
| ≥16.88 | 325 (24.6) | 46 (30.7) | 66 (37.7) | 0.73 (0.46–1.16) | 0.18 | |
| Fasting glucose (mg/dl) | ||||||
| 95–109 | 939 (73.2) | 142 (30.0) | 178 (38.4) | 0.68 (0.52–0.90) | 0.006 | 0.09 |
| 110–125 | 344 (26.8) | 59 (34.9) | 58 (33.1) | 1.08 (0.69–1.69) | 0.73 | |
| 2-h postchallenge glucose (mg/dl) | ||||||
| 140–153 | 444 (33.6) | 62 (27.8) | 81 (36.7) | 0.66 (0.45–0.99) | 0.05 | 0.20 |
| 154–172 | 438 (33.2) | 70 (32.4) | 86 (38.7) | 0.76 (0.51–1.12) | 0.17 | |
| 173–199 | 439 (33.2) | 74 (33.6) | 75 (34.6) | 0.96 (0.65–1.42) | 0.84 |
Data are n (%) unless otherwise indicated.
Test for trend for treatment effects across the categories; for race, we performed a test for heterogeneity.
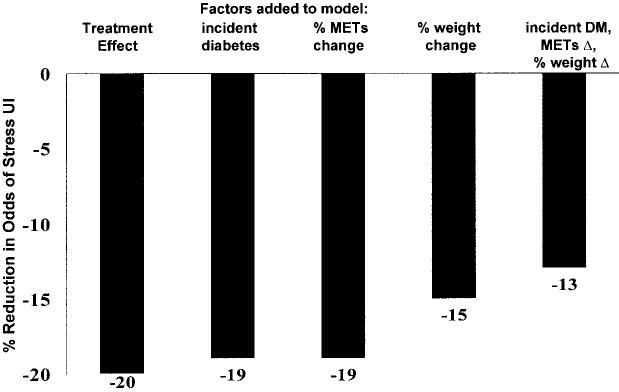
Intensive lifestyle intervention was associated with a 20% reduction in the odds (OR 0.80 [95% CI 0.64–1.01]) of weekly stress incontinence compared with placebo after adjusting for baseline covariates. We performed sequential models adjusting for possible mediators of this association. In combination, incident diabetes, percent changes in physical activity, and weight explained 35% of the treatment effect (Fig. 1). Percent change in weight accounted for almost all of the treatment effect explained, with change in exercise and incident diabetes each explaining ∼5%. Similar results were obtained when absolute change or end-of-study values rather than percent change in the mediators were added to the models.
Figure 1.
Percent reduction in odds for weekly or more stress urinary incontinence in the intensive lifestyle intervention group versus the placebo/standard lifestyle group. Each model is adjusted for baseline hormone therapy use, general health status, and 2-h postchallenge glucose test results. Sequential models are adjusted for the indicated change variables. DM, diabetes.
CONCLUSIONS
After ∼3 years, overweight women at risk for diabetes who were assigned to intensive lifestyle modification had significantly lower prevalence of total (stress or urge) weekly urinary incontinence compared with women assigned to metformin or placebo. This result appeared to be due to differences in weekly stress incontinence. The beneficial effect was comparable across subgroups.
Weight loss was the most important mediator of the beneficial effect of the intensive lifestyle change program on stress incontinence. Weight reduction has been shown to improve incontinence in morbidly obese women undergoing bariatric surgery and in moderately obese women in weight-reduction programs (11-13). Presumably, increasing body weight causes increased abdominal weight, increased intra-abdominal pressure, and increased intravesicular pressure and urethral mobility, resulting in incontinence (12). Studies have shown that a 5–10% weight loss in women with urinary incontinence resulted in marked improvement of incontinence, and it has been suggested that improved incontinence may motivate overweight and obese women to lose weight (11,13).
Increased physical activity did not significantly affect the beneficial treatment effect of the intensive lifestyle intervention on urinary incontinence. In other studies, over 40% of middle-aged women reported leaking urine during exercise, and a similar proportion reported avoiding exercise because of urinary incontinence (14). Running and high-impact aerobics have been reported as the most common forms of exercise associated with incontinence (15). It has been suggested that walking is less likely to provoke incontinence and therefore is a more common form of exercise chosen by women with incontinence. A majority of the subjects in the intensive lifestyle intervention arm of the DPP chose walking as their physical activity.
Although type 2 diabetes increases risk for incontinence, it is not surprising that incident diabetes among the DPP treatment groups did not mediate the benefit observed in urinary incontinence with lifestyle intervention. The early detection of diabetes in the DPP was associated with blood glucose levels that are not usually high enough to cause an osmotic diuresis. Moreover, microvascular complications associated with type 2 diabetes that may damage the innervation of the bladder or alter detrusor muscle function (23-25) have not occurred in early disease. For similar reasons, pre-diabetes most likely does not promote incontinence.
The DPP had a diverse population of women in a wide age range and excellent measures of body weight, physical activity, and incident diabetes through the trial, but we did not have baseline measures of urinary incontinence and could not examine net improvement in incontinence symptoms. However, since the groups were balanced with respect to most variables, there is no reason to suspect that these groups would have differed in relation to urinary incontinence prevalence at baseline. Adjustment for the three variables that were imbalanced by group at baseline did not materially alter the results. Another potential limitation includes limited power to evaluate modification of treatment effect by various baseline covariates.
In conclusion, women at high risk for diabetes who were randomly assigned to an intensive lifestyle intervention involving weight loss and exercise had a substantially lower prevalence of stress urinary incontinence. Increased low-impact exercise did not appear to have an adverse effect on incontinence. Health care providers may use the message that weight loss and lifestyle intervention may lower the risk of urinary incontinence. Lower prevalence of urinary incontinence may be a powerful motivating factor for women with pre-diabetes to choose a lifestyle modification in order to prevent diabetes.
Acknowledgments
This study was supported by the following: The Diabetes Prevention Program, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Child Health and Human Development, the National Institute on Aging, the Office of Research on Minority Health and Health Disparities, the Office of Women's Health, the Indian Health Service, the Centers for Disease Control and Prevention, the General Clinical Research Program, the National Center for Research Resources, the American Diabetes Association, Bristol-Myers Squibb, Lipha Pharmaceuticals, and Parke-Davis.
LifeScan, Health O Meter, Hoechst Marion Roussel, Merck-Medco Managed Care, Merck, Nike Sports Marketing, Slim Fast Foods, and Quaker Oats donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices, Matthews Media Group, and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center.
We thank the thousands of volunteers in this program for their devotion to the goal of diabetes prevention.
Abbreviations
- DPP
Diabetes Prevention Program
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care. 2003;26:3329–3330. doi: 10.2337/diacare.26.12.3329. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin SM, Valdez R, Geiss LS, Rolka DB, Narayan KMV. Estimated number of adults with prediabetes in the U.S. in 2000: opportunities for prevention. Diabetes Care. 2003;26:645–649. doi: 10.2337/diacare.26.3.645. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Haämaälaäinen H, Ilanne-Parikka P, Keinaänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 5.Wetle T, Scherr P, Branch LG, Resnick NM, Harris T, Evans D, Taylor JO. Difficulty with holding urine among older persons in a geographically defined community: prevalence and correlates. J Am Geriatr Soc. 1995;43:349–355. doi: 10.1111/j.1532-5415.1995.tb05806.x. [DOI] [PubMed] [Google Scholar]
- 6.Sampselle CM, Harlow SD, Skurnick J, Brubaker L, Bondarenko I. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol. 2002;100:1230–1238. doi: 10.1016/s0029-7844(02)02241-x. [DOI] [PubMed] [Google Scholar]
- 7.Varmus H. Disease-Specific Estimates of Direct and Indirect Costs of Illness and NIH Support. U.S. Govt. Printing Office, Department of Health and Human Services, National Institutes of Health, Office of the Director; Washington, DC: 1997. Appendix. [Google Scholar]
- 8.Wilson L, Brown JS, Shin GP, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 9.Jackson RA, Vittinghoff E, Kanaya AM, Miles TP, Resnick HE, Kritchevsky SB, Simonsick EM, Brown JS. Urinary incontinence in elderly women: findings from the Health, Aging, and Body Composition Study. Obstet Gynecol. 2004;104:301–307. doi: 10.1097/01.AOG.0000133482.20685.d1. [DOI] [PubMed] [Google Scholar]
- 10.Brown J, Grady D, Ouslander J, Herzog A, Varner R, Posner S. Prevalence of urinary incontinence and associated risk factors in postmenopausal women: Heart & Estrogen/Progestin Replacement Study (HERS) Research Group. Obstet Gynecol. 1999;94:66–70. doi: 10.1016/s0029-7844(99)00263-x. [DOI] [PubMed] [Google Scholar]
- 11.Subak LL, Johnson C, Whitcomb E, Boban D, Saxton J, Brown JS. Does weight loss improve incontinence in moderately obese women? Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:40–43. doi: 10.1007/s001920200008. [DOI] [PubMed] [Google Scholar]
- 12.Bump R, Sugerman H, Fantl J, McClish D. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;166:392–399. doi: 10.1016/s0002-9378(11)91418-5. [DOI] [PubMed] [Google Scholar]
- 13.Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174:190–195. doi: 10.1097/01.ju.0000162056.30326.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown WJ, Miller YD. Too wet to exercise? Leaking urine as a barrier to physical activity in women. J Sci Med Sport. 2001;4:373–378. doi: 10.1016/s1440-2440(01)80046-3. [DOI] [PubMed] [Google Scholar]
- 15.Nygaard I, DeLancey J, Arnsdorf L, Murphy E. Exercise and incontinence. Obstet Gynecol. 1990;75:848–851. [PubMed] [Google Scholar]
- 16.The Diabetes Prevention Program Research Group Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22:623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Diabetes Prevention Program (DPP) Research Group Description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kriska AM, Caspersen CJ. Introduction to a collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29:S5–S9. [PubMed] [Google Scholar]
- 19.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 20.Grady D, Brown JS, Vittinghoff E, Apple-gate W, Varner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116–120. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 21.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study: epidemiology of incontinence in the county of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 22.Sandvik H, Hunskaar S, Vanvik A, Bratt H, Seim A, Hermstad R. Diagnostic classification of female urinary incontinence: an epidemiological survey corrected for validity. J Clin Epidemiol. 1995;48:339–343. doi: 10.1016/0895-4356(94)00147-i. [DOI] [PubMed] [Google Scholar]
- 23.Ellenberg M. Development of urinary bladder dysfunction in diabetes mellitus. Ann Intern Med. 1980;92:321–323. doi: 10.7326/0003-4819-92-2-321. [DOI] [PubMed] [Google Scholar]
- 24.Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol. 1997;157:580–584. doi: 10.1016/s0022-5347(01)65209-1. [DOI] [PubMed] [Google Scholar]
- 25.Brown JS, Nyberg LM, Kusek JW, Burgio KL, Diokno AC, Foldspang A, Fultz NH, Herzog AR, Hunskaar S, Milsom I, Nygaard I, Subak LL, Thom DH. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on Epidemiologic Issues in Urinary Incontinence in Women. Am J Obstet Gynecol. 2003;188:S77–S88. doi: 10.1067/mob.2003.353. [DOI] [PubMed] [Google Scholar]