Abstract
Prematurity is more prevalent in African Americans than in European Americans. We investigated the contribution of a functional SNP in the promoter of the SERPINH1 gene, enriched among those of African ancestry, to preterm premature rupture of membranes (PPROM), the leading identifiable cause of preterm birth. SERPINH1 encodes heat-shock protein 47, a chaperone essential for collagen synthesis. The SERPINH1 −656 minor T allele had a greater frequency in African populations and African Americans than in European Americans (12.4% vs. 4.1%). The −656 T allele displayed significantly reduced promoter activity compared to the major −656 C allele in amnion fibroblasts, which lay down the fibrillar collagen that gives tensile strength to the amnion. An initial case-control study demonstrated that the −656 T allele is significantly more frequent in African-American neonates (P < 0.0009) born from pregnancies complicated by PPROM compared with controls (odds ratio of 3.22, 95% confidence interval 1.50, 7.22). There was no significant difference in ancestry among cases and controls using a dihybrid model based on 29 ancestry-informative markers. Adjusting the results of the case-control study for admixture still yielded a statistically significant association between the −656 T allele and PPROM (P < 0.002). A follow-up case-control study gave similar results. The combined case-control findings showed a highly significant (P < 0.0000045) association between the −656 T allele and PPROM. The SERPINH1 −656 T allele is the first example of an ancestry-informative marker associated with preterm birth in African Americans.
Keywords: Hsp47
Prematurity is a major problem in the United States, costing the health care system >14 billion dollars each year for care of premature infants. Long-term disabilities associated with prematurity add additional costs. There are significant racial/ethnic disparities in the incidence of prematurity, with African-American women experiencing a disproportionate number of preterm births (2- to 3-fold more) than European-American women (1, 2). This disparity cannot be explained by socioeconomic status or access to health care (3). Several factors are thought to contribute to premature birth including infection, decidual bleeding/hemorrhage, stress, lifestyle (e.g., smoking and illicit drug use), and genetics. There is emerging evidence suggesting that interactions among these factors (i.e., gene–environment interactions) can increase the likelihood of a preterm birth (4, 5). However, no known genetic variation can account for the greater risk of preterm birth in African Americans.
One approach to the elucidation of the ethnic disparity in risk for prematurity is to identify genetic variants with different allele frequencies among populations that are associated or linked to this outcome. Knowledge of such a genotype could be used to identify subjects who might benefit from therapeutic interventions. This knowledge would allow preventative measures (e.g., lifestyle change or medical therapy) to be instituted before the onset of preterm labor. It could also facilitate clinical trials of prevention therapies.
Preterm births cluster in families, and the strongest predictor of a preterm birth is a prior preterm delivery (6, 7). The notion that genetic factors, particularly genes involved in collagen metabolism, play a role in preterm birth is supported by the frequent occurrence of preterm birth after preterm premature rupture of membranes (PPROM) when the fetus is affected with Ehlers–Danlos syndrome (8). Candidate gene studies revealed that polymorphisms that increase the promoter activity of genes encoding matrix metalloproteinases, enzymes that break down collagen, are associated with risk of PPROM, the leading identifiable cause of preterm delivery (9–11). Although these studies support the idea that genetic variation contributes to preterm delivery, none of the known variants can account for why African Americans are at greater risk.
Heat-shock protein 47 (Hsp47), encoded by the SERPINH1 gene, is a heat-shock protein localized to the endoplasmic reticulum that serves as a chaperone stabilizing the collagen triple helix. Hsp47 appears to be essential for collagen synthesis, and mutant mice lacking Hsp47 die before birth with ruptured blood vessels and a marked reduction in type I collagen. There is a direct relationship between Hsp47 expression and collagen production; type I procollagen increases in proportion to the increase in Hsp47 levels (12). Thus, Hsp47 could be a factor that influences the amount of fibrillar collagen laid down in the amnion, which gives the fetal membranes tensile strength. Notably, PPROM fetal membranes have reduced collagen content, which could be the result of reduced synthesis or increased catabolism (13, 14).
Rocnik et al. (12) identified a functional SNP in the promoter of the SERPINH1 gene (−656 C/T), and they reported that the T allele is highly enriched in African ancestry. Here we evaluated the potential contribution of this SNP to risk of PPROM in an African-American population.
Results
In a study of ethnic/racial distribution, we found that the SERPINH1−656 T allele was most frequent in African populations, particularly those living in Sierra Leone (Table 1). Moreover, the −656 T allele was found in greater frequency in African Americans (n = 323) than in European Americans (n = 148) (12.4% vs. 4.1%, P < 0.024). These observations confirm the higher T allele frequency among those with African ancestry.
Table 1.
Ethnic distribution of the −656 minor T allele in the SERPINH1 gene
| Ethnic group (country) | No. of tested samples | T allele frequency |
|---|---|---|
| Caucasian (Western European) | 148 | 0.041 |
| Bolivian | 92 | 0.046 |
| Mayan (Guatemala) | 40 | 0.075 |
| Mexican | 144 | 0.056 |
| South Asian | 140 | 0.046 |
| Chinese | 43 | 0.083 |
| Nigerian | 76 | 0.11 |
| Creole (Sierra Leone) | 37 | 0.24 |
| Fula (Sierra Leone) | 7 | 0.21 |
| Limba (Sierra Leone) | 23 | 0.24 |
| Loko (Sierra Leone) | 9 | 0.17 |
| Mandigo (Sierra Leone) | 8 | 0.125 |
| Mende (Sierra Leone) | 93 | 0.13 |
| Temne (Sierra Leone) | 59 | 0.25 |
To determine the ancestry of the SERPINH1 alleles, panels of DNA from well characterized populations were genotyped. The analysis indicates that the SERPINH1 −656 T allele is more common in African populations than in South America (Bolivia and Guatemala), Mexico, and Asia (China). Western European populations have a −656 T allele frequency of 0.041.
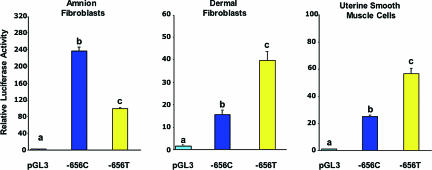
We next performed promoter function studies comparing the activities of the two −656 alleles, which revealed host cell-specific differences between the minor T and major C alleles (Fig. 1). The minor T allele displayed significantly reduced promoter activity in amnion fibroblasts, which lay down the fibrillar collagen of the amnion. In contrast, the activity of the minor T allele promoter was significantly greater than that of the major C allele in the context of dermal fibroblasts and uterine smooth muscle cells. These findings raised the possibility that fetuses that inherit the SERPINH1 −656 T allele might be at greater risk for preterm birth due to PPROM because of a reduced amnion collagen synthesis and, consequently, reduced tensile strength.
Fig. 1.
Effect of the −656 SERPINH1 SNP on promoter activity. SERPINH1 promoter fragments representing the −656 C and T alleles were cloned into the pGL3 basic vector. The relative Photinus luciferase activities ± SE (n = 3 separate experiments performed with triplicate wells for each cell host) standardized to Renilla luciferase were compared by using the Tukey–Kramer test. Values with different letters are significantly different (P < 0.05) from each other within each cell host.
Measurement of nascent SERPINH1 RNA and mRNA from amnion samples with a −656 C/C and −656 C/T genotype by real-time PCR revealed significantly lower quantities of both nascent transcripts, a reflection of transcription rate, and mRNA in the amnion samples with a −656 C/T genotype (Table 2). This result is consistent with the notion that, in vivo, the −656 T allele has reduced promoter activity. This finding was confirmed by using chromatin immunoprecipitation performed on nuclear DNA isolated from cultured amnion fibroblasts heterozygous for the −656 SNP (see Fig. 3, which is published as supporting information on the PNAS web site).
Table 2.
In vivo expression of SERPINH1 in amnion depends on the −656 genotype
| RNA expression | −656 C/C | −656 C/T | P value |
|---|---|---|---|
| Nascent transcripts | 3.42 ± 0.70 (6) | 0.56 ± 0.16 (4) | <0.02 |
| mRNA | 31.91 ± 0.47 (6) | 7.41 ± 1.33 (4) | <0.01 |
Quantitative PCR analysis of nascent and mature SERPINH1 RNAs in human amnion tissue. Values are means ± SE. Numbers in parentheses indicate the number of samples analyzed.
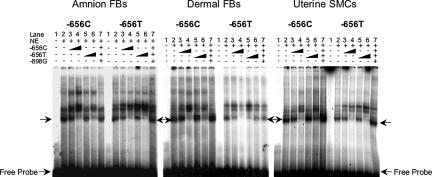
EMSAs revealed a specific complex formed with nuclear extracts from the three different cell types, with oligonucleotides representing the −656 C and T alleles (Fig. 2). The minor T allele oligonucleotide probe had lower affinity for the nuclear proteins in dermal fibroblasts and uterine smooth muscle cells, but equivalent affinity to the major C allele oligonucleotide in amnion fibroblasts. Despite the significant differences in promoter activities among the three cell types, there were no clear differences in the gel shift patterns. This finding suggests that either posttranslational modifications or association of transcriptional coactivators or corepressors with the nuclear factor binding to the −656 region explains the differential activities of the two promoter alleles in different cell types.
Fig. 2.
The −656 region specifically binds a nuclear protein. EMSA studies were performed with nuclear extracts prepared from amnion fibroblasts (FBs) (Left), dermal fibroblasts (Center), and uterine smooth muscle cells (SMCs) (Right) and 32P-labeled double-strand oligonucleotides representing the −656 C and T alleles. Specific binding was established by addition of increasing amounts of unlabeled double-strand oligonucleotide representing the two SERPINH1 alleles or an irrelevant nucleotide sequence. Arrowheads indicate specific complexes and free probe.
To determine whether the SERPINH1 −656 minor T allele contributes to risk of PPROM, we performed an initial case-control study in African Americans. The study focused on the genotype of the offspring based on the hypothesis that the genotype of the extraembryonic tissues (fetal membranes) represents the primary determinant of risk of PPROM. Analysis of the demographic characteristics of the controls (n = 174), neonates born at term from normal pregnancies, and the cases (n = 152), neonates from pregnancies complicated by PPROM, revealed no significant differences in maternal age, gravidity, and parity. However, the length of gestation (controls, 39.1 ± 1.3 weeks; cases, 31.9 ± 2.8 weeks; P < 0.0001) and birth weight (controls, 3,309 ± 483 g; cases, 1,937 ± 502 g; P < 0.0001) were significantly lower in the PPROM group, as expected.
The minor SERPINH1−656 T allele was found significantly more frequently in African Americans (11.51% allele frequency; P < 0.0009) in pregnancies complicated by PPROM compared with controls (4.05%) with an odds ratio of 3.22 [95% confidence interval (CI) 1.50, 7.22]. There was a statistically significant allele dose effect for the −656 T allele in a test for trend (P < 0.002) with respect to risk of PPROM. Heterozygotes for the T allele had an odds ratio of 2.68 (95% CI 1.45, 4.95) for PPROM compared with homozygotes for the common C allele, and homozygotes for the T allele had yet an additionally greater risk with an odds ratio of 2.68 compared with heterozygotes for the T allele.
The urban African-American population from which our subjects were drawn is heterogeneous, and this heterogeneity could have confounded the findings of the association study as a result of population stratification. Consequently, we performed analyses using 29 ancestry-informative markers to determine whether population stratification could have affected our findings. There was no significant difference in ancestry among cases and controls using a dihybrid model (percentage African ancestry: cases, 0.840 ± 0.135; controls, 0.831 ± 0.144; P = 0.571). Moreover, using logistic regression analysis we adjusted the results of the case-control study for admixture and still observed a statistically significant association between the minor −656 T allele and PPROM (P < 0.002 and an odds ratio of 3.14, 95% CI 1.53, 4.47). The population-attributable risk for the unadjusted data was 12% (95% CI 3.6%, 19.9%) and 12.3% (95% CI 5.2%, 20.6%) for the admixture-adjusted data.
A second case-control study was conducted on a different sample of 184 controls and 92 cases. This result demonstrated again that there was a significant association of PPROM with neonates carrying the −656 T allele (−656 T allele frequency: cases, 11.41%; controls, 5.16%; P < 0.0076; odds ratio 2.37; 95% CI 1.17, 4.79). Combining the two case-control studies (cases = 244; controls = 358) we obtained a highly significant association between the −656 T allele and PPROM (−656 T allele frequency: cases, 11.48%; control, 4.47%; P < 0.0000045; odds ratio 2.77; 95% CI 1.73, 4.95).
In previous studies, associations between SNPs in the promoters of the MMP1 (10) and MMP8 (11) genes and PPROM were reported. These genes encoding matrix metalloproteinases are located on chromosome 11q22.2 ≈27 megabases away from the SERPINH1 gene, raising the theoretical possibility that linkage disequilibrium between the MMP alleles and the −656 T allele accounted for our findings. We determined the MMP1 genotypes and MMP8 haplotypes of the samples analyzed in this study SNP and used the Haploview 3.2 program to test for linkage disequilibrium with the −656 SERPINH1 SNP. There was no evidence for linkage disequilibrium between the SERPINH1 SNP and the MMP1 and MMP8 alleles (P > 0.47), although the previously described linkage disequilibrium within the MMP8haplotype (+17C/G and −799C/T) was confirmed (Fig. 4, which is published as supporting information on the PNAS web site).
Discussion
We found that a SNP in the promoter of the SERPINH1 gene, which reduces promoter function in amnion fibroblast cells, is strongly associated with risk of PPROM, the leading identifiable cause of preterm birth. Amnion samples carrying the −656 T allele have reduced amounts of nascent SERPINH1transcripts and mRNA, which would result in reduced Hsp47 protein and consequently reduced interstitial collagen synthesis and a weaker amnion, more prone to rupture. The −656 T allele is enriched in individuals of African ancestry. Our use of a panel of ancestry-informative markers to assess population structure argues against the possibility that population stratification confounded our analysis. Thus, it appears that the SERPINH1 −656 T allele or a gene in linkage disequilibrium with it represents a significant risk factor for preterm birth in African Americans.
The cell host-specific differential activities of the −656 C/T SERPINH1 promoter genotypes is also of interest in that the minor T allele, which displayed lower activity in amnion fibroblasts compared with the major C allele, displayed higher activity in dermal fibroblasts and uterine smooth muscle cells. Because both keloids, a fibrotic response in skin, and uterine fibroid tumors, which have a dense collagen matrix, are more prevalent in African Americans, it is intriguing to speculate that a single SNP could contribute to multiple phenotypes (15, 16).
Methods
Study Populations.
A number of population samples were used for the estimation of the −656 minor T allele frequency. These included European Americans from State College, PA (n = 148); Bolivians from La Paz, Bolivia (n = 92); Mayans from Guatemala (n = 40); Mexicans from Tlapa, Mexico (n = 144); South Asian Indians (n = 140); Han Chinese (n = 43); Benin from Nigeria (n = 76); and seven different ethnic groups from Sierra Leone (Creole, n = 37; Fula, n = 7; Limba, n = 23; Loko, n = 9; Mandingo, n = 8; Mende, n = 93; Temne, n = 59). All of these DNA samples were collected with informed consent for biomedical research.
Subjects in the case-control study were African-American women and their neonates receiving obstetrical care at the Hospital of the University of Pennsylvania or Hutzel Hospital. The study was approved by the respective institutional review boards as well as the Institutional Review Board of the National Institute of Child Health and Human Development, and written informed consent was obtained from mothers before collection of the samples. Control samples (n = 358) were obtained from neonates of singleton pregnancies delivered at term of mothers with no prior history of PPROM or preterm labor. Cases of PPROM (n = 244) were defined as neonates from pregnancies complicated by rupture of membranes before 37 weeks of gestation. The diagnosis of membrane rupture was based on pooling of amniotic fluid in the vagina, amniotic fluid ferning patterns, and a positive nitrazine test. Women with multiple gestations, fetal anomalies, trauma, connective tissue diseases, and medical complications of pregnancy requiring induction of labor were excluded.
Genotyping.
Genomic DNA was extracted from umbilical cords, cord blood, or neonate cheek swabs as previously described (11). The −656 C/T SNP in the SERPINH1 promoter was genotyped by using PCR products generated with forward primer 5′-CCACTGTCGCCCAGATTATTTA-3′ and reverse primer 5′-CAGTGCCCTTCTCCATACTTGT-3′ (12). After initial denaturation at 94°C for 5 min, PCR was performed for 35 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 1 min, followed by a final 10-min extension step at 72°C. The 624-bp product was digested with endonuclease ApaL1, which yielded one fragment for the −656 T allele and two fragments with sizes of 446 and 178 bp for the −656 C allele. The fragments were resolved in 1% agarose gels. MMP1 (10) and MMP8 (11) promoter genotypes were determined as previously reported.
Assessment of Population Structure.
Because many African-American populations have substantial admixture that is not evenly distributed throughout the population, there is a chance that some of the observed association could be the result of admixture stratification. To control for admixture stratification, we typed 29 ancestry-informative markers, which are useful for calculating gene flow between West African and Western European populations (11). These markers were used to calculate the individual biogeographical ancestry levels of the persons in the study in the context of the two primary parental populations (West African and Western European) using parental allele frequencies and the maximum likelihood as previously reported (11). These biogeographical ancestry estimates were then used as conditioning variables in the logistic regression analyses to control for any effects that admixture stratification could have on the phenotype. The unadjusted odds ratio for the SERPINH1 genotype and PPROM association was estimated. Next, adjusted odds ratio estimates were computed by incorporating the admixture estimates into the model. The resulting odds ratio is an average of the SERPINH1 genotype PPROM association over subjects with like genetic profiles.
Analysis of Linkage Disequilibrium.
The Haploview 3.2 program (www.broad.mit.edu/mpg/haploview) was used to test for linkage disequilibrium. We also evaluated the association among SNPs in the controls and found no significant association between the SERPINH1 and MMP8 genotypes (Fisher's exact test, P < 0.47).
Construction of Promoter–Reporter Plasmids.
To determine whether the −656 C/T SNP influences transcription of the SERPINH1 gene, we obtained a 1,176-bp fragment from −1104 to +72 bp of the SERPINH1 promoter, amplified by using forward and reverse primers with the indicated sequences (forward primer, 5′-CCACTGTCGCCCAGATTATTTA-3′; reverse primer, 5′-GTCTCCCGCCCCTCACCT-3′). A mutagenesis kit (Stratagene, La Jolla, CA) was used to create the targeted alleles with a uniform backbone sequence. Promoter fragments were cloned into the pGL3-Basic vector (Promega, Madison, WI), which contains the firefly luciferase gene as a reporter. The DNA sequences of the promoter constructs were confirmed before use, and three different plasmid preparations for each construct were tested.
Cell Culture and Transfection.
Primary cultures of human amnion fibroblasts and human dermal fibroblast cells were cultured in DMEM (10). Human uterine smooth muscle cells were cultured in smooth muscle cell basal medium with growth factors as previously described (17). The media were supplemented with 10% FBS and antibiotics [100 units/ml penicillin G, 100 units/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B (Gibco/BRL, Gaithersburg, MD)]. All cells were maintained at 37°C in a water-saturated atmosphere under 5% CO2 in air.
For transfection, 100 × 105 amnion fibroblasts, 20 × 105 uterine smooth muscle cells, and 40 × 105 dermal fibroblasts were seeded in individual wells of a 12-well culture plate. Cells were transfected by using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN) with 0.5 μg of the pGL3 vector containing a 1,176-bp SERPINH1 promoter fragment coupled to the firefly luciferase reporter gene. In each transfection, 25 ng of pRL-TK (Promega), a control plasmid expressing Renilla reniformis luciferase, was used to correct for transfection efficiency. The medium was changed 24 h after transfection, and cultures were continued for an additional 24 h in serum-free medium before collecting cells for the luciferase assays.
Luciferase Assays.
After 36–48 h culture, the transfected cells were lysed in lysis buffer and 20-μl aliquots of supernatant were then assayed for luciferase activity by using the Dual-Luciferase Reporter Assay System (Promega) as previously described (11). Promoter activities were expressed as the ratio between Photinus luciferase and Renilla luciferase activities.
EMSA.
Nuclear proteins were extracted as described previously (11). The following double-stranded oligonucleotide probes (SNP identified in bold) were constructed: −656 C sense, 5′-TTCCTTCCTGTGCACTCCTCCAAGC-3′; −656 C antisense, 5′-GCTTGGAGGAGTGCACAGGAAGGAA-3′; −656 T sense, 5′-TTCCTTCCTGTGTACTCCTCCAAGC-3′; −656 T antisense, 5′- GCTTGGAGGAGTACACAGGAAGGAA-3′; sense unrelated competitor, 5′-ATGCTGTGAACCTCAGGGTGCTCG-3′; antisense unrelated competitor, 5′-CGAGCACCCTGAGGTTCACAGCAT-3′. The double-stranded synthetic oligonucleotides were labeled with T4 polynucleotide kinase and [γ-32P]ATP. The EMSA binding reaction was mixed in 1× binding buffer (Promega) with 10 μg of nuclear protein and 1 × 105 cpm of 32P-labeled double-stranded oligonucleotide probe (1 ng) with or without unlabeled competitor probe in a total volume of 10 μl. Reaction mixtures were incubated at room temperature for 30 min and then subjected to 8% PAGE at 250 V for 4 h. The dried gels were then exposed to x-ray film.
Quantitation of SERPINH1 Nascent Transcripts and mRNA.
TRIzol Reagent (Invitrogen, Carlsbad, CA) was used for total RNA preparation from the freshly frozen human amnion tissue. RNA was DNase-treated with DNA-free reagent (Promega). Total RNA (1–5 μg) was used in 20–40 μl of reverse transcriptase reaction with random hexamers (Roche Diagnostics) and Moloney murine leukemia virus reverse transcriptase (Promega). RT reaction (1 μl, 1:10 diluted) was added into 20 μl of real-time PCR. The SYBR green dye (Applied Biosystems, Foster City, CA) was used for the amplification of cDNA. Reactions were run in triplicate on a Prism 7000 Real-Time PCR machine (Applied Biosystems). To measure nascent SERPINH1transcript levels, total RNA was reversed transcribed by using 150 nmol of a SERPINH1-specific primer (5-ACGAAATTCGGTCGGAATACA-3) together with 1.5 nmol of a GAPDH-specific primer (5-TAGAGGCAGGGATGATGTTCTGGA-3). Relative fold changes were calculated by using the standard curve or the ΔCt method. Primer sets were designed by using PRIMER EXPRESS software (Applied Biosystems). Primers for mature mRNAs were designed to span the exon–exon junction, and primers for nascent RNA were designed to be in the next exon and intron. The sequences used were as follows: for SERPINH1 mRNA 5-AACGCCATGTTCTTCAAGCCACACT-3 (forward) and 5-TAGTTGTAGAGGCCTGTCCGGTGCAT-3 (reverse); for SERPINH1nascent RNA, 5-GACGGCGCCCTGCT-3 (forward) and 5-AGCATAAATGAGAGGCAGTGAAGA-3 (reverse). The internal control GAPDHprimers were 5-GTATCGTGGAAGGACTCATGACCA-3 and 5-TAGAGGCAGGGATGATGTTCTGGA-3.
Statistics.
Tests of association were conducted using Pearson's χ2 test and Fisher's exact test as needed to account for small sample sizes. Odds ratio estimates and exact binomial confidence intervals were computed by using Stata 8.0 (Stata, College Station, TX). Dose–response modeling was done by using logistic regression. Estimates of population-attributable risk were calculated as described by Bruzzi et al. (18). Significant differences in activities among the different promoter constructs were evaluated using the Tukey–Kramer test with P < 0.05 considered as significant.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Child Health and Human Development Grant HD34612 and grants from the March of Dimes Foundation. This research was supported in part by the Intramural Research Program of the National Institute of Child Health and Human Development, Department of Health and Human Services, and National Institutes of Health.
Abbreviations
- PPROM
preterm premature rupture of membranes
- Hsp47
heat-shock protein 47
- CI
confidence interval
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
See Commentary on page 13267.
References
- 1.Kempe A., Wise P. H., Barkan S. E., Sappenfield W. M., Sachs B., Gortmaker S. L., Sobol A. M., First L. R., Pursley D., Rinehart H., et al. N. Engl. J. Med. 1992;327:969–973. doi: 10.1056/NEJM199210013271401. [DOI] [PubMed] [Google Scholar]
- 2.Ahern J., Pickett K. E., Selvin S., Abrams B. J. Epidemiol. Community Health. 2003;57:606–611. doi: 10.1136/jech.57.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meis P. J., Goldenberg R. L., Mercer B. M., Iams J. D., Moawad A. H., Miodovnik M., Menard M. K., Caritis S. N., Thurnau G. R., Dombrowski M. P., et al. Am. J. Perinatol. 2000;17:41–45. doi: 10.1055/s-2000-7292. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Zuckerman B., Pearson C., Kaufman G., Chen C., Wang G., Niu T., Wise P. H., Bauchner H., Xu X. J. Am. Med. Assoc. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Macones G. A., Parry S., Elkousy M., Clothier B., Ural S. H., Strauss J. F., III Am. J. Obstet. Gynecol. 2004;190:1504–1508. doi: 10.1016/j.ajog.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Winkvist A., Mogren I., Hogberg U. Int. J. Epidemiol. 1998;27:248–254. doi: 10.1093/ije/27.2.248. [DOI] [PubMed] [Google Scholar]
- 7.Carr-Hill R. A., Hall M. H. Br. J. Obstet. Gynaecol. 1985;92:921–928. doi: 10.1111/j.1471-0528.1985.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 8.Parry S., Strauss J. F., III N. Engl. J. Med. 1998;338:663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 9.Ferrand P. E., Parry S., Sammel M., Macones G. A., Kuivaniemi H., Romero R., Strauss J. F., III Mol. Hum. Reprod. 2002;8:494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto T., Parry S., Urbanek M., Sammel M., Macones G., Kuivaniemi H., Romero R., Strauss J. F., III J. Biol. Chem. 2002;277:6296–6302. doi: 10.1074/jbc.M107865200. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Parry S., Macones G., Sammel M. D., Ferrand P. E., Kuivaniemi H., Tromp G., Halder I., Shriver M. D., Romero R., Strauss J. F., III Hum. Mol. Genet. 2004;13:2659–2669. doi: 10.1093/hmg/ddh287. [DOI] [PubMed] [Google Scholar]
- 12.Rocnik E. F., van der Veer E., Cao H., Hegele R. A., Pickering J. G. J. Biol. Chem. 2002;277:38571–38578. doi: 10.1074/jbc.M206689200. [DOI] [PubMed] [Google Scholar]
- 13.Skinner S. J., Campos G. A., Liggins G. C. Obstet. Gynecol. 1981;57:487–489. [PubMed] [Google Scholar]
- 14.Hampson V., Liu D., Billett E., Kirk S. Br. J. Obstet. Gynaecol. 1997;104:1087–1091. doi: 10.1111/j.1471-0528.1997.tb12073.x. [DOI] [PubMed] [Google Scholar]
- 15.Marshall L. M., Spiegelman D., Barbieri R. L., Goldman M. B., Manson J. E., Colditz G. A., Willett W. C., Hunter D. J. Obstet. Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 16.Taylor S. C. Cutis. 2003;71:271–275. [PubMed] [Google Scholar]
- 17.Leite R. S., Brown A. G., Strauss J. F., III FASEB J. 2004;18:1418–1420. doi: 10.1096/fj.04-1684fje. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzi P., Green S. B., Byar D. P., Brinton L. A., Schairer C. Am. J. Epidemiol. 1985;122:904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.