Abstract
We characterized the expression of a putative toxin of Bacillus anthracis, a member of the cholesterol-dependent cytolysin (CDC) family, which includes listeriolysin O, perfringolysin O, and streptolysin O. We named this cytotoxin anthrolysin O (ALO). Although B. anthracis expresses minimal hemolytic activity in clinical settings, we show that Sterne strain 7702 expresses hemolytic activity when grown in brain heart infusion broth or in other rich bacteriologic media, but it secretes barely detectable amounts of hemolysin when grown in Luria-Bertani (LB) broth. Glucose supplementation of LB broth increases the amount of secreted hemolytic activity. Expression of hemolytic activity is maximal during mid- to late-log phase and decreases in the stationary phase. These observations are supported, in part, by semiquantitative reverse transcriptase PCR of alo mRNA. Hemolytic activity in growth supernatants was increased in the presence of reducing agent and almost totally inhibited in a dose-dependent manner by cholesterol; both of these activities are characteristic of a CDC toxin. A mutant of Sterne strain 7702, strain UT231, in which the alo gene was deleted and replaced by a kanamycin cassette, secreted barely detectable hemolytic activity into the growth medium. When strain UT231 was complemented in trans with native alo on a low-copy-number plasmid [strain UT231(pUTE554)], it regained the ability to secrete hemolytic activity, indicating that ALO is the major hemolysin secreted by this strain of B. anthracis in rich media in vitro. To further support the alo gene product being a hemolysin, recombinant B. anthracis ALO (rALO) purified from Escherichia coli was extremely active against washed human erythrocytes, with complete hemolysis detected at ∼30 molecules of rALO per erythrocyte. Considering the virulence roles of CDCs for other gram-positive bacteria, we speculate that ALO may have a role in anthrax virulence.
The fact that Bacillus anthracis spores can be produced and disseminated as biological weapons of warfare or terrorism (2, 18, 23) increases the need for a better understanding of the virulence factors of this organism. Although the study of B. anthracis dates back at least to the late nineteenth century (20), important aspects of anthrax pathogenesis remain to be elucidated. In addition to a polypeptide capsule, B. anthracis produces at least two toxins, lethal toxin and edema toxin, which are thought to be responsible for the majority of the pathology, morbidity, and mortality associated with anthrax and thus have been the focus of a great deal of research and recent review (4, 20, 30). These toxins likely come into play late in the course of infection (8, 12, 14, 19). Few studies have examined the early events in the establishment of B. anthracis infection.
Experiments by Guidi-Rontani and colleagues identified alveolar macrophages as the primary site of B. anthracis spore germination in animal models of inhalation anthrax (10-12). After germination, the bacteria quickly escape from the phagolysosome into the cytoplasm and are eventually released from the macrophage into the extracellular milieu (8). The mechanisms by which these early membrane disruptive events occur remain to be studied and elucidated.
A BLAST search performed in our laboratory of the B. anthracis genomic sequence data made available by The Institute for Genomic Research (Rockville, Md.) revealed an open reading frame (ORF) predicted to encode a protein with a high degree of homology to listeriolysin O (LLO) of Listeria monocytogenes and cereolysin O of Bacillus cereus. This gene has also been identified in the B. anthracis genome by Read et al. (24). LLO is encoded by the gene hly and contributes to the virulence of L. monocytogenes by allowing this organism to escape from the phagolysosome of its host cell (7). This led us to hypothesize that the B. anthracis homologue of LLO, which we call anthrolysin O (ALO), may play a similar role in the pathogenesis of anthrax. We characterize here the activity and expression of ALO.
LLO is a member of the cholesterol-dependent cytolysin (CDC) family of proteins, an expansive family of highly conserved, pore-forming proteins that are found in many gram-positive organisms. At present, >20 species possess a gene encoding a CDC sharing 40 to 70% homology with other members of the family. CDCs are secreted by bacteria, apparently seek cholesterol in host cell plasma membranes, insert into the membranes, and oligomerize to form large pores (21). Formation of the pores is responsible for the gross hemolytic and cytolytic properties of CDCs and, in more subtle fashion, has recently been proposed as a secretion system—called cytolysin-mediated translocation—for delivery of bacterial virulence factors directly into the host cell cytoplasm by gram-positive bacteria (16).
Since CDCs can be potent hemolysins, a search for a CDC family member in B. anthracis has most likely been discouraged by the fact that B. anthracis is nonhemolytic or weakly hemolytic on standard sheep blood agar plates. However, Guttmann and Ellar observed weak hemolysis at 48 h on washed-human-blood agar plates in two of three attenuated B. anthracis strains (13). Indeed, in initial characterization in the clinical microbiology laboratory, beta-hemolysis is considered to be a characteristic that specifically rules out B. anthracis (25). Analysis of the B. anthracis genome sequence with the subsequent identification of a putative CDC led us to take a closer look for a cytolysin or hemolysin produced by B. anthracis. Our studies reveal that B. anthracis produces biologically relevant amounts of hemolysin in culture supernatants, whose expression is dependent on growth conditions, growth phase, and the type of bacteriologic media used. The hemolysin secreted by B. anthracis possesses characteristics of a CDC. The culture supernatant of a B. anthracis strain, in which the alo gene has been disrupted, expresses barely detectable hemolytic activity, indicating that the CDC of B. anthracis is the predominant hemolysin secreted by these bacteria in culture under the conditions used in our studies.
MATERIALS AND METHODS
Bacterial strains and growth.
We used B. anthracis strain 7702, the capsule-negative toxigenic (pXO1+, pXO2−) Sterne strain (5). Unless otherwise indicated, bacteria were grown either in brain heart infusion broth (BHI; Difco, Detroit, Mich.), without added bicarbonate, with shaking (200 rpm) at 37°C in an air shaker incubator (New Brunswick Scientific, Edison, N.J.) or on BHI agar in a humidified 5% CO2 incubator.
Hemolysis assay.
Two hemolysis assays were used. The first was a procedure for measurement of hemolytic activity in culture supernatants and was adapted from the study by Alouf et al. (1). Broth cultures of B. anthracis, started from plate-grown bacteria, were grown at 37°C until the desired density was reached. Culture supernatants were harvested by centrifugation (8,000 × g for 1 min) and filtered through a 0.45-μm (pore-size) polyethersulfone membrane (Millipore Corp., Bedford, Mass.). Supernatants were assayed directly or were diluted in sterile medium before being assayed. The hemolytic activity of ALO was increased by the addition of 100 μl of cysteine (200 mM stock) to 900 μl of culture supernatant and incubated at room temperature for 10 min (in experiments in which cysteine was omitted, it was replaced with 100 μl of double-distilled H2O). Erythrocytes (500 μl, ∼106 cells/ml) were added, and samples were incubated at 37°C for 45 min. After incubation, samples were centrifuged for 1 min at 8,000 × g, and the A541 of the supernatant was determined. The 100% hemolysis reference was prepared by adding 0.1% (wt/vol [final concentration]) sodium carbonate to 500 μl of sheep erythrocytes or 1 ml of double-distilled H2O to 500 μl of human erythrocytes to yield a final A541 of ∼1.0. Uninoculated broth, with or without cysteine, served as a negative control.
The second hemolysis assay was performed in 96-well plates. One microliter of 0.6 M cysteine in phosphate-buffered saline (PBS) was added to 100 μl of 2-fold (or 10-fold) dilutions of the ALO samples and allowed to incubate at room temperature for 10 min in a 96-well round-bottom plate. After incubation, 35 μl of PBS and 15 μl of a 10% solution of human red blood cells washed four times with PBS were added to each well. The plate was allowed to incubate at 37°C for 1 h with gentle and occasional rocking and then centrifuged at 700 rpm for 1 min. The results were read by placing the plate on a horizontal X-ray light box and then viewing (and photographing) the plate from above. The hemolysis endpoint in a series of dilutions was the most-concentrated fraction, i.e., the least-diluted fraction, that gave any degree of hemolysis.
For cholesterol inhibition assays, a concentrated stock of cholesterol (Sigma, St. Louis, Mo.) in 100% ethanol was diluted in 100% ethanol so that 10 μl could be added to the assay to give the appropriate final concentration. Addition of 10 μl of 100% ethanol alone served as a control.
Reverse transcriptase PCR (RT-PCR).
Cells were grown in Luria-Bertani (LB) or BHI broth as described above, and 1-ml samples for RNA isolation were taken at mid-exponential phase (optical density at 600 nm [OD600] of ∼1.0), transition phase (OD600 of ∼1.5), or stationary phase (OD600 of ∼1.8). The GramCracker kit (Ambion, Austin, Tex.) and RNAwiz (Ambion) were used to isolate total RNA according to the manufacturer's instructions. Purified RNA was treated with a DNA-free kit (Ambion). The treated RNA (1.0 μg) was subjected to reverse transcription with a RETROscript kit (Ambion). RT mixtures (0.1 to 2.0 μl) were used as cDNA templates for PCRs. Primers for amplification of a 643-bp DNA fragment internal to the alo gene were as follows: alo forward primer (5′-TTAGCACTTGAATATTCTGTAGTTG-3′) and alo reverse primer (5′-TGCAGTATACAGAATCTATGGTTTA-3′). To verify that equivalent amounts of cDNA were used in the PCRs, reactions were also performed with primers designed to amplify an 899-bp fragment internal to the 16S rRNA gene (accession no. AF290552) of B.anthracis: rnn forward primer (5′-CACACTGGGACTGAGACACG-3′) and rnn reverse primer (5′-AAGGGGCATGATGATTTGAC-3′). Negative controls included no-RT samples assembled for each PCR set.
Construction of an alo-null mutant UT231 and its complemented strain UT231(pUTE554).
An alo-null mutant (UT231) in which sequences from 102 nucleotides downstream of the translational start to 37 nucleotides upstream of the translational stop were replaced with the an omega cassette conferring kanamycin resistance, Ω-km2, was created by using a method described previously (6). Briefly, DNA sequences flanking the internal region of alo were amplified by using the PCR and the following oligonucleotide primers: for the 803-bp upstream flanking region, forward primer 5′-GGATCCGCATTACCGGCTTGTGTTTC-3′ and reverse primer 5′-CTGCAGTTTGGTAGTCTTGCCGCTTT-3′ were used, and for the 1,154-bp downstream flanking region, forward primer 5′-GTCGACTTCCCATACCTGACCAAACC-3′ and reverse primer 5′-GGATCCGGTTTCAATTGGAGGAACAA-3′ were used. A pUTE29 delivery vector harboring the Ω-km2 cassette flanked by the PCR products was cloned in Escherichia coli. The construct was subsequently electroporated into B. anthracis 7702 with selection for the vector-encoded tetracycline resistance gene (15). After growth in LB broth without antibiotics, cultures were screened for kanamycin-resistant, tetracycline-sensitive clones. A kanamycin-resistant, tetracycline-sensitive isolate in which a double-crossover recombination event occurred was confirmed by PCR with primers corresponding to regions flanking the alo gene and sequences within the Ω-km2 element.
For complementation of the UT231 mutation, plasmid pUTE554 containing the alo gene was created as follows. A 2,111-bp PCR product with DNA sequence corresponding to alo and including 513 bp upstream of the translational start codon and 59 bp downstream of the translational stop codon was generated by using the oligonucleotide primers 5′-GCCGATCTACTTTCACTTAA-3′ (forward) and 5′-ATGCCGAAGAAAATAACAAA-3′ (reverse). The PCR product was cloned initially into pGEMT-Easy (Promega, Madison, Wis.) and transformed into E. coli TG1. The alo gene was then subcloned into SacI /SalI-digested plasmid pUTE29 (6) to create pUTE554. B. anthracis UT231 was electroporated with pUTE554 or the pUTE29 vector (as a negative control), with the vector-encoded tetracycline resistance gene for selection. The presence of plasmids was confirmed by PCR with primers that amplify internal sequences of alo and the tetracycline resistance gene of pUTE29.
Expression and purification of rALO.
The gene for ALO was amplified from B. anthracis chromosomal DNA with the primer set 5-CGGGGGATCCGAAACACAAGCC (forward) and 5-CCGGGAATTCCCTAATGACTAATAGTAGC (reverse) that placed a signal peptide deficient alo gene (starting at E35 of the ALO primary structure) into the pTrcHisA expression vector (Invitrogen, Carlsbad, Calif.), which facilitated the intracellular expression of ALO. The gene was cloned between the BamHI and EcoRI sites of pTrcHisA. Recombinant ALO (rALO) was expressed and purified as previously described for related recombinant perfringolysin O (PFO) (28).
RESULTS
Identification of a gene encoding a CDC in B. anthracis.
A BLAST analysis of the B. anthracis genome sequence data (www.tigr.org) revealed an ORF with more than 60% similarity to the LLO protein of L. monocytogenes at the amino acid level (Fig. 1). The presence of a gene in the B. anthracis genome encoding a putative CDC family member has been reported previously (8, 17, 24), but to our knowledge studies have not been performed on the regulation of expression of this gene product by environmental variables or on the characterization of rALO (we have named the gene alo).
FIG. 1.
Hemolytic activity of decreasing concentrations of B. anthracis 7702 BHI broth culture supernatants. Bacteria were grown in BHI at 37°C to stationary phase (OD600 of >1.0). Supernatants were harvested by centrifugation (10,000 × g, 10 min) and passed through a 0.45-μm (pore-size) membrane filter to remove the remaining bacteria. The sterile supernatants, or appropriate twofold dilutions, were added to 106 washed sheep erythrocytes. Hemolysin was activated by addition of cysteine to the supernatants to a final concentration of 20 mM. 100% hemolysis (positive control) was achieved by the addition of a final concentration of 0.1% (wt/vol) sodium carbonate to the sheep erythrocytes. The A541 of the assay supernatant was determined and used to calculate the percent hemolysis. The results shown are the average from three independent experiments, each in duplicate. The error bars represent the standard deviation.
The amino acid sequence of ALO is nearly identical to the amino acid sequences of CDCs possessed by other closely related Bacillus species. Table 1 shows the percent similarity and percent identity between ALO and several other CDC family members. Sequence alignment of LLO and ALO shows that these proteins are closely related and share several conserved sequences, most notably the undecapeptide sequence present near the C terminus of both ALO and LLO, which is 100% conserved in all CDCs (3).
TABLE 1.
Similarity and identity of ALO to other CDCs
| Organism | CDCa | ALO
|
|
|---|---|---|---|
| % Homology | % Identity | ||
| Bacillus cereus | Cereolysin O | 98 | 98 |
| Bacillus weihenstephanensis | Cereolysin O (put.) | 98 | 98 |
| Bacillus thuringiensis | Cereolysin O (put.) | 98 | 97 |
| Bacillus mycoides | Cereolysin O (put.) | 97 | 94 |
| Clostridium perfringens | PFO | 87 | 74 |
| Bacillus alvei | Alvcolysin | 87 | 73 |
| Streptococcus pyogenes | Streptolysin O | 80 | 64 |
| Streptococcus canis | Streptolysin O | 79 | 63 |
| Listeria ivanovii | Ivanolysin | 66 | 46 |
| Listeria seeligeriolysin | Seeligeriolysin | 66 | 45 |
| Streptococcus pneumoniae | Pneumolysin | 67 | 44 |
| Listeria monocytogenes | LLO | 64 | 43 |
put., putative.
B. anthracis culture supernatants possess hemolytic activity.
The presence of a gene encoding a putative CDC in B. anthracis raises some interesting questions. Is this gene expressed by B. anthracis and, if so, why isn't B. anthracis hemolytic on sheep blood agar plates? At least some strains of B. anthracis are, as observed by Guttmann and Ellar, hemolytic on washed human blood agar plates (13). To begin to address these questions, we determined the hemolytic activity present in culture supernatants of B. anthracis grown in BHI broth. We used a modified hemolysis assay developed by Alouf et al. (1). B. anthracis culture supernatants expressed dose-dependent hemolytic activity against sheep erythrocytes (Fig. 1). BHI broth alone was not hemolytic. We conclude that although B. anthracis is not hemolytic on commercially available sheep blood agar plates, B. anthracis BHI culture supernatants possess active, dose-dependent hemolytic activity against sheep and human (reported below) erythrocytes.
Hemolytic activity of B. anthracis supernatants is activated by reducing agents and inhibited by cholesterol.
Members of the CDC family have also been called thiol-activated cytolysins since many of them exhibit increased hemolytic activity after treatment with sulfhydryl-containing reducing agents (e.g., cysteine, β-mercaptoethanol, and dithiothreitol) (3). We examined the hemolytic activity against sheep and human erythrocytes of culture supernatants with or without pretreatment with 20 mM cysteine. There was a significant increase in the hemolytic activity of B. anthracis supernatants after treatment with cysteine (53% ± 6% with cysteine versus 32% ± 5% without cysteine [n = 3] for sheep erythrocytes).
The CDCs are so named because they associate with cholesterol in cell membranes and insert themselves into the membrane, creating large pores. The presence of free cholesterol significantly inhibits the hemolytic activity of members of the CDC family of proteins (21, 26, 32). To support our hypothesis that a CDC was causing the observed hemolysis, the effect of free cholesterol on hemolytic activity of B. anthracis culture supernatants was examined. In dose-response experiments, cholesterol concentrations of ≥50 ng/ml inhibited the hemolytic activity of B. anthracis supernatants to background levels (Fig. 2). These data indicate that a CDC, presumably ALO, is the predominant hemolysin present in B. anthracis culture supernatants.
FIG. 2.
Dose-dependent cholesterol inhibition of B. anthracis hemolysin. B. anthracis 7702 was grown in BHI broth at 37°C until stationary phase, as described in Fig. 1, supernatants were harvested by centrifugation, passed through a 0.45-μm (pore-size) membrane filter, and assayed for hemolytic activity. Cholesterol was diluted in absolute ethanol so that 10 μl could be added to the supernatant to yield the indicated final concentrations of cholesterol. Absolute ethanol alone (10 μl) was added as a control. The results shown are the average from three independent experiments, each in duplicate. The error bars represent the standard deviation.
Culture supernatants of B. anthracis Sterne strain 7702 Δalo (strain UT231) express barely detectable hemolytic activity.
To confirm that ALO is responsible for the hemolytic activity present in B. anthracis culture supernatants, we constructed an alo deletion mutant of Sterne strain 7702. Internal sequences of the alo gene were replaced with a kanamycin resistance cassette as described in Materials and Methods and as shown in Fig. 3. B. anthracis UT231 was indistinguishable from the parent strain in terms of growth rate, colony morphology, and sporulation efficiency (data not shown). Culture supernatants of BHI-grown B. anthracis UT231 contained barely detectable hemolytic activity against sheep or human erythrocytes when grown under conditions identical to the parent strain (34% ± 4% hemolysis for 7702 versus 2.5% ± 0.7% hemolysis for UT231 [n = 3] for sheep erythrocytes). We next complemented UT231 in trans with a native B. anthracis alo gene inserted into the low-copy-number plasmid pUTE29. Plasmid pUTE29 containing the alo gene is called plasmid pUTE554. The hemolytic activities against washed human erythrocytes of undiluted BHI supernatants of B. anthracis strains 7702, UT231, UT231(pUTE29), and UT231(pUTE554) are presented in Fig. 4. Strains UT231 (the Δalo strain) and UT231 transformed with pUTE29 (with no alo insert) showed no hemolytic activity, whereas strains 7702 (parent) and UT231(pUTE554) (the complemented Δalo strain), showed complete hemolysis. Thus, alo supplied in trans to the Δalo mutant restored the ability of the complemented strain to express hemolytic activity. These data support our hypothesis that the alo gene product is the hemolysin detected in B. anthracis culture supernatants and support the results of our cholesterol inhibition assays, which indicate that ALO is the predominant hemolysin produced by B. anthracis under the culture conditions used in these studies.
FIG. 3.
Diagram of the genetic organization of B. anthracis 7702 Δalo mutant, UT231. Construction of the mutant is described in Materials and Methods. Putative functions of proteins encoded by flanking ORFs are shown. The vertical lines represent potential stem-loop structures, each with ΔG values of −16.4 kcal/mol. Sequences replaced by the Ω cassette are indicated.
FIG. 4.
Hemolytic activity of undiluted BHI growth supernatants of B. anthracis strains 7702, the parent ALO+ strain; UT231, the ALO knockout of 7702; UT231(pUTE29), UT231 transformed with plasmid pUTE29 containing no alo gene; and UT231(pUTE554), UT231 transformed with pUTE29 containing the parent alo gene, i.e., the alo complemented UT231 strain. Results are shown from a representative experiment of four independent experiments.
Activity of rALO.
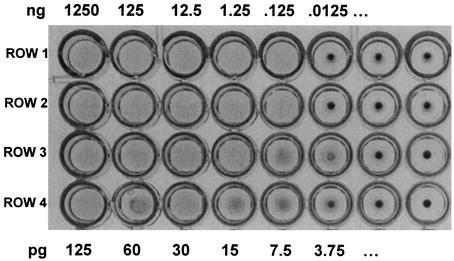
To further show the hemolytic activity of the alo gene product, we measured the hemolytic activity of rALO in the 96-well dilution plate assay by using washed human erythrocytes (Fig. 5). As little as 7.5 pg of rALO caused complete hemolysis. This correlates to ∼1.5 × 10−16 mol of ALO, or ∼9 × 107 molecules, causing the lysis of 4 × 106 erythrocytes (used in each well of the assay), which equals ∼24 molecules of ALO per erythrocyte. This is equivalent to one or two pores, if we assume that ALO is similar in function to other well-studied CDCs, such as PFO (27, 29).
FIG. 5.
Hemolytic activity of rALO. rALO was diluted and tested for hemolytic activity against washed human erythrocytes. In the first two rows (duplicates of each other), 10-fold dilutions were assayed, starting from 1,250 ng of rALO. In the second two rows (duplicates of each other), twofold dilutions were assayed, starting with 125 pg of rALO. Dilutions of rALO were in PBS containing 0.1% bovine serum albumin. Results are shown from a representative experiment of six independent experiments.
Effect of growth medium on B. anthracis hemolysin production.
We investigated the role of growth conditions on ALO expression. Our own microarray data (T. M. Koehler, unpublished data) indicate that alo is only weakly expressed in a Casamino Acids medium often used to assess anthrax toxin gene expression. We first measured hemolytic activity of culture supernatants from B. anthracis grown in different types of media to determine whether the medium used had an effect on hemolysin production. Our results showed a dramatic difference in hemolysis detectable in culture supernatants of B. anthracis grown in LB broth compared to BHI broth (Fig. 6); there was barely detectable hemolytic activity present in LB culture supernatants. Semiquantitative RT-PCR data at least partially support these observations (Fig. 7), indicating higher steady-state levels of alo mRNA when cells are grown in BHI compared to growth in LB medium. However, the differences in alo mRNA detected in BHI versus LB medium were not as dramatic as the hemolytic activity observed in supernatants. These data together suggest that ALO expression may be transcriptionally and posttranscriptionally regulated. Studies of this regulation are currently under way. Next, we showed that the addition of dextrose to LB broth, in amounts equal that present in BHI broth, increased the hemolytic activity detectable in culture supernatants, almost to levels seen in BHI broth. We also tested Super Broth (25 g of tryptone [Difco], 15 g of yeast extract [Difco], and 5 g of NaCl/liter), which has the same ingredients as LB medium but at higher concentrations, with less NaCl, and GC broth plus supplements, which is a rich nutrient medium commonly used for the culture of fastidious organisms such as Neisseria sp. Super Broth and GC broth culture supernatants contained hemolytic activity comparable to that observed when bacteria were grown in BHI broth. Thus, rich medium or significant concentrations of glucose in LB are required for optimal expression of hemolytic activity.
FIG. 6.
Effect of growth medium on hemolysin production. B. anthracis 7702 was grown in BHI medium, LB medium, LB medium plus 2 g of dextrose/liter, Super Broth, or GC broth at 37°C until stationary phase. Supernatants were harvested by centrifugation, passed through 0.45-μm (pore-size) membrane filters and assayed for hemolytic activity against sheep erythrocytes in the presence of cysteine, as described in Fig. 1. The results shown are the average from three independent experiments, each carried out in duplicate. The error bars represent the standard deviation.
FIG. 7.
RT-PCR of alo mRNA isolated from B. anthracis strain 7702 grown in BHI or LB broth at mid-log, transition (trans), or early stationary phase as described in Materials and Methods.
ALO is expressed maximally during mid- and late-log-phase growth.
To further characterize the expression of ALO by B. anthracis, we measured hemolytic activity in culture supernatants from lag to stationary phase as the bacteria grew in BHI broth. Hemolytic activity was first observed in mid-log phase and increased through late log phase, essentially following the growth curve (Fig. 8). Little further increase in hemolytic activity of culture supernatants was observed during stationary phase, suggesting that ALO expression is decreased in the stationary phase. Semiquantitative RT-PCR of alo mRNA from log-, transition-, and stationary-phase cells grown in BHI or LB broth support these observations (Fig. 7).
FIG. 8.
Expression of hemolytic activity in culture supernatants during growth of B. anthracis in BHI broth. Growth of B. anthracis (OD600) and percent hemolysis, measured as described in Fig. 1 and in Materials and Methods, are plotted against each other to visualize hemolysin production at various growth phases. One experiment representative of three independent experiments.
DISCUSSION
ALO, previously identified in the genome of B. anthracis, is highly homologous to other CDCs. The CDCs, including cereolysin O, streptolysin O, LLO, pneumolysin, and PFO, are a family of highly conserved proteins found in many diverse gram-positive species. These proteins appear to play a significant role in the pathogenesis of their respective organisms (3, 9, 21, 26, 32). The expression of a CDC gene in B. anthracis may have implications regarding our understanding of the pathogenic mechanisms of the organism, including its ability to escape from the macrophage and to affect other cells during systemic disease.
We demonstrated that B. anthracis produces readily detectable hemolytic activity against sheep and human erythrocytes when grown in BHI broth culture. We also show that the hemolysin produced by B. anthracis is activated by cysteine and inhibited by cholesterol, which are both characteristics of CDCs. Furthermore, we demonstrate that a Δalo mutant (UT231) expresses almost no hemolytic activity in culture supernatants when grown under the same conditions as the parent strain and that the alo-complemented strain regains the ability to express hemolytic activity. To our knowledge, this is the first time regulation of the alo gene has been shown to be influenced by environmental conditions.
Our observations on ALO expression differ in some respects from those of Mignot et al. (17), who recently reported a lack of hemolytic activity against sheep erythrocytes in B. anthracis culture supernatants. These investigators proposed that the lack of expression of significant hemolytic activity was due to the lack of the functional transcriptional regulator, PlcR, in B. anthracis. The alo gene is preceded by a consensus site for binding of the pleiotropic regulator PlcR, found in B. cereus and B. thuringiensis. However, the plcR gene of B. anthracis is predicted to encode a nonfunctional protein. Mignot et al. (17) demonstrated that a recombinant B. anthracis strain, 9131(pXO1−, pXO2−) harboring a multicopy plasmid containing the plcR gene from B. cereus expresses significantly increased hemolytic activity against both sheep and human erythrocytes compared to the parental strain and that the hemolysis appeared to be due to a CDC.
There are several possible explanations for the differences between what we are seeing and what Mignot et al. reported. (i) The growth conditions, e.g., aeration, temperature, media, and medium manufacturer, were different. This seems quite feasible since, in our present studies, we have shown differences in ALO expression in various growth conditions. (ii) For their in vitro work, Mignot et al. used a B. anthracis strain, strain 9131, that lacked both pXO1 and pX02. In our studies, we used strain 7702, which contains pXO1 but not pXO2. pXO1 contains almost 150 genes, some of which are regulatory. It is possible that there are as-yet-unknown complex interactions between pXO1 genes and their products and the regulation of alo expression. (iii) It appears that Mignot et al. grew their B. anthracis 9131 in media containing erythrocytes. The erythrocytes may have bound secreted ALO during B. anthracis growth, thus removing measurable, soluble ALO as it was being expressed. When the growth cultures were centrifuged to obtain supernatants free of bacteria and erythrocytes, they would also be free of ALO; thus, it would appear as if no or little hemolysin was being produced. A broader implication of these observations is that Mignot et al. suggested that there are many genes in the B. anthracis genome that appear to be silent when studied in silico. This has also observed by others (T. Koehler and colleagues, data not shown). However, the expression of detectable ALO in vitro suggests that such in silico data should be interpreted with caution. It is possible that many of the proposed silent genes are indeed expressed to some degree, at least under certain environmental conditions.
Our experiments show that the amount of hemolysin expressed in culture supernatants is dependent on the growth medium used and suggest that expression and/or secretion of ALO is under environmental control, as evidenced by the fact that we saw significantly decreased hemolytic activity when B. anthracis was grown in LB broth. After an examination of the obvious differences in ingredients in the media tested, it appears that it may simply be the richness of the medium and/or the glucose concentration that is having this effect on expression. Further studies are warranted.
Work by Guidi-Rontani et al. (12) identified alveolar macrophages as the primary site of B. anthracis spore germination in a mouse model of inhalation anthrax. The spores are phagocytosed by the macrophages and germinate within the phagosome (8, 12). At some point—and this is not clear in the literature—the vegetative bacilli escape from the phagolysosome into the cytosol, where they may multiply (8). The mechanism by which this phagolysosomal escape occurs is not known. Like B. anthracis, L. monocytogenes must also escape from the phagolysosome after infection of a host cell. It accomplishes this by producing LLO, as well as two phospholipase C proteins (PLC-A and PLC-B) (22, 31). It is believed that these proteins act together in a coordinated fashion to release L. monocytogenes from the intracellular vesicle. Interestingly, in addition to possessing ALO, a homologue of LLO, B. anthracis also possesses homologues of both PLC proteins of L. monocytogenes. We hypothesize that ALO and the PLCs of B. anthracis mediate the escape of this organism from the phagolysosome of a host macrophage by a mechanism similar to that of L. monocytogenes. We do not know how much ALO is expressed by B. anthracis within macrophages or when growing in human blood, as occurs during late stages of anthrax. However, the very high specific activity of rALO that we observed—with as little as 24 molecules of rALO causing the complete lysis of a human erythrocyte—combined with the small volume of a phagocytic vacuole suggests that ALO might act to effect escape from the phagocytic vacuole or from the macrophage.
The observation of a gene encoding a CDC in the genome of B. anthracis and the finding that this gene appears to be expressed at significant levels in vitro may represent important insights into our understanding of B. anthracis pathogenesis.
Acknowledgments
This work was supported in part by NIH grant AI033505 and internal (Drexel University) funding to R.F.R. and by NIH grant AI033537 and Department of the Army grant DAMD 17-01-2-0047 to T.M.K.
We thank Alexandra Bortnick and Elise Bifano for excellent technical assistance and dedication to this project. We thank Rodney K. Tweten, University of Oklahoma Health Sciences Center, for his generous gift of rALO.
Editor: D. L. Burns
REFERENCES
- 1.Alouf, J. E., and C. Geoffroy. 1988. Production, purification, and assay of streptolysin O. Methods Enzymol. 165:52-59. [DOI] [PubMed] [Google Scholar]
- 2.Barakat, L. A., H. L. Quentzel, J. A. Jernigan, D. L. Kirschke, K. Griffith, S. M. Spear, K. Kelley, D. Barden, D. Mayo, D. S. Stephens, T. Popovic, C. Marston, S. R. Zaki, J. Guarner, W. J. Shieh, H. W. Carver II, R. F. Meyer, D. L. Swerdlow, E. E. Mast, and J. L. Hadler. 2002. Fatal inhalational anthrax in a 94-year-old Connecticut woman. JAMA 287:863-868. [DOI] [PubMed] [Google Scholar]
- 3.Billington, S. J., J. G. Songer, and B. H. Jost. 2001. Molecular characterization of the pore-forming toxin, pyolysin, a major virulence determinant of Arcanobacterium pyogenes. Vet. Microbiol. 82:261-274. [DOI] [PubMed] [Google Scholar]
- 4.Brossier, F., M. Weber-Levy, M. Mock, and J. C. Sirard. 2000. Role of toxin functional domains in anthrax pathogenesis. Infect. Immun. 68:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataldi, A., E. Labruyere, and M. Mock. 1990. Construction and characterization of a protective antigen-deficient Bacillus anthracis strain. Mol. Microbiol. 4:1111-1117. [DOI] [PubMed] [Google Scholar]
- 6.Dai, Z., J. C. Sirard, M. Mock, and T. M. Koehler. 1995. The atxA gene product activates transcription of the anthrax toxin genes and is essential for virulence. Mol. Microbiol. 16:1171-1181. [DOI] [PubMed] [Google Scholar]
- 7.Decatur, A. L., and D. A. Portnoy. 2000. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science 290:992-995. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival survival and escape. Cell Microbiol. 2:453-463. [DOI] [PubMed] [Google Scholar]
- 9.Goebel, W., and J. Kreft. 1997. Cytolysins and the intracellular life of bacteria. Trends Microbiol. 5:86-88. [DOI] [PubMed] [Google Scholar]
- 10.Guidi-Rontani, C. 2001. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol. 10:405-409. [DOI] [PubMed] [Google Scholar]
- 11.Guidi-Rontani, C., M. Levy, H. Ohayon, and M. Mock. 2001. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 42:931-938. [DOI] [PubMed] [Google Scholar]
- 12.Guidi-Rontani, C., M. Weber-Levy, E. Labruyere, and M. Mock. 1999. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31:9-17. [DOI] [PubMed] [Google Scholar]
- 13.Guttmann, D. M., and D. J. Ellar. 2000. Phenotypic and genotypic comparisons of 23 strains from the Bacillus cereus complex for a selection of known and putative B. thuringiensis virulence factors. FEMS Microbiol. Lett. 188:7-13. [DOI] [PubMed] [Google Scholar]
- 14.Hanna, P. 1998. Anthrax pathogenesis and host response. Curr. Top. Microbiol. Immunol. 225:13-35. [DOI] [PubMed] [Google Scholar]
- 15.Koehler, T. M., Z. Dai, and M. Kaufman-Yarbray. 1994. Regulation of the Bacillus anthracis protective antigen gene: CO2 and a trans-acting element activate transcription from one of two promoters. J. Bacteriol. 176:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 17.Mignot, T., M. Mock, D. Robichon, A. Landier, D. Lereclus, and A. Fouet. 2001. The incompatibility between the PlcR- and AtxA-controlled regulons may have selected a nonsense mutation in Bacillus anthracis. Mol. Microbiol. 42:1189-1198. [DOI] [PubMed] [Google Scholar]
- 18.Mina, B., J. P. Dym, F. Kuepper, R. Tso, C. Arrastia, I. Kaplounova, H. Faraj, A. Kwapniewski, C. M. Krol, M. Grosser, J. Glick, S. Fochios, A. Remolina, L. Vasovic, J. Moses, T. Robin, M. DeVita, and M. L. Tapper. 2002. Fatal inhalational anthrax with unknown source of exposure in a 61-year-old woman in New York City. JAMA 287:858-862. [DOI] [PubMed] [Google Scholar]
- 19.Mock, M., and A. Fouet. 2001. Anthrax. Annu. Rev. Microbiol. 55:647-671. [DOI] [PubMed] [Google Scholar]
- 20.Mock, M., and T. Mignot. 2003. Anthrax toxins and the host: a story of intimacy. Cell. Microbiol. 5:15-23. [DOI] [PubMed] [Google Scholar]
- 21.Palmer, M. 2001. The family of thiol-activated, cholesterol-binding cytolysins. Toxicon 39:1681-1689. [DOI] [PubMed] [Google Scholar]
- 22.Portnoy, D. A., G. A. Smith, and H. Goldfine. 1994. Phospholipases C and the pathogenesis of Listeria. Braz. J. Med. Biol. Res. 27:357-361. [PubMed] [Google Scholar]
- 23.Quintiliani, R., Jr., and R. Quintiliani. 2002. Fatal case of inhalational anthrax mimicking intra-abdominal sepsis. Conn. Med. 66:261-267. [PubMed] [Google Scholar]
- 24.Read, T. D., S. L. Salzberg, M. Pop, M. Shumway, L. Umayam, L. Jiang, E. Holtzapple, J. D. Busch, K. L. Smith, J. M. Schupp, D. Solomon, P. Keim, and C. M. Fraser. 2002. Comparative genome sequencing for discovery of novel polymorphisms in Bacillus anthracis. Science 296:2028-2033. [DOI] [PubMed] [Google Scholar]
- 25.Robinson-Dunn, B. 2002. The microbiology laboratory's role in response to bioterrorism. Arch. Pathol. Lab. Med. 126:291-294. [DOI] [PubMed] [Google Scholar]
- 26.Schindel, C., A. Zitzer, B. Schulte, A. Gerhards, P. Stanley, C. Hughes, V. Koronakis, S. Bhakdi, and M. Palmer. 2001. Interaction of Escherichia coli hemolysin with biological membranes: a study using cysteine scanning mutagenesis. Eur. J. Biochem. 268:800-808. [DOI] [PubMed] [Google Scholar]
- 27.Shatursky, O., A. P. Heuck, L. A. Shepard, J. Rossjohn, M. W. Parker, A. E. Johnson, and R. K. Tweten. 1999. The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99:293-299. [DOI] [PubMed] [Google Scholar]
- 28.Shepard, L. A., A. P. Heuck, B. D. Hamman, J. Rossjohn, M. W. Parker, K. R. Ryan, A. E. Johnson, and R. K. Tweten. 1998. Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry 37:14563-14574. [DOI] [PubMed] [Google Scholar]
- 29.Shepard, L. A., O. Shatursky, A. E. Johnson, and R. K. Tweten. 2000. The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins. Biochemistry 39:10284-10293. [DOI] [PubMed] [Google Scholar]
- 30.Smith, H. 2002. Discovery of the anthrax toxin: the beginning of studies of virulence determinants regulated in vivo. Int. J. Med. Microbiol. 291:411-417. [DOI] [PubMed] [Google Scholar]
- 31.Wadsworth, S. J., and H. Goldfine. 1999. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect. Immun. 67:1770-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weis, S., and M. Palmer. 2001. Streptolysin O: the C-terminal, tryptophan-rich domain carries functional sites for both membrane binding and self-interaction but not for stable oligomerization. Biochim. Biophys. Acta 1510:292-299. [DOI] [PubMed] [Google Scholar]