Abstract
l-Arginine is the principal physiological precursor of nitric oxide (NO, a key neurotransmitter) that plays a versatile role in the physiology of the gastrointestinal tract. In this study, the efficacy of l-arginine in enhancing intestinal absorption of ardeparin, a low-molecular-weight heparin (LMWH) was investigated in Caco-2 cell monolayers and a rat model. Regional permeability studies using rat intestine were performed using a modified Ussing chamber. Cell viability in the presence of various concentrations of enhancer was determined by MTT assay. Furthermore, the eventual mucosal epithelial damage was histologically evaluated. LMWH formulated with l-arginine was administered orally to mate Sprague-Dawley rats and the absorption of LMWH was determined by measuring plasma anti-factor Xa activity. Higher ardeparin in-vitro permeability (~3 fold) compared with control was observed in the presence of 2% l-arginine. Regional permeability studies indicated predominant absorption in the colon region. Cell viability studies showed no significant cytotoxicity below 0.8% l-arginine. The oral bioavailability of ardeparin formulated with l-arginine (250 mg kg−1) was increased by ~2 fold compared with control. The formulation was well tolerated by the rats and no abnormal histopathological findings were observed in intestinal tissues of rats exposed to l-arginine. These results suggest that l-arginine may be useful in enhancing the intestinal absorption of LMWHs.
Introduction
Low-molecular-weight heparins (LMWHs) in their current clinical application are administered parenterally, as their physicochemical properties limit their absorption from the gastrointestinal tract. Although this route of administration is suitable for the short-term treatment of inpatients, use of heparin in longer-term therapy requires a non-invasive method for delivery. LMWHs exhibit a relatively low oral bioavailability, primarily because of their large molecular size and high water solubility/low permeability characteristics. Their vulnerability to degradation in the gastrointestinal tract also presents an obstacle to their oral absorption. Acceptance of oral heparin as a clinically useful agent for thrombotic disorders is dependent on a formulation approach which provides improved bioavailability and systemic efficacy.
Absorption enhancers have often been adopted to improve the absorption of poorly absorbable drugs, including hydrophilic antibiotics and biotechnology-derived drugs. These absorption enhancers include surfactants, bile salts, chelating agents and fatty acids (Yamamoto et al 1996; Uchiyama et al 1999). However, absorption enhancers often cause damage and irritate the intestinal mucosal membrane (Uchiyama et al 1996; Yamamoto et al 1996). Therefore, there is a need for the development of effective and less toxic absorption enhancers for use in clinical practice.
l-Arginine, the principal physiological precursor of nitric oxide (NO), is a non-essential amino acid. It has become apparent that NO also plays a versatile role in the physiology and pathophysiology of the gastrointestinal tract (Stark & Szurszewski 1992). The goal of this study was to determine whether l-arginine, an NO donor, would enhance the in-vitro permeability and in-vivo absorption of the LMWH ardeparin. Transport of ardeparin across Caco-2 cell monolayers was conducted in the absence and presence of increasing concentrations of l-arginine. The intestinal membrane toxicity of l-arginine has been investigated by means of Caco-2 cell culture and pathophysiological studies in rats. Regional permeability studies were also performed.
Materials and Methods
LMWH, typically ardeparin (68 U mg−1, anti-factor Xa activity), was obtained from Celcus Laboratories Inc. (Cincinnati, OH, USA). l-Arginine was obtained from Sigma Chemicals Co. (St Louis, MO, USA). Caco-2 cells (C2BBel clone), Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, phosphate-buffered saline (PBS) and trypsin-EDTA were obtained from American Tissue Culture Collection (ATCC, Rockville, MD, USA). Human transferrin was purchased from Gibeo SRL (Los Angeles, CA, USA). MTT reagent was purchased from Sigma Chemicals Co. (St Louis, MO, USA). Sodium dodecyl sulfate (SDS) was purchased from Bio-Rad Laboratories (Hercules, CA, USA). Radioactive 14C mannitol and 3H ardeparin were obtained from American Radiolabeled Chemicals Inc. (St Louis, MO, USA).
Caco-2 cell culture
Human colon adenocarcinoma Caco-2 cells (C2BBel clone), were maintained in culture medium (DMEM supplemented with 10% FBS, 100UmL−1 penicillin. 100 μg mL−1 streptomycin and 10 μg mL−1 human transferrin) at 37°C in 5% CO2 and at 90% relative humidity. The medium was changed every other day until the flasks reached 90% confluence, which was determined by microscopy in the case of 96-well plates and transepithelial electrical resistance (TEER) in the case of Transwells. The cells were harvested with trypsin-EDTA, resuspended in culture medium and seeded at a density of 2000 cells/well in flat-bottom 96-well microtitre tissue culture plates and 200 000 cells/well for transwells and allowed to grow in a humidified 37°C incubator (5% CO2). Culture medium was changed every 48 h.
Transport studies across Caco-2 cell monolayers
Human colon adenocarcinoma (Caco-2) cells were seeded at a density of 200 000 cells/well onto collagen-treated polycarbonate Transwell inserts (0.4 μm pore size, 0.33 cm2 area) and allowed to grow at 37°C. Culture medium was changed every 48 h. Cell monolayer integrity was evaluated by monitoring TEER using an EVOM voltohmmeter (World Precision Instruments, Sarasota, FL, USA). TEER values of more than 500 Ωcm2 in representative cell monolayers were indicative of monolayer integrity. Before each experiment, membranes were rinsed twice with warm PBS solution. The inserts were then immersed into transport buffer. After equilibration in the incubator for 30min, measurements of the TEER values of the inserts were performed. For the transport studies, 3H ardeparin or 14C mannitol, with or without enhancer, was added to the apical chamber. The amount of radioactive 3H ardeparin and 14C mannitol used was 0.045 μCi in each chamber. The concentrations of l-arginine used were 0, 0.5, 1 and 2%. Samples were withdrawn from the basolateral chamber at predetermined time intervals. The amount of 3H ardeparin and 14C mannitol transported across the cell monolayer was determined by scintillation counting (Beckman LS 6500 liquid scintillation counter; Beckman instruments Inc., Fullerton, CA, USA). At the end of the experiment. TEER values were measured to establish the effect of enhancer on monolayer integrity.
In-vitro cytotoxicity studies
Caco-2 cells were plated at a density of 4.0 × 103 cells/well in 96-well flat-bottomed microtitre plates and incubated for 48 h. After washing with PBS, the cells were incubated with 200 μL of test sample and controls. DMEM media alone was used as negative control and SDS (0.1%) as positive control. The permeation enhancer l-arginine in DMEM media was incubated with the Caco-2 monolayer in 96-well plates at various concentrations of the enhancer (0.437, 0.875, 1.87, 3.5 and 7%). The cells were exposed to the compounds for 6 and 12 h. After specified periods of incubation (5% CO2, 37°C) with the test compounds, the cell viability was assessed with the colorimetric MTT (3-(4,5-dimethylihiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and the absorbance was measured at 570 nm with a microplate reader (Tecan SpectraFlour Plus, Hayward, CA, USA). This assay is based on the reduction of MTT tetrazolium by the mitochondrial dehydrogenase in viable cells to coloured formazan dye. The cell viability was expressed as the percentage absorbance of test compound relative to positive control.
Gastrointestinal permeability studies
Gastrointestinal permeability of ardeparin and l-arginine was examined in a modified Ussing chamber (surface area 0.7 cm2) using rat intestine for 3h. Male Sprague-Dawley rats (Charles River Laboratories, Charlotte, NC, USA), 250–300 g, were used. The rats were anaesthetized and the gastrointestinal-tract tissues were isolated using a previously reported method (Asada et al 1995). The rats were anaesthetized by an intramuscular injection of an anaesthetic mixture containing xylazine (10 mg kg−1)and ketamine(100 mg kg−1). The duodenal and ileal segments were removed from top and bottom (13 cm on either side) and the residual small intestine was designated as jejunum. In this study, the central part of the jejunum was used. The colon region was removed following the caecum and was used for the permeability experiments as well. The experimental segments were obtained and the underlying muscularis was removed before mounting onto a modified Ussing chamber. PBS was added to the serosal side. The tissues were exposed to ardeparin either alone or in the presence of enhancer to the luminal side. Mixing was performed by means of a magnetic stirrer and by bubbling with 95% O2 and 5% CO2 gas. The solution was maintained at 37°C by means of water-jacketted reservoirs connected to a constant-temperature circulating pump. The concentration of ardeparin and l-arginine used was 2400 IU kg−1 and 250 mg kg−1, respectively. At predetermined time intervals up to 180 min, samples of 100 μL were taken from the serosal side and replaced with an equal volume of fresh transport medium. Ardeparin appearing in the receiver compartment was analysed by colorimetric detection (Teien et al 1976).
The apparent permeability coefficient (Papp) was calculated by equation 1.
| (1) |
dM/dt is the flux across the tissue, A is the surface area of the membrane and Co is the initial drug concentration. The results of experiments, performed at least in triplicate, are presented as mean ± s.d. Transport enhancement ratios were calculated from Papp values according to equation 2 (Thanou et al 2001).
| (2) |
All studies involving the use of rats in this manuscript were approved by the TTUHSC Institutional Animal Care and Use Committee (IACUC) (protocol approval No. 03013-05) and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
In-vivo studies in rats
Male Sprague-Dawley rats (Charles River laboratories, Charlotte, NC, USA), 250–350g, were used for the in-vivo absorption experiments (3–6 rats in each group). The rats were fasted for at least 12 h before the experiment, with free access to water. The rats were anaesthetized by an intramuscular injection of an anaesthetic mixture containing xylazine (10 mg kg−1) and ketamine (100 mg kg−1) to obtain the control blood sample from the tail vein at 0 time point. Anaesthesia was maintained with additional intramuscular injections of anaesthetic solution as needed throughout the experiments. The rats then received one of the following treatments: oral ardeparin (1200 IU kg−1) in 400 μL of NaHCO3 solution (1.5 g/100 cm3, pH 8.2) so as to neutralize the gastric acidity; oral ardeparin (1200 IU kg−1) plus l-argininc (250 mg kg−1) in 400 μL of NaHCO3 solution; intravenous ardeparin; and subcutaneous ardeparin. Oral administration was carried out by placing the feeding tube deeply into the throat to initiate the swallow reflex. The gavage tube was made of stainless steel with a blunt end so as to avoid causing lesions on the tissue surface. Serial blood samples were collected from the tip of the anaesthetized rat tail at 0, 30, 60, 90, 120, 240, 360 and 480 min in citrated microcentrifuge tubes and plama was harvested by centrifugation (1600g for 5min) and stored at −20°C for further analysis. Ardeparin absorption was determined by measuring plasma anti-factor Xa levels using a colorimetric assay kit (Teien et al 1976) (Chromogenix Coatest Heparin Kit; Diapharma Group Inc., West Chester, OH, USA)
Data analysis
Pharmacokinetic parameters of different formulations were compared by analysis of variance. P < 0.05 was considered statistically significant. Standard non-compartmental analysis (Kinetica, Version 4.0; Innaphase Corp., Philadelphia, PA) was performed for ardeparin absorption profiles. The area under the plasma concentration versus time curve (AUCo.4–480) was determined by the linear trapezoidal rule. Absolute and relative (compared with s.c.) bioavailabilities (Fabsolute and Frelative) were estimated by comparing AUC0–480 for orally administered ardeparin with that of intravenously and subcutaneously administered ardeparin, respectively.
Histological evaluation of gastrointestinal tissues from rats
Formulations containing 1200 IU kg−1 ardeparin and 250 mg kg−1 of l-arginine were administered to rats by oral gavage as described above. The gastrointestinal tissues before administration of formulation were prepared as control samples. At the end of the in-vivo experiment (8h after administration), the gastric and intestinal tissues were isolated from the rats and fixed in neutral buffered formalin for processing. The tissue specimens were washed with alcohol to remove any tissue water. Specimens were embedded in paraffin and cut into sections with a thickness of approximately 5 microns by a microtome at −20°C. The sections were stained with haematoxylin and eosin (H&E) and examined under an optical microscope (Olympus, Melville, NY, USA).
Results and Discussion
Transport of ardeparin across Caco-2 cell monolayers
The apparent permeability (Papp) of ardeparin across Caco-2 cell monolayers and the enhancement ratio are listed in Table 1. l-Arginine at concentrations of 0.5, 1 and 2% enhanced the permeability of ardeparin. The corresponding Papp with 2% l-arginine was significantly higher (~3 fold) than that of the control. Recently, it was reported that NO donors increased the permeability of water-soluble compounds across Caco-2 cell monolayers (Salzman et al 1995). That study showed that incubation with sodium nitroprusside, an NO donor, resulted in a concentration-dependent increase in transepithelial transport of fluorescein sulfonic acid in Caco-2 cells. Thus, our findings are in agreement with the previous results of absorption-enhancing effects of other NO donors. The Papp values for 14C mannitol in all instances were higher than that of ardeparin (Table 1). Mannitol is a highly hydrophilic compound that does not permeate transcellularly. When tight junctions are disrupted, it shows higher permeability as it leaks through the paracellular route. The ardeparin permeability was significantly different from that of mannitol probably due to the difference in their hydrodynamic radius. The hydrodynamic radius of mannitol is approximately 4.1 Å (Kaskel et al 1987; Knipp et al 1997), while that of ardeparin as determined by size exclusion chromatography using a triple detector is 19 Å (Motlekar et al 2005). Tight junctions are generally reported to be impermeable to molecules with radii larger than 11–15 Å (Artursson 1990). It is speculated that l-arginine increases the permeability of ardeparin by modulating the paracellular permeability. It has been reported that the absorption-enhancing mechanism of l-arginine may be based on the intestinal epithelial actions of the chemical mediator NO. It has been reported (Unno et al 1997) that co-incubation of Caco-2 monolayers with several free-radical scavengers and peroxynitrous acid scavengers ameliorated the hyperpermeability induced by a NO donor. NO is capable of modulating the function of tight junctions between adjacent enterocytes. Results from earlier studies have suggested that NO gas or NO donors dilate the paracellular channel at the level of the zonula occludens as observed by electron microscopy (Salzman et al 1995). The mechanism responsible for the widening of the tight junctions may involve a loss of junctional F-actin as observed using confocal microscopy (Salzman et al 1995).
Table 1.
Effect of l-arginine on 3H ardeparin and 14C mannitol fluxes across Caco-2 cell monolayer
|
Papp(× 10−7cms−1 |
|||
|---|---|---|---|
| l-Arginine (%) | 3H Ardeparin | 14C Mannitol | Enhancement ratio(ER) |
| 0 | 2.83±0.62 | 7.50±3.10 | 1.0 |
| 0.5 | 6.00±0.93 | 9.78±1.70 | 2.1 |
| 1 | 7.03±0.48 | 16.95±1.00 | 2.5 |
| 2 | 8.49±1.21 | 21.68±3.18 | 3.0 |
P < 0.05, compared with control.
Although a detailed explanation of the increase in LMWH uptake and the mechanism of action of l-arginine remain to be elucidated, it is believed that subtle changes in the cell membrane caused by cytoskeletal changes result in enhanced permeability to ardeparin. Hence, l-arginine may be used as a permeation enhancer to increase the oral absorption of ardeparin.
Cytotoxicity of l-arginine in Caco-2 cell monolayers
NO has been noted to impair cell proliferation, DNA synthesis and protein synthesis (Garg & Hassid 1993). Indeed, as a result of ATP depletion or other effects, exposure to NO leads to cytotoxicity in some cell types (Hibbs et al 1988). Accordingly, we considered the possibility that NO-induced hyperpermeability in Caco-2 monolayers might merely be a manifestation of cytotoxicity rather than an alteration in the behaviour of tight junctions. We investigated the effect of various concentrations of l-arginine on cell viability. Mitochondrial dehydrogenase (MDH) activity, in the presence of 0.437, 0.875, 1.75 and 3.5% l-arginine for 6h, was similar to that of the negative control (no enhancer) during the same period. However, 7% l-arginine significantly decreased MDH activity at 6h, while 7, 3.5 and 1.75% decreased it after 12h exposure (Figure 1). Recently, it has been reported that NO donors increase the permeability of compounds across Caco-2 cell monolayers with neither loss of cell viability nor lactate dehydrogenase (LDH) release (Salzman et al 1995). Also demonstrated was the low cytotoxicity of other NO donors S and NO gas as evaluated by the cell detachment and LDH release studies in Caco-2 cells. Additionally, confocal and ultrastructural microscopy following exposure to NO donors indicated no cellular injury (Salzman et al 1995). This suggests that the hyperpermeability that resulted from exposure to l-arginine was not due to loss of cellular viability.
Figure 1.
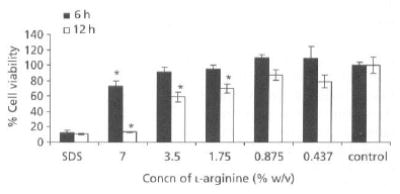
Caco-2 cell viability after exposure to various concentrations of l-arginine for 6 and 12 h. The values are means of three independent experiments. *P < 0.05, compared with control.
Although l-arginine was found to be cytotoxic to the Caco-2 system at 7%, no perceptible evidence of mucosal irritation or damage was obtained when the oral formulation containing 250 mg kg−1 (~15%) of l-arginine was delivered to the rat in-vivo as shown by histological studies. This discrepancy could be partly explained by assuming that the enhancer is diluted in-vivo to concentrations tolerable to the intestinal mucosa. In addition, the intact tissue produces a protective mucous layer not found in Caco-2 monolayers. The in-vivo intestinal tissue also possesses mechanisms allowing recovery from trauma over time, which may not be present in cell cultures.
Regional permeability studies using rat gastrointestinal section
In this study, the interest was to identify the region of maximum permeability for ardeparin in the gastrointestinal tract. The in-vitro permeability coefficient of ardeparin in the absence or presence of l-arginine (250 mg) in the rat gastrointestinal tract (stomach, duodenum, jejunum, ileum and colon) was determined (Figure 2). The results of permeability experiments through rat isolated gastrointestinal tissues suggested that there is a regional difference in the permeation of ardeparin with l-arginine and hence its absorption. It is clear that in the presence of l-arginine, the permeation of ardeparin through the colon and jejunum was higher than that through the other regions. However, the permeation was relatively higher in the colon than in the jejunum. This observation was consistent with the results obtained from a study by Yamamoto et al (2001); using a similar technique with other NO donor compounds, these authors found that the intestinal absorption of carboxyfluorescein was higher in the colon than in the jejunum. NO donor compounds have been shown to stimulate electrolyte transport in guinea-pig intestine in-vitro (MacNaughton 1993). The discrepancy between the Papp values obtained for rat isolated gastrointestinal tract and Caco-2 cell monolayers may be attributed to the species and morphological differences between the two models. Overall, in-vitro studies indicate that absorption of ardeparin in the gastrointestinal tract is site-dependent and that the colon may be a preferred site for the oral delivery of ardeparin with l-arginine. This argument is supported by another study in which the investigators demonstrated that the rectal absorption of insulin was remarkably enhanced in the presence of the NO donor S-nitroso-N-acetyl-penicillamine (Utoguchi et al 1998).
Figure 2.
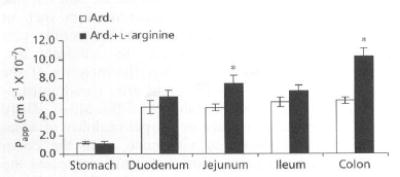
Regional permeability of ardeparin (Ard. 2400 IU kg−1), with or without l-arginine, across rat gastrointestinal membrane. Data are shown us the mean concentration, and error bars represent the s.d., n = 3. *P < 0.05, compared with control.
Effect of l-arginine on the intestinal absorption of ardeparin in rats
Since LMWH levels in plasma cannot be measured directly, anti-factor Xa was used as a surrogate marker for estimating plasma levels of LMWH (Hirsh et al 1998). The anti-factor Xa activity of ardeparin in rat plasma is shown in Figure 3. It has been reported that the plasma anti-factor Xa activity required for obtaining 50% of anti-thrombotic effect is 0.12 IU mL−1 (Bianchini et al 1995). In male Sprague-Dawley rats, a plasma anti-factor Xa level of 0.2 IU mL−1 or higher results in anti-thrombotic effects (Bianchini et al 1995). The oral administration of LMWH alone (1200 IU kg−1) did not significantly affect the anti-factor Xa level in rat plasma and failed to attain therapeutic levels. However, administration of l-arginine (250 mg kg−1) with ardeparin (1200 IU kg−1) produced a significant increase in plasma anti-factor Xa levels within 90 min to 0.26 IU mL−1 (~2 fold compared with the control, 0.13 IU mL−1), indicating that the intestinal absorption of ardeparin was enhanced by treatment with l-arginine at the above dose. The area under anti-factor Xa activity in rat plasma-versus-time curve from 0 to 8 h (AUC 0–8h) in the presence of 250 mg kg−1 of l-arginine was 89.36 IU min mL−1. The oral administration of ardeparin (1200 mg kg−1) with either lower (125 mg kg−1) or higher concentration of l-arginine (500 mg kg−1) did not increase the oral bioavailability (data not shown). The Tmax of the formulation was shorter in the presence of enhancer at a dose of 250 mg kg−1, while the Cmax was significantly higher (Table 2). Many absorption enhancers stimulate drug absorption immediately. The l-arginine-containing formulation had a relatively longer Tmax compared with that of sodium caprate, which we previously tested (Tmax = 30 min; data not shown). Several enhancers mediate their effects via induction of cellular injury. However, NO dilates tight junctions and increases permeability without cellular injury (Salzman et al 1995).
Figure 3.
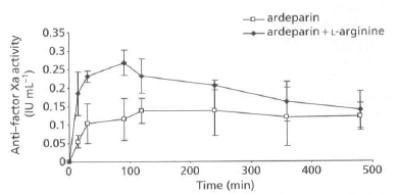
Anti-factor Xa activity-time profiles of ardeparin in rats after oral administration of formulations. Data are shown as the mean concentration, and error bars represent the s.d., n = 4–6.
Table 2.
Pharmacokinetic parameters following oral administration of ardeparin in rats
| Formulation (route) | Cmax(IU mL−1) | Tmax(min) | AUC0–8h(IU min mL−1) | Fabs(%) | Frel(%) |
|---|---|---|---|---|---|
| Ardeparin alone (oral) | 0.13 ± 0.01 | 232 ± 55 | 58.5 ± 9.8 | 2.6 ± 0.4 | 8.6 ± 1.4 |
| Ardeparin + l-arginine (oral) | 0.26 ± 0.01* | 90 ± 16* | 89.3 ± 7.6* | 4.1 ± 0.4* | 13.7 ± 1.3* |
| Ardeparin (i.v.) | 0.68 ± 0.02 | 0 | 750.3 ± 8.5 | 100 | N/A |
| Ardeparin (s.c.) | 0.61 ± 0.03 | 120 ± 15 | 679.9 ± 32.7 | 90.6 | 100 |
P < 0.05, compared with ardeparin alone. N/A, not applicable.
The relatively longer Tmax observed with l-arginine may be related to the absorption enhancement mechanism that depends on the reaction kinetic of a series of biochemical process. However, the results indicate that the addition of l-arginine can induce a positive increment in the rate of absorption of LMWH at relatively lower (250 mg kg−1) but not at higher doses (500 mg kg−1). These results are consistent with those reported in an earlier study (Wapnir et al 1997), which indicate that NO acts in a concentration-dependent manner - at low levels as a vasodilator and stimulator of absorption, and at higher levels as a vasoconstrictor and inhibitor of absorption (Wapnir et al 1997).
The increase in anti-factor Xa levels may be explained by the ability of l-arginine to affect transport mechanisms in the intestinal epithelium. As discussed earlier, the primary mechanism responsible for this permeation-enhancing effect seems to be based on the opening process of the tight junctions mediated by NO (Salzman et al 1995), although active transport and ion-pair formation are two other possible mechanisms.
Histological evaluation of gastrointestinal tissues
A major concern regarding intestinal absorption enhancers is their potential to cause epithelial damage. To address this concern, the effect of l-arginine on gastrointestinal tissues was examined using H&E staining. For the formulation used in these studies, the dose of l-arginine and ardeparin used was 250 mg kg−1 and 1200 IU kg−1, respectively. As shown in Figure 4, the morphology of the gastrointestinal tissues, including villi fusion, occasional epithelial cell shedding and congestion of mucosal capillary, was not visibly affected by the oral administration of l-arginine. In addition, no inflammatory symptoms were detected in the l-arginine-treated group. The formulation was therefore well tolerated by the rats. These results suggest that the ability of l-arginine to increase ardeparin permeability is not a direct result of its tissue toxicity. In addition, due to the short half-life of NO gas in solution (3–5 s) and the relatively large accessible surface area (225 Å) and hydrophilicity (Lehrman 1990) not favourable for absorption, l-arginine may act mainly at the site of administration in the mucosa, leading to fewer side effects occurring elsewhere in the body.
Figure 4.
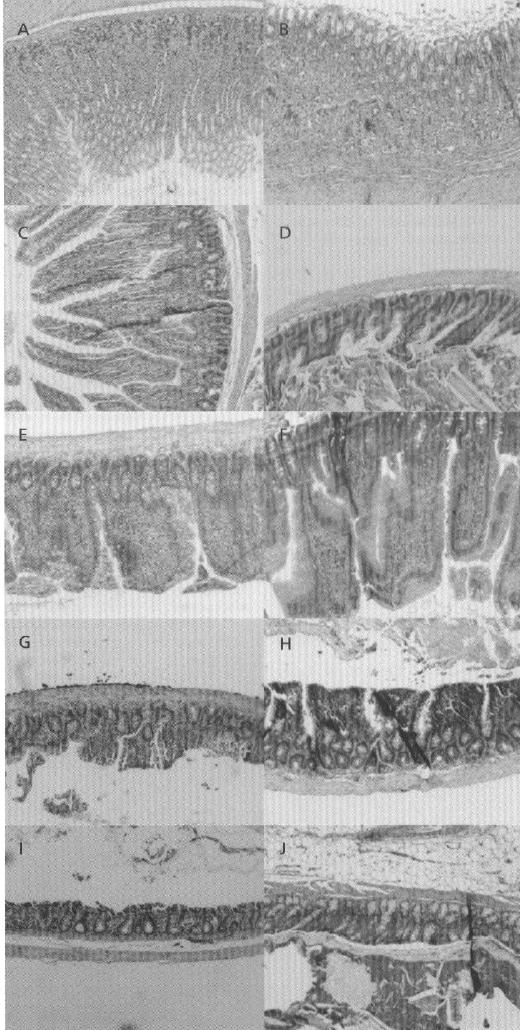
H&E photomicrographs of rat gastric and intestinal tissue sections after oral administration of l-arginine (250 mg kg−1 and LMWH 1200 IU kg−1). All panels represent cross-sections of gastric and intestinal tissues. The original magnification was 100× for all panels. A, B. Stomach (control and test, respectively). C, D. Duodenum (control and test, respectively). E, F. Jejunum (control and test, respectively), G, H. Ileum (control and test, respectively). I, J. Colon (control and test, respectively).
Several studies support the safe and protective effect of l-arginine in the gastrointestinal tract. Also, l-arginine has been used extensively in dietary and pharmacological products. The toxicological and behavioural effects of arginine have been evaluated in rats in an earlier report in the form of a 13-week oral toxicity study. The study estimated the no-observed-adverse-effect level for this amino acid at 5.0% (w/w) in rats (Tsubuku et al 2004). These observations, in addition to ours, suggest that the naturally occurring amino acid l-arginine is non-toxic at the dose tested in the rat model. Therefore, it is a promising absorption promoter for LMWHs if future studies were conclusive in other animal species and man.
Conclusions
This study suggests that the intestinal absorption of ardeparin, a low-molecular-weight heparin, was enhanced by l-arginine at a dose of 250 mg kg−1. It is important to point out that the dose of l-arginine used in the rat model in this study may be too high for human acceptability. Hence, further means to elicit a better response at lower doses, and acute and chronic toxicological studies involving other markers such as LDH, should be investigated before the results presented here are extrapolated to clinical use. Histological studies indicated that the integrity of the intestinal epithelium was preserved after co-administration of LMWH with l-arginine at this dose. There exists a regional difference in the permeability of ardeparin in the presence of l-arginine, with the colon showing maximal permeation. l-Arginine increased the transport of ardeparin across Caco-2 cell monolayers without severe cytotoxicity at relatively lower concentrations. The use of l-arginine as an absorption promoter may be useful to improve the oral bioavailability of LMWHs and other poorly absorbable macromolecules.
Acknowledgments
This study was supported by grants from Texas Tech University Health Sciences Center School of Pharmacy and National Institutes of Health (GM 069397-01A2). The authors are also grateful to Dr Thomas Abbruscato for kindly providing assistance with Ussing chamber experiments and Dr Surendra Gupta (American Radiolabeled Chemicals Inc.) for his generous contribution of radiolabelled ardeparin.
References
- Artursson P. Epithelial transport of drugs in cell culture. I: a model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J Pharm Sci. 1990;79:476–482. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- Asada H, Douen T, Waki M, Adachi S, Fujita T, Yamamoto A, Muranishi S. Absorption characteristics of chemically modified-insulin derivatives with various fatty acids in the small and large intestine. J Pharm Sci. 1995;84:682–687. doi: 10.1002/jps.2600840604. [DOI] [PubMed] [Google Scholar]
- Bianchini P, Bergonzini GL, Parma B, Osima B. Relationship between plasma antifactor Xa activity and the antithrombotic activity of heparins of different molecular mass. Haemostasis. 1995;25:288–298. doi: 10.1159/000217175. [DOI] [PubMed] [Google Scholar]
- Garg UC, Hassid A. Mechanisms of nitrosothiol-induced antimitogenesis in aortic smooth muscle cells. Eur J Pharmacol. 1993;237:243–249. doi: 10.1016/0014-2999(93)90275-m. [DOI] [PubMed] [Google Scholar]
- Hibbs JB, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hirsh J, Warkentin TE, Raschke R, Granger C, Ohman EM, Dalen JE. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1998;114:489S–510S. doi: 10.1378/chest.114.5_supplement.489s. [DOI] [PubMed] [Google Scholar]
- Kaskel FJ, Kumar AM, Lockhart EA, Evan A, Spitzer A. Factors affecting proximal tubular reabsorption during development. Am J Physiol. 1987;252:F188–F197. doi: 10.1152/ajprenal.1987.252.1.F188. [DOI] [PubMed] [Google Scholar]
- Knipp GT, Ho NF, Barsuhn CL, Borchardt RT. Paracellular diffusion in Caco-2 cell monolayers: effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci. 1997;86:1105–1110. doi: 10.1021/js9700309. [DOI] [PubMed] [Google Scholar]
- Lehrman S. Fundamentals of protein biotechnology. Marcel Dekker Inc; New York: 1990. [Google Scholar]
- MacNaughton WK. Nitric oxide-donating compounds stimulate electrolyte transport in the guinea pig intestine in vitro. Life Sci. 1993;53:585–593. doi: 10.1016/0024-3205(93)90716-g. [DOI] [PubMed] [Google Scholar]
- Motlekar NA, Srivenugopal KS, Wachtel MS, Youan BB. Oral delivery of low-molecular-weight heparin using sodium caprate as absorption enhancer reaches therapeutic levels. J Drug Targeting. 2005;13:573–583. doi: 10.1080/10611860500471906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Salzman AL, Menconi MJ, Unno N, Ezzell RM, Casey DM, Gonzalez PK, Fink MP. Nitric oxide dilates tight junctions and depletes ATP in cultured Caco-2BBe intestinal epithelial monolayers. Am J Physiol. 1995;268:G361–G373. doi: 10.1152/ajpgi.1995.268.2.G361. [DOI] [PubMed] [Google Scholar]
- Stark ME, Szurszewski JH. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992;103:1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- Teien AN, Lie M, Abildgaard U. Assay of heparin in plasma using a chromogenic substrate for activated factor X. Thromb Res. 1976;8:413–4l6. doi: 10.1016/0049-3848(76)90034-7. [DOI] [PubMed] [Google Scholar]
- Thanou M, Verhoef JC, Nihot MT, Verheijden JH, Junginger HE. Enhancement of the intestinal absorption of low molecular weight heparin (LMWH) in rats and pigs using Carbopol 934P. Pharm. Res. 2001;18:1638–1641. doi: 10.1023/a:1013055120007. [DOI] [PubMed] [Google Scholar]
- Tsubuku S, Hatayama K, Mawatari K, Smriga M, Kimura T. Thirteen-week oral toxicity study of L-arginine in rats. Int J Toxicol. 2004;23:101–105. doi: 10.1080/10915810490435622. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Yamamoto A, Hatano H, Fujita T, Muranishi S. Effectiveness and toxicity screening of various absorption enhancers in the large intestine: intestinal absorption of phenol red and protein and phospholipid release from the intestinal membrane. Biol Pharm Bull. 1996;19:1618–1621. doi: 10.1248/bpb.19.1618. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Sugiyama T, Quan YS, Kotani A, Okada N, Fujita T, Muranishi S, Yamamolo A. Enhanced permeability of insulin across the rat intestinal membrane by various absorption enhancers: their intestinal mucosal toxicity and absorption-enhancing mechanism of n-lauryl-beta-D-maltopyranoside. J Pharm Pharmacol. 1999;51:1241–1250. doi: 10.1211/0022357991776976. [DOI] [PubMed] [Google Scholar]
- Unno N, Menconi MJ, Fink MP. Nitric oxide-induced hyperpermeability of human intestinal epithelial monolayers is augmented by inhibition of the amiloride-sensitive Na(+)-H+ antiport: potential role of peroxynitrons acid. Surgery. 1997;122:485–491. 491–482. doi: 10.1016/s0039-6060(97)90042-8. [DOI] [PubMed] [Google Scholar]
- Utoguchi N, Watanabe Y, Shida T, Matsumoto M. Nitric oxide donors enhance rectal absorption of macromolecules in rabbits. Pharm Res. 1998;15:870–876. doi: 10.1023/a:1011920530771. [DOI] [PubMed] [Google Scholar]
- Wapnir RA, Wingertzahn MA, Teichberg S. L-arginine in low concentration improves rat intestinal water and sodium absorption from oral rehydration solutions. Gut. 1997;40:602–607. doi: 10.1136/gut.40.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Uchiyama T, Nishikawa R, Fujita T, Muranishi S. Effectiveness and toxicity screening of various absorption enhancers in the rat small intestine: effects of absorption enhancers on the intestinal absorption of phenol red and the release of protein and phospholipids from the intestinal membrane. J Pharm Pharmacol. 1996;48:1285–1289. doi: 10.1111/j.2042-7158.1996.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Tatsumi H, Maruyama M, Uchiyama T, Okada N, Fujita T. Modulation of intestinal permeability by nitric oxide donors: implications in intestinal delivery of poorly absorbable drugs. J Pharmacol Exp Ther. 2001;296:84–90. [PubMed] [Google Scholar]


