Abstract
It has been reported that Helicobacter hepaticus infection of mice leads to chronic hepatitis and hepatocarcinoma. Our aim was to monitor a cohort of 80 conventional A/J mice in which half of the mice were infected by H. hepaticus in order to study the evolution of the infection and the pathological changes in comparison to uninfected mice. H. hepaticus was detected by culture only in some colon and cecum specimens after 17 months of age, while PCR detected H. hepaticus in the intestines of all inoculated mice after only 5 months of infection. The percentage of mice in which H. hepaticus was detected in the gallbladder, bile ducts, and liver by PCR, as well as the number of bacteria present in the liver, tended to increase with increasing age and longer infection time. Anti-H. hepaticus immunoglobulin G antibodies were positive by enzyme-linked immunosorbent assay only in inoculated mice. Pathological findings were also more frequent as the mice grew older: fibrosis was present (especially in the peripheral part of the liver), and significant portal inflammation including lymphoid nodules was present in almost all infected animals. Biliary lesions of neutrophilic acute cholangitis or lymphocytic cholangitis were noted. However, lesions were also observed in uninfected animals, although at a significantly lower level, and the only hepatocellular carcinoma occurred in an uninfected mouse. The evolution towards hepatocarcinoma is not always the endpoint and may depend on the bacterial strain and on the environmental conditions.
Helicobacter species are responsible for chronic infections. In humans, Helicobacter pylori induces gastritis, which may last for decades and may be a lifelong condition. This infection is the first bacterial infection found to be involved in the development of a carcinogenic process in humans (8).
After the occurrence of an unexpected and high level of hepatocellular tumors in mice with chronic active hepatitis, H. hepaticus was cultured from the liver and identified as a new species of Helicobacter that induces carcinoma (5). This bacterium has been the topic of extensive research since then. It was the second Helicobacter after H. pylori to have its complete genome sequenced (S. Suerbaum, C. Josenhans, M. Frosch, M. Bell, K. Braig, P. Brandt, B. Chevreux, G. Dietrich, B. Drescher, M. Droege, B. Fartmann, H. P. Fischer, Z. Ge, R. Holland, W. Mann, G. Nyakatura, O. Pfannes, D. Schauer, J. Weber, and J. G. Fox, abstract, 14th International Workshop on Gastroduodenal Pathology and Helicobacter pylori, Strasbourg, France, 5 to 8 September 2001, Gut 49:A9, 2001).
There have been studies on diagnostic methods (4, 13, 16, 17), pathogenic properties (2, 18), and the process of carcinogenesis (3, 9, 15) but few prospective studies of the natural history of H. hepaticus infection and liver pathology in mice.
Therefore, our aim was to study the natural history of this infection for 17 months and to use the LightCycler methodology to quantitate the bacteria present in the liver.
Study design.
Eighty male A/J mice (3 to 4 weeks old) (not specific pathogen free), obtained from Jackson Laboratory (Bar Harbor, Maine), were maintained in microisolator cages and fed conventional food (Usine d’Alimentation Rationnelle, Villemoisson-sur-Orge, France). The mice were cared for in accordance with the National Institutes of Health guidelines. Half of the mice were orally inoculated with H. hepaticus (group I), and the other half (group II) were used as controls. H. hepaticus type strain CCUG 33637 (equivalent to ATCC 51448) was grown for 4 days under microaerobic conditions at 37°C on a selective medium developed for culture of H. pylori. Bacteria were harvested and resuspended in brucella broth to approximately 107 CFU/ml. Mice were inoculated by oral gavage with 100 μl of this suspension. Five to 10 mice from each group were euthanized at 5, 11, 15, and 17 months of age after weight measurement. At necropsy, sections of liver, colon, cecum (two samples), gallbladder, bile ducts, and feces were ground and used for culture and PCR. Liver samples were also prepared for histological examination. Serum was collected and tested for anti-H. hepaticus antibodies by enzyme-linked immunosorbent assay (ELISA).
Culture.
Tissue samples were ground in brucella broth and plated on nonselective Columbia agar medium supplemented with 10% human blood and pylori agar prepared in house (14). Ground samples (feces, colon, and cecum) were filtered before culture (pore size, 0.45 μm). Plates were incubated at 37°C for up to 12 days in a microaerobic atmosphere generated by using GasPaks in jars. H. hepaticus was characterized by morphology, Gram stain, and catalase and urease production.
Histological examination.
After sacrifice, tissue samples were taken both from the peripheral part of the left lateral, left middle, right middle, and quadrate lobes and from the central or perihilar part of the liver of each animal. After 10 to 24 h of fixation in 10% formaldehyde, the specimens were routinely processed and embedded in paraffin. Sections (5 μm thick) were stained with hematein-eosin-safran, with trichrome stain for better evaluation of fibrosis, with modified Giemsa stain, and in certain cases, with Warthin-Starry stain to detect bacteria.
Various histological parameters were evaluated and graded semiquantitatively in both peripheral and central specimens as follows: fibrosis (0, absence; 1, mild portal fibrosis; 2, portal fibrosis with occasional septa; 3, septal fibrosis; 4, cirrhosis), portal inflammation (0, no inflammation; 1, mild infiltrate in a minority of portal tracts; 2, mild infiltrate in almost all portal tracts; 3, moderate infiltrate in most portal tracts; 4, severe infiltrate in most portal tracts), lymphoid nodules in portal tracts (0, no nodules found in portal tracts; 1, nodules occasionally found; 2, nodules found in a majority of portal tracts; 3, large nodules in all portal tracts), biliary lesions (0, no lesions; 1, occasional or mild cholangitis; 2, severe cholangitis), lobular activity (0, absence of activity; 1, less than one small focus with hepatic necrosis and/or inflammation per lobule; 2, one or two small foci with hepatic necrosis and/or inflammation per lobule; 3, many foci with hepatic necrosis and/or inflammation per lobule; 4, many large and confluent foci per lobule), and H. hepaticus-like organism (0, absence of organism; 1, suspected presence of bacteria with morphology consistent with H. hepaticus as determined by using special stains). When tumors were present, their types and locations were noted.
Semiquantitative parameters of the two groups were compared using the chi-square test. A P of <0.05 was considered to be significant.
Molecular detection.
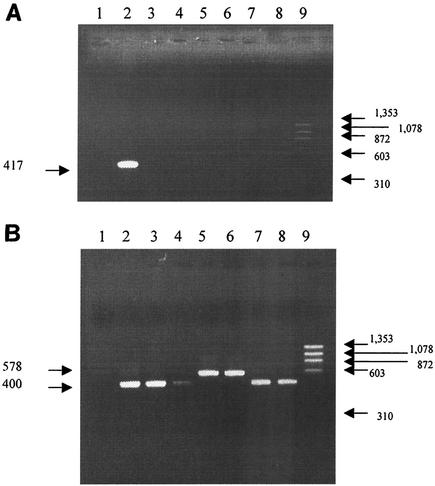
Genomic DNA was isolated by using the QIAamp DNA mini kit (Qiagen SA, Courtaboeuf, France) in accordance with the manufacturer's instructions. Two sets of primers were selected (16). The first set of primers amplified a 417-bp region of the 16S rRNA gene specific for H. hepaticus: HhpF (5′ GCA TTT GAA ACT GTT ACT CTG 3′) and HhpR (5′ CTG TTT TCA AGC TCC CC 3′). The specificity of the primers was determined by testing DNA from six different Helicobacter species: Helicobacter bilis, H. pylori, Helicobacter rappini, Helicobacter pullorum, Helicobacter muridarum, and Helicobacter felis. The expected 417-bp DNA fragment was amplified only from 16S rRNA from H. hepaticus (Fig. 1A). The second set of primers used (HS1 [5′ AAC GAT GAA GCT TCT AGC TTG CTA G 3′] and HS2 [5′ GTG CTT ATT CGT TAG ATA CCG TCA T 3′]) was specific to the 16S ribosomal DNA of the Helicobacter genus. A 400-bp amplicon was amplified from Helicobacter species (Fig. 1B), except for H. bilis and H. rappini (578 bp). All PCR amplifications were performed in a 50-μl volume using a thermal cycler (model 480; Perkin-Elmer Biosystems, Foster City, Calif.). The reaction mixture contained 1× Taq polymerase buffer, 200 μM (each) oligonucleotide, 0.5 μM (each) primer, 1.5 mM MgCl2, 2 U of Taq polymerase, and 5 μl of DNA. Samples were heated at 94°C for 5 min and subjected to 35 cycles of PCR, with 1 cycle consisting of denaturation at 94°C for 1 min, annealing at 59°C for 2 min (primers HhpF and HhpR) or 60°C for 1.5 min (primers HS1 and HS2), and elongation at 72°C for 1 min. The final incubation was at 72°C for 7 min. PCR products (5 μl) were electrophoretically separated in a 1% agarose gel stained with ethidium bromide and visualized with UV light.
FIG. 1.
Amplicons obtained with different primers for H. hepaticus detection. (A) Primers HhpF and HhpR were used to detect H. hepaticus. (B) Primers HS1 and HS2 were used to detect all Helicobacter species. Lane 1, negative control (water instead of DNA); lane 2, DNA from H. hepaticus type strain CCUG 33637 (equivalent to ATCC 51448); lanes 3 to 8, DNA from H. pylori, H. felis, H. bilis, H. rappini, H. muridarum, and H. pullorum, respectively; lane 9, HaeIII-digested φX174 DNA molecular size markers (Eurobio). The positions of molecular size markers (in base pairs) are shown at the sides of the gels.
LightCycler PCR assay.
The LightCycler PCR assay was used to quantify Helicobacter bacteria. Primers used were the same as those used for the qualitative PCR (primers HhpF and HhpR). For amplicon detection, the LightCycler Faststart DNA Master Sybr Green I kit was used according to the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, Ind.).
ELISA for serum anti-H. hepaticus antibody.
H. hepaticus sonicate was prepared as described elsewhere for H. felis (4). Wells of microtiter plates were coated with 0.5 μg of H. hepaticus sonicate per ml in carbonate buffer. After the plates had been washed, twofold dilutions of sera from H. hepaticus-infected or control mice were incubated for 60 min at 37°C. Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) was then added to each well. Serum IgG antibody titers of <0.1 optical density (OD) unit at 405 nm were considered negative.
Detection of the cag PAI.
Ten different regions of the cag pathogenicity island (PAI) (5′ and 3′ ends of cagA, cagC, cagE/cagF, cagL/cagN/cagM, cagP/cagQ/cagR, cagS/cagT, virB9, virB10, virB11, and virD4) and IS605 (tnpA and tnpB) were sought by PCR and dot blot hybridization as described for H. pylori (14).
The weight of the animals at the time of sacrifice was not significantly different for infected and uninfected mice.
Detection of H. hepaticus.
H. hepaticus was detected by culture only after 17 months in some samples of cecum and colon (Table 1), while it was detected by PCR in the feces, cecum, and colon regularly throughout the experiment. For the other tissues (gallbladder, bile ducts, and liver), H. hepaticus detection increased over the 17-month period (Table 1).
TABLE 1.
Detection of H. hepaticus in inoculated mice by PCR at various time points and by culture
| Method | Mouse age (mo) | Detection of H. hepaticus (no. of mice positive/no. tested)a in:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Feces | Cecum
|
Colon | Gall- bladder | Bile ducts | Liver | |||
| Sample 1 | Sample 2 | |||||||
| PCR | 5 | 5/5 | 4/4 | 5/5 | 3/5 | NR | 2/5 | 3/5 |
| 11 | 5/5 | 5/5 | 5/5 | 4/5 | 1/5 | 5/5 | 4/5 | |
| 15 | 5/5 | 5/5 | 5/5 | 3/5 | 4/5 | 1/5 | 2/5 | |
| 17 | 19/19 | 19/19 | 19/19 | 19/19 | 16/19 | 17/19 | 17/19 | |
| Culture | 17 | NR | 4/19b | 5/19c | 3/19d | NR | NR | NR |
NR, no results.
Significantly different from the value for 17-month-old mice by PCR (P = 0.046 by the McNemar test).
Significantly different from the value for 17-month-old mice by PCR (P = 0.025 by the McNemar test).
Significantly different from the value for 17-month-old mice by PCR (P = 0.083 by the McNemar test).
The mean copy number of target DNA in each inoculated mouse was monitored for 5 to 17 months using the LightCycler PCR assay. The number of bacteria was low and stable for up to 11 months (4.3 × 103 [95% confidence interval {95% CI}, 3.1 × 103 to 5.4 × 103]). It increased notably during the last 6 months of infection to reach 6.4 × 104 (95% CI, 4.2 × 104 to 8.5 × 104) at 17 months.
Histology.
At autopsy, organs and the liver in particular were grossly normal, except for those of two animals with hepatic and retroperitoneal tumors.
(i) Fibrosis.
Significant fibrosis (score of ≥2), consisting of occasional septa (3 cases) or septal fibrosis (2 cases) but never of cirrhosis, was observed in 5 of 59 animals. Four were infected animals, three of them at 17 months, and one fibrotic animal was not infected (not significant [NS]). The two cases of more-severe fibrosis (score 3) were found in two infected animals at 17 months, also displaying very severe inflammation (NS). Fibrosis was usually more severe in the peripheral part of the liver. Focal lobular postnecrotic fibrosis was occasionally observed.
(ii) Inflammation of portal tracts.
Significant portal inflammation (score of ≥2) was found frequently (33 of 59 animals), even at the early stage (7 of 10 animals at 5 months). Inflammation was observed in 25 of 29 infected animals versus 13 of 30 uninfected animals (P < 0.001) (relative risk, 2.89; 95% CI, 1.3 to 6.47). Major infiltrates (score 4) were observed in only three infected animals and at 11 (n = 1) and 17 (n = 2) months (NS), and severe infiltrates (score of 3 or 4) were observed in both groups, 7 of 29 infected animals versus 4 of 30 uninfected animals (NS). This infiltrate consisted mainly of lymphocytes, sometimes grouped in lymphoid nodules (see below), associated with a variable number of plasma cells, neutrophils, and occasional small granulomas with giant cells or foamy macrophages (Fig. 2A).
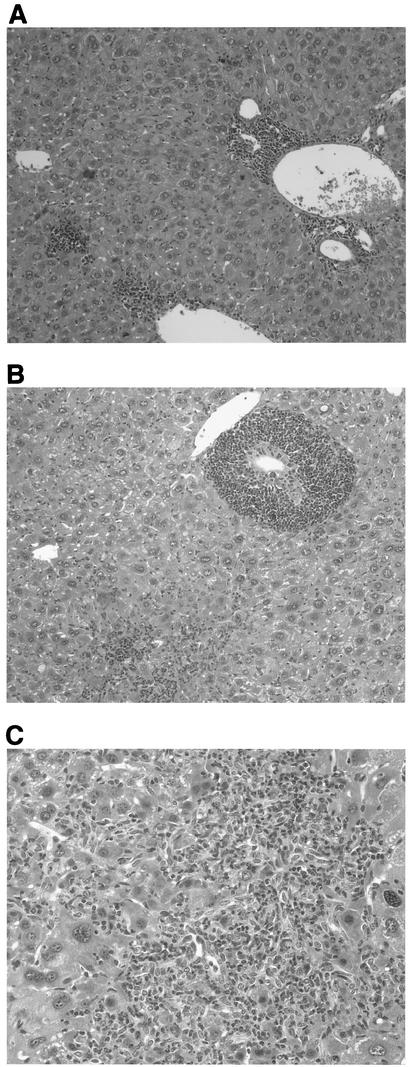
FIG. 2.
Histologies of liver sections from infected mice sacrificed at 17 months of age. (A) Lymphoplasmocytic inflammatory infiltrates located in the portal tracts and in the lobules near the central vein. There is also focal necrosis of hepatocytes. (B) Portal tracts containing follicular lymphoid infiltrate and lymphocytic cholangitis. Focal necroinflammatory changes are visible in the lobules. (C) Portal tract containing moderate lymphoplasmocytic inflammatory infiltrate and surrounded by oval cell proliferation. There is focal necrosis of hepatocytes. Original magnifications, ×10 (A and B) and ×5 (C).
(iii) Lymphoid nodules in portal tracts.
The presence of lymphoid nodules in portal tracts was not uncommon (21 of 59 mice), both in infected (13 of 29 mice) and uninfected (8 of 30 mice) animals (NS), but was usually discrete. Of eight animals with prominent nodules (score of 2 or 3), five were infected (two sacrificed at 5 months, the others at 11 or 17 months) (NS). Nodules were more numerous in the central part of the liver. Some were centered by bile ducts (Fig. 2B).
(iv) Biliary changes.
Biliary lesions were noted in only 14 of 59 animals (8 of 29 infected mice and 6 of 30 uninfected mice) (NS) and two at the first time point (5 months). Of seven animals with severe lesions (score 2), five were infected animals (NS). The lesions were heterogeneous and consisted of neutrophilic acute cholangitis with various degrees of neoductular proliferation (either oval cell hyperplasia [Fig. 2C] or typical ductular proliferation) or lymphocytic cholangitis with occasional destruction of the biliary duct epithelium.
(v) Lobular necrosis or inflammation changes.
Lobular inflammation was consistently found in both groups (26 of 29 infected animals and 22 of 30 uninfected animals) (NS), although it was usually very weak and focal (29 of 48). Noticeable lobular inflammation (score of 2 or 3) was found in 16 of 29 infected mice and 10 of 30 uninfected mice (NS), and major lobular inflammation (score 3) was noted in only eight animals (5 of 29 infected mice and 3 of 30 uninfected mice [NS]). The lobular inflammation appeared as small isolated groups of lymphoid cells or larger foci of lymphocytes and histiocytes intermingled with variable numbers of apoptotic or necrotic hepatocytes (Fig. 2C). Occasionally, pure macrophagic granulomas were present. Hepatocytes often showed subsequent regenerative changes (mitosis and binucleation) and dystrophic changes (vesicular nuclei and nuclear pseudoinclusions). Two microscopic infarcts with purulent reaction were present in a noninoculated animal.
(vi) Helicobacter.
Bacilliform elements compatible with H. hepaticus were observed only in the lumen of interlobular biliary ducts of eight animals, all infected.
(vii) Tumors.
Two tumors were identified at the macroscopic level. One infected animal had a huge retroperitoneal spindle cell sarcoma at 17 months, while one uninfected mouse had a 1-cm well-differentiated hepatocellular carcinoma in the right hepatic lobe, also found at 17 months. Additionally, a 2-mm benign hepatocellular nodule (either hyperplastic or benign adenoma) was discovered at histological examination in an uninfected animal. Some animals also displayed atypical regeneration or dysplastic hepatocytes.
Serological results.
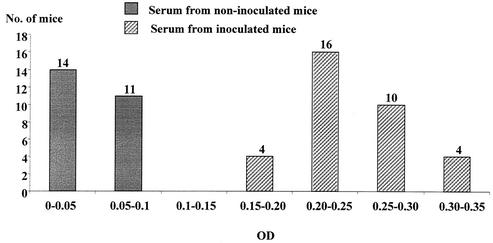
ELISA results have been broken down in increments of 0.05 OD units (Fig. 3). The results clearly show that two groups are present. The first group (<0.1 OD unit) corresponds to the noninoculated mice, and the second group, which features a normal distribution, corresponds to the inoculated mice. No association between the level of antibodies and quantitative PCR results was observed.
FIG. 3.
Distribution of mice according to the ODs of anti-H. hepaticus antibodies by ELISA at sacrifice.
Detection of cag PAI genes in H. hepaticus.
None of the genes of the cag PAI were detected in H. hepaticus in agreement with the genome sequence data (Suerbaum et al., Gut 49:A9, 2001).
This long-term follow-up study of H. hepaticus infection in mice confirms that the bacterium originally colonizes the intestinal tracts of mice. It is possible to find it by PCR in all animals at any time point (5 to 17 months) and in any part of the intestine. However, the number of organisms must be low during most of the mouse's life, since only PCR and not culture was positive at up to 15 months of life. This result can be explained by an eventual, immunologically mediated suppression of the organism. Later, H. hepaticus is found in the gallbladder and liver (also by PCR). It was even possible to visualize H. hepaticus-like organisms in the livers of animals. An original finding in this study is the quantification of the bacteria present in the liver. While bacteria were not detected until the mice were 11 months old, they were detected at 15 months and increased to >105 DNA target copies by the end of the study.
The results of the histological analysis were less convincing than the results of previous histological studies with H. hepaticus (6, 19, 20). In the previous studies, almost all male mice of certain strains, spontaneously or after intraperitoneal injection of a suspension of H. hepaticus into the liver, developed a quite unique chronic active hepatitis with very high incidence (>90%) of benign or malignant hepatocellular tumors. Hepatitis started when the mice were 2 to 6 months old with focal hepatic necrosis and focal subacute nonsuppurative hepatic inflammation with necrosis of single hepatocytes. When the mice were 6 to 10 months old, lesions were often visible at gross examination, and there were additional changes in histology. These changes included the following: oval cell hyperplasia; lymphoplasmocytic, portal infiltrates around bile ducts; postnecrotic fibrosis or mild postnecrotic cirrhosis (usually without regenerative nodules); hepatocellular regeneration and dystrophy; and cholangitis. Spiral bacteria could be identified within hepatocyte canaliculi using special stains, immunohistochemistry, and electron microscopy.
In our study, most of these lesions were found in infected animals, but to a lesser degree, and generally without specificity compared with the controls. This low severity of hepatic lesions could be due to the type of inoculation (oral gavage versus intraperitoneal injection), a lower load of bacteria, or the attenuation of bacterial pathogenicity.
Interestingly, previous studies of H. hepaticus-infected, interleukin-10-deficient mice reported high levels of intestinal inflammation when the mice were raised in conventional facilities, while little inflammation was found when they were raised under specific pathogen-free conditions (10-12). However, our mice were raised in conventional facilities, so the facilities the mice were raised in do not account for the mild lesions observed in the liver.
The unexpectedly high number of hepatic lesions in control animals with a pattern similar to that in infected mice is also puzzling. Contamination by Helicobacter species was excluded by specific tests. We cannot totally rule out the improbable possibility that the animals had other forms of infectious hepatitis, especially of viral origin (mouse hepatitis virus, ectromelia virus, etc.) (19, 20). Environmental chemical contaminants, food or water hepatotoxins, or immunotoxins capable of inducing chronic hepatitis or cholangitis also appear unlikely, due to the study design.
Fibrosis was generally mild, and no cirrhosis and no hepatocellular tumor was observed in the infected mice. Lesions with hepatic necrosis or inflammation affecting the parenchyma and the biliary structures were observed in both groups of animals but were not statistically significant, except for portal inflammation, which was defined as at least moderate inflammatory infiltrate in most portal tracts. Indeed, this aspect was more frequent in the infected group. The presence of Helicobacter organisms was suspected in a minority of infected animals; however, A/JCr mice were shown to harbor less bacteria than other strains of mice (20).
Molecular analysis was shown to be more efficient than histological study, notably the detection of H. hepaticus by PCR.
Fox et al. were the first to study the natural history of H. hepaticus infection in axenic Swiss mice that were experimentally infected (7). Our findings, using conventional A/J mice, are in agreement with their results, i.e., H. hepaticus can be found both in the intestinal tract and in the liver particularly by PCR (culture tended to be more difficult). Similarly, the consequences of H. hepaticus colonization appear progressively throughout the lifetime of the animals. The main difference was that by using conventional mice and not axenic mice, pathology was also observed in the uninfected group. However, while there were no significant differences concerning lymphoid nodules in the portal tract, lobular inflammation, biliary changes, and fibrosis, inflammation of the portal tract was more frequent in infected mice (relative risk, 2.89). Surprisingly, one tumor compatible with an H. hepaticus origin was found in an uninfected mouse, while one sarcoma-type tumor was found in an infected mouse. However, the clear-cut difference in antibody response to H. hepaticus in mice of the two groups does not support spread of the infection among control mice.
In the study by Fox et al. (7), only one hepatocellular carcinoma was diagnosed from 30 animals. These results are quite different from the original data presented by Ward et al. where the tumor incidence was very high (19). Like Fox et al., we used the type strain of H. hepaticus. It may be that laboratory passages have attenuated the virulence of this strain. It has been reported that in another Helicobacter species, H. pylori, chromosomal rearrangements are frequent and may lead to differences in pathogenicity. One of the major pathogenic factors of this bacterium, the cag PAI, was also looked for in our H. hepaticus strain and was not present on the basis of several cag genes screened.
In humans, hepatocellular carcinoma usually occurs in the context of cirrhosis with or without chronic hepatitis; carcinomas arising in noncirrhotic livers are much more rare but seem to be more and more frequent in recent studies from Western countries. We think that Helicobacter infection of the liver can be considered a causative agent of chronic hepatitis, but its role in hepatocarcinogenesis was not confirmed in this study. More studies must be performed to explain the role of Helicobacter in human carcinogenesis of the liver (1).
Acknowledgments
This work was supported in part by the Ligue Nationale Contre le Cancer (Périgueux, France) and the Conseil Régional d'Aquitaine. Philippe Avenaud was the recipient of a fellowship from the Association pour la Recherche contre le Cancer (ARC).
We thank Maria Winnock from INSERM U330 for the statistics.
Editor: A. D. O'Brien
REFERENCES
- 1.Avenaud, P., A. Marais, L. Monteiro, B. Le Bail, P. Bioulac Sage, C. Balabaud, and F. Mégraud. 2000. Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer 13:39-46. [PubMed] [Google Scholar]
- 2.Chien, C. C., N. S. Taylor, Z. Ge, D. B. Schauer, V. B. Young, and J. G. Fox. 2000. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J. Med. Microbiol. 49:525-534. [DOI] [PubMed] [Google Scholar]
- 3.Diwan, B. A., G. Ramakrishna, L. M. Anderson, and D. Ramljak. 2000. Overexpression of Grb2 in inflammatory lesions and preneoplastic foci and tumors induced by N-nitrosodimethylamine in Helicobacter hepaticus-infected and -non-infected A/J mice. Toxicol. Pathol. 28:548-554. [DOI] [PubMed] [Google Scholar]
- 4.Fox, J. G., M. Blanco, J. C. Murphy, N. S. Taylor, A. Lee, Z. Kabok, and J. Pappo. 1993. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect. Immun. 61:2309-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox, J. G., F. E. Dewhirst, J. G. Tully, B. J. Paster, L. Yan, N. S. Taylor, M. J. Collins, P. L. Gorelick, and J. M. Ward. 1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 32:1238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox, J. G., X. Li, L. Yan, R. J. Cahill, R. Hurley, R. Lewis, and J. C. Murphy. 1996. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of Helicobacter-induced carcinogenesis. Infect. Immun. 64:1548-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox, J. G., L. Yan, B. Shames, J. Campbell, J. C. Murphy, and X. Li. 1996. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect. Immun. 64:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 1994. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 61:1-241. [PMC free article] [PubMed] [Google Scholar]
- 9.Josyula, S., H. A. Schut, B. A. Diwan, M. R. Anver, and L. M. Anderson. 2000. Age-related alterations in 32P-postlabeled DNA adducts in livers of mice infected with the tumorigenic bacterial pathogen, Helicobacter hepaticus. Int. J. Oncol. 17:811-818. [DOI] [PubMed] [Google Scholar]
- 10.Kullberg, M. C., J. M. Ward, P. L. Gorelick, P. Caspar, S. Hieny, A. Cheever, D. Jankovic, and A. Sher. 1998. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect. Immun. 66:5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kullberg, M. C., A. G. Rothfuchs, D. Jankovic, P. Caspar, T. A. Wynn, P. L. Gorelick, A. W. Cheever, and A. Sher. 2001. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect. Immun. 69:4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kullberg, M. C., D. Jankovic, P. L. Gorelick, P. Caspar, J. J. Letterio, A. W. Cheever, and A. Sher. 2002. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. J. Exp. Med. 196:505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livingston, R. S., L. K. Riley, E. K. Steffen, C. L. Besch-Williford, R. R. Hook, Jr., and C. L. Franklin. 1997. Serodiagnosis of Helicobacter hepaticus infection in mice by an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 35:1236-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Occhialini, A., A. Marais, M. Urdaci, R. Serra, N. Munoz, A. Covacci, and F. Mégraud. 2001. Composition and gene expression of the cag pathogenicity island in Helicobacter pylori strains isolated from gastric carcinoma and gastritis patients in Costa Rica. Infect. Immun. 69:1902-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramljak, D., A. B. Jones, B. A. Diwan, A. O. Perantoni, J. F. Hochadel, and L. M. Anderson. 1998. Epidermal growth factor and transforming growth factor-alpha-associated overexpression of cyclin D1, Cdk4, and c-Myc during hepatocarcinogenesis in Helicobacter hepaticus-infected A/JCr mice. Cancer Res. 58:3590-3597. [PubMed] [Google Scholar]
- 16.Shames, B., J. G. Fox, F. Dewhirst, L. Yan, Z. Shen, and N. S. Taylor. 1995. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J. Clin. Microbiol. 33:2968-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen, Z., D. B. Schauer, H. L. Mobley, and J. G. Fox. 1998. Development of a PCR-restriction fragment length polymorphism assay using the nucleotide sequence of the Helicobacter hepaticus urease structural genes ureAB. J. Clin. Microbiol. 36:2447-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor, N. S., J. G. Fox, and L. Yan. 1995. In-vitro hepatotoxic factor in Helicobacter hepaticus, H. pylori and other Helicobacter species. J. Med. Microbiol. 42:48-52. [DOI] [PubMed] [Google Scholar]
- 19.Ward, J. M., J. G. Fox, M. R. Anver, D. C. Haines, C. V. George, M. J. Collins, Jr., P. L. Gorelick, K. Nagashima, M. A. Gonda, R. V. Gilden, J. G. Tully, R. J. Russel, R. E. Benveniste, B. J. Paster, F. E. Dewhirst, J. C. Donovan, L. M. Anderson, and J. M. Rice. 1994. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J. Natl. Cancer Inst. 86:1222-1227. [DOI] [PubMed] [Google Scholar]
- 20.Ward, J. M., M. R. Anver, D. C. Haines, and R. E. Benveniste. 1994. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am. J. Pathol. 145:959-968. [PMC free article] [PubMed] [Google Scholar]