Abstract
Leishmania mexicana mutants deficient in the multicopy CPB gene array have reduced virulence, demonstrated by poor lesion growth in BALB/c mice and induction of a protective Th1 response. Reinsertion of the amastigote-specific CPB2.8 or metacyclic stage-specific CPB2 gene into a CPB-deficient mutant L. mexicana failed to restore either a Th2 response or sustained virulence. However, reexpression of multiple CPB genes from a cosmid significantly restored virulence. This was characterized by increased lesion and parasite growth and the acquisition of a Th2 response, as determined by measuring interleukin-4 production and immunoglobulin G1 (IgG1) and IgE levels. These studies confirm that L. mexicana cysteine proteases are important virulence factors and provide an explanation for the presence in L. mexicana of a multicopy tandem array of CPB genes.
Parasite cysteine proteases have been implicated in several processes including differentiation, nutrition, host cell infection, and evasion of the host immune response (10, 17, 19). Leishmania mexicana possesses three cysteine proteases of the papain family (designated clan CA), namely, the cathepsin L-like CPA (12) and CPB (25) and the cathepsin B-like CPC (4). The CPB genes are multicopy and are located in a single locus of 19 copies arranged in a tandem repeat (13, 14) (Fig. 1a). The first two copies of CPB, CPB1 and CPB2, are expressed in the infective metacyclic stage of the parasite, while the others are expressed predominantly in the intracellular amastigote stage (6, 13, 14). Information about the roles and importance of the enzymes in host-parasite interactions was obtained by the generation of a CPB-deficient (Δcpb) mutant. It was shown that Δcpb promastigotes are less infective to macrophages than wild-type parasites in vitro and that they are able to form only small, slow-growing lesions in BALB/c mice (8, 13). In contrast, Δcpb amastigotes were able to infect macrophages in vitro with the same kinetics as wild-type parasites but, in a similar manner to Δcpb promastigotes, formed only small, slow-growing lesions in mice. Subsequent studies indicated that the absence of the CPB genes resulted in a protective Th1 immune response, contrary to the Th2 response normally observed when the CPB isoenzymes are present (1, 2). The pivotal role of interleukin-4 (IL-4) and the Th2 response in establishing nonhealing L. mexicana infections is well documented (3, 21), and significantly we have recently found that enzymatically active CPB is a potent stimulator of IL-4 and a Th2 response (15).
FIG. 1.
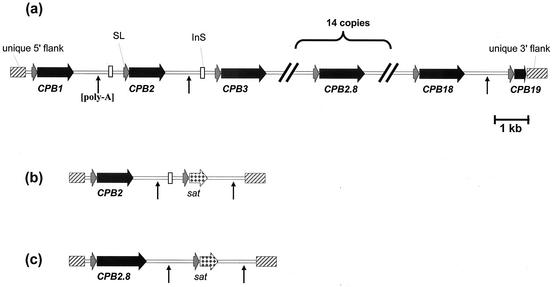
Map of the CPB array and reintegration constructs. (a) Map of the L. mexicana CPB locus. InS, insertion sequence present in the intercistronic sequence downstream of CPB1 and CPB2; SL, CPB splice leader acceptor site present upstream of each gene; vertical arrows, polyadenylation addition sites. (b and c) Maps of the CPB2 and CPB2.8 reintegration cassettes. The CPB genes, their 3′ intergenic sequences, and the sat gene were cloned between the unique 5′ and 3′ CPB flanking regions. Symbols are as in panel a.
Reexpression of individual CPB genes from an extrachromosomal episome in the Δcpb mutant has allowed further analysis of the importance of individual CPB gene products. In particular, differences in substrate specificity among the individual isoenzymes were demonstrated (8, 14) and insights into the processing and the trafficking of these enzymes were provided (5). However, only marginal recovery of lesion growth, at best, could be restored to Δcpb mutants by reinserting individual CPB genes on episomes (8, 13). This inability to restore virulence could have been due to the loss of the CPB gene-containing episome by amastigotes in vivo, in the absence of antibiotic pressure. An alternative possibility is that higher CPB activity is required to enhance infectivity and that this is achieved only when several isoenzymes are present. To test these possibilities, we reintegrated individual CPB genes into the endogenous CPB locus of the Δcpb mutant and also reintroduced multiple CPBs of the array into the Δcpb mutant on an extrachromosomal cosmid. The results of our studies indicate that the expression of multiple CPB genes is key to the development of a Th2 response and consequently the virulence of L. mexicana to mice.
MATERIALS AND METHODS
Leishmania cultivation and transfections.
The lines studied in this work were derived from L. mexicana (MNYC/BZ/62/M379) as described elsewhere (13). L. mexicana promastigotes were routinely grown in modified Eagle’s medium (designated complete HOMEM medium), pH 7.5, containing 10% (vol/vol) heat-inactivated fetal bovine serum at 25°C as described elsewhere (14). L. mexicana parasites were transformed in vitro into axenic amastigotes as described previously (8).
All transfections were carried out as previously described (13) with 10 μg of linear DNA fragments or 5 μg of cosmid DNA. After transfection, the cells were selected in complete HOMEM medium containing the appropriate antibiotic for selection (hygromycin [50 μg/ml], nourseothricin [10 μg/ml], bleomycin [10 μg/ml], or puromycin [10 μg/ml]). Individual clones were isolated on 1% (wt/vol) agar-complete HOMEM plates and then directly transferred to complete HOMEM medium. The individual CPB genes were reintegrated into the CPB locus of L. mexicana as described previously (6).
Infectivity studies.
Groups of five mice were inoculated in the footpad with 5 × 105 stationary-phase L. mexicana parasites resuspended in 0.025 ml of phosphate-buffered saline, pH 7.4. The resultant lesions were monitored over an 8-week period. All experiments were carried out on at least two occasions.
Screening of the L. mexicana cosmid library.
An L. mexicana M379 cosmid library (D. C. Barker, Cambridge, United Kingdom) was screened with three [32P]dCTP-labeled probes prepared with a Prime-it random-priming kit (Stratagene). One probe recognized the unique 5′ flanking region (5′ flank), another recognized the CPB gene (CPB), and the third recognized the unique 3′ flanking region (3′ flank), as previously described (13). Hybridization took place overnight at 65°C in 1 M NaCl-1% sodium dodecyl sulfate (SDS) with 100 μg of salmon sperm DNA/ml. The filters were washed stringently with 0.2× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 0.1% SDS at 60°C and exposed to photographic film (Agfa) at −70°C.
A cosmid (designated pGL263) containing 5′ flank, 3′ flank, and CPB sequences was isolated from a selected bacterial clone with a Wizard midiprep kit by following the manufacturer's instructions (Promega, Southampton, United Kingdom). After mouse infection, the cosmid was recovered from the induced lesions as described elsewhere (7).
Gelatin SDS-PAGE analysis.
Cysteine protease activity was assayed by gelatin SDS-polyacrylamide gel electrophoresis (PAGE) as previously described (16). Coomassie blue stain was used to visualize the hydrolysis of 0.2% gelatin copolymerized in the 12% separating gel.
Protease activity assays.
Lysates of stationary-phase promastigotes were prepared as for gelatin SDS-PAGE analyses and assayed for their ability to hydrolyze Z-Phe-Arg-7-amido-4-methylcoumarin (ZFR-AMC) at 30°C in 0.1 M sodium acetate, pH 5.2, with 2 mM EDTA and 1 mM dithiothreitol. Lysate samples were preincubated for 10 min at 30°C in assay buffer only or in the presence of cysteine protease inhibitor E-64 (10 μM; Sigma). The reaction rate was measured on a Perkin-Elmer LS 55 spectrofluorometer, with 380 nm as the excitation wavelength and 440 nm as the emission wavelength.
Detection of Leishmania-specific IgG1, IgG2a, and total IgE.
Peripheral blood was collected from infected animals by tail bleeding into heparinized capillary tubes at 6 to 8 weeks postinfection. All plasma samples were stored at −20°C before analysis. Leishmania-specific immunoglobulin G1 (IgG1), IgG2a, and total IgE were measured by enzyme-linked immunosorbent assay and end point dilution as previously described (2, 20). The end point dilution represents the final plasma concentration that yielded an absorbance higher than that yielded by a negative-control plasma sample included in the assay.
Proliferation assays.
Spleens and popliteal lymph nodes from L. mexicana-infected mice were removed aseptically, and splenocytes and lymph node suspensions (5 × 105/well) were incubated in 96-well plates at 37°C in complete tissue culture medium (RPMI 1640 [Life Technologies, Paisley, United Kingdom] with 10% [vol/vol] heat-inactivated fetal bovine serum) with or without soluble Leishmania antigen for 3 days. Soluble Leishmania antigen was prepared as described previously (24). Supernatants were assayed for IL-4 as previously described (2). All assays were conducted in triplicate.
RESULTS
Expression of CPB genes in the Δcpb mutant.
We have shown previously that the L. mexicana CPB locus contains 19 genes in a tandem array (Fig. 1a). To isolate cosmids containing the CPB array, a CPB gene-specific probe (13) was used to screen an L. mexicana cosmid genomic library and several independent clones were isolated. Analysis using specific probes revealed that cosmid pGL263 hybridized to the 5′ and 3′ flank probes, as well as the CPB gene itself, supporting evidence that it contained the CPB array. Restriction mapping, coupled to pulsed-field gel electrophoresis and sequence analysis, revealed that the cosmid contained no complete open reading frames apart from the CPB array.
The Δcpb mutant was generated with hyg and ble drug resistance genes as selectable markers (13). As the cosmid pGL263 contains the hyg resistance gene (18), the Δcpb mutant was reengineered to contain the pac gene, which confers puromycin resistance, in place of the hyg gene. Confirmation that pac had replaced hyg in the Δcpbpac cell line was obtained by assessing the sensitivity of clones to hygromycin and by PCR analysis of genomic DNA with combinations of oligonucleotide primers designed to anneal to pac and CPB flanking regions outside of the integration cassette (data not shown).
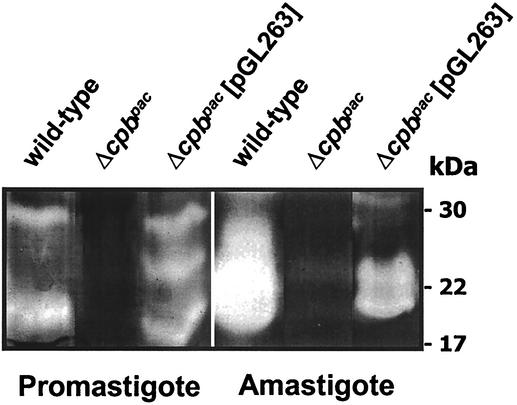
The Δcpbpac mutant was transfected with pGL263, and a clone resistant to hygromycin, designated the Δcpbpac[pGL263] mutant, was characterized. Extracts of promastigote and amastigote stages were assessed for cysteine protease activity by gelatin SDS-PAGE (Fig. 2). The Δcpbpac[pGL263] mutant was found to exhibit cysteine protease activity in both life cycle stages, with higher levels detected in the amastigote. In contrast, the Δcpb mutant had no cysteine protease activity that was detectable by this method.
FIG. 2.
Gelatin SDS-PAGE analysis of the Δcpbpac[pGL263] mutant. Lysates of 107 wild-type, Δcpbpac, and Δcpbpac[pGL263] promastigotes and 4 × 106 axenic amastigotes were analyzed by gelatin SDS-PAGE. Molecular mass standards are indicated.
In addition to the Δcpbpac[pGL263] cell line, which expresses multiple copies of CPB, two cell lines containing single copies of CPB reintroduced into the CPB locus of the Δcpb mutant were generated (by using the constructs detailed in Fig. 1b and c) and analyzed. The Δcpb::CPB2.8 mutant expresses the amastigote-specific CPB2.8 gene, while the Δcpb::CPB2 mutant expresses the metacyclic stage-specific CPB2 gene (6). The stage-specific expression of CPB2 and CPB2.8 has been described previously (6, 14). In vitro, none of these cell lines have an impaired growth rate compared with wild-type parasites. Analysis of the cysteine protease activity in stationary-phase promastigotes of these cell lines with the peptidyl substrate ZFR-AMC showed that the Δcpbpac[pGL263] parasites had 32% of the cysteine protease activity found in wild-type promastigotes, whereas the corresponding activities in the Δcpb::CPB2.8 and Δcpb mutants were only 13 and 10%, respectively. These protease activity data are consistent with a relatively high level of CPB gene expression from the CPB cosmid, albeit less than that which occurs in wild-type parasites, but only a low level from the reintegrated CPB gene.
Infectivity of promastigotes of the mutant parasites.
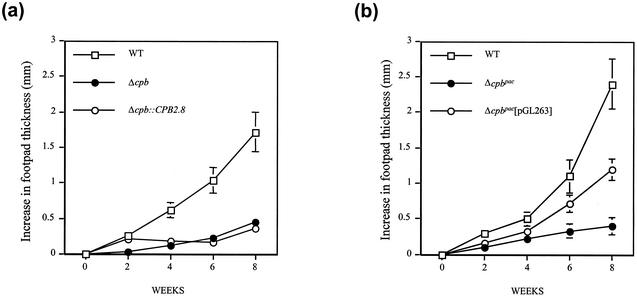
As previously observed (2), the Δcpb mutants produced small and slow-growing lesions in mice compared with the rapidly growing lesions produced by wild-type L. mexicana (Fig. 3). The Δcpb::CPB2.8 mutant induced early lesion growth (2 weeks), similar to wild-type parasites. Lesions were significantly larger at 2 weeks than those produced by Δcpb mutants (P < 0.01), but this was not sustained, and lesion size thereafter was similar to that for Δcpb mutant-infected animals (Fig. 3a) and significantly less than that of wild-type L. mexicana lesions. In contrast, the increase in footpad thickness induced by the Δcpb::CPB2 mutant line was at no stage greater than that produced by Δcpb mutants (data not shown). However, Δcpbpac[pGL263] promastigotes induced lesions which were significantly larger than those produced by Δcpbpac parasites at 4, 6, and 8 weeks (P < 0.05) postinfection and approximately 50% of the size of wild-type parasite-induced lesions (Fig. 3b). The total parasite counts at 8 weeks reflected differences in lesion size between mutants and wild-type parasites (wild-type, [8.0 ± 1.2] × 108; Δcpbpac[pGL263] mutant, [1.1 ± 0.6] × 108; Δcpbpac mutant, [5.0 ± 1.3] × 106). These results show that the presence of multiple CPB genes facilitates the establishment and sustained development of promastigote-initiated infection by L. mexicana.
FIG. 3.
Lesion development in BALB/c mice infected with L. mexicana mutants. (a) BALB/c mice were inoculated with 5 × 105 wild-type, Δcpb, and Δcpb::CPB2.8 parasites. Footpad thickness was monitored for 8 weeks. Values represent means ± standard errors of the means (n = 5). (b) Same as panel a except that mice were infected with Δcpbpac and Δcpbpac[pGL263] mutants.
As no selection pressure for the cosmid was maintained during in vivo infection, we verified that the pGL263 cosmid could be isolated from Δcpbpac[pGL263] amastigotes isolated from a mouse lesion at 8 weeks. Isolated cosmid pGL263 DNA was analyzed by agarose gel electrophoresis and by PCR and was confirmed to contain CPB genes (data not shown). We also verified that the promastigote parasites, obtained after transformation in vitro of the amastigotes, were resistant to hygromycin and thus still contained the pGL263 cosmid.
Expression of multicopy CPB restores the Th2 response, as determined by measuring IL-4 production and IgG1 and IgE levels.
We have previously shown that when BALB/c mice are infected with the Δcpb mutant, they are able to mount a protective Th1 response, rather than the nonprotective Th2 response normally observed in wild-type infections (2). The latter response is characterized by elevated IL-4 production and comparatively high titers of IgG1 and IgE. To determine the type of immune response induced by the different mutant parasite lines, we measured the Ig titers in the plasma of infected mice.
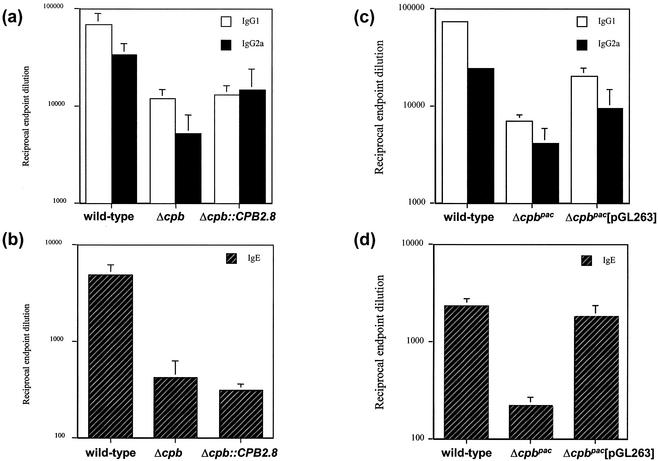
As previously demonstrated, mice infected with wild-type parasites exhibited a high level of IgG1 at 8 weeks postinfection, characteristic of a Th2 response, while Δcpb mutant infections led to a relatively low titer, indicative of a Th1 response (Fig. 4a). Infection with the Δcpb::CPB2.8 mutant was similar to infection with the Δcpb mutant in terms of IgG1 and IgG2a antibody production. While BALB/c mice infected with wild-type parasites had significant IgE production, again indicative of IL-4 production and a Th2 response, IgE levels were significantly lower (P < 0.001) in mice infected with Δcpb and Δcpb::CPB2.8 parasites (Fig. 4b). Conversely, following infection with the Δcpbpac[pGL263] mutant, BALB/c mice produced significantly more IgG1 (P < 0.01) than mice infected with Δcpbpac parasites but significantly less (P < 0.001) than mice infected with the wild type (Fig. 4c). Importantly, infection with the Δcpbpac[pGL263] mutant increased IgE levels so that they were equivalent to that produced by wild-type infection and significantly greater (P < 0.001) than that produced by infection with the Δcpbpac mutant (Fig. 4d).
FIG. 4.
Analysis of parasite-specific IgG1 and IgG2a levels (a and c) and total IgE levels (b and d) in BALB/c mice infected with L. mexicana mutants. The antibody titers were determined in the plasma of mice infected with wild-type, Δcpb, Δcpb::CPB2.8, Δcpbpac, and Δcpbpac[pGL263] strains at 8 weeks postinfection. Values represent the mean end point dilutions ± standard errors of the means (n = 5).
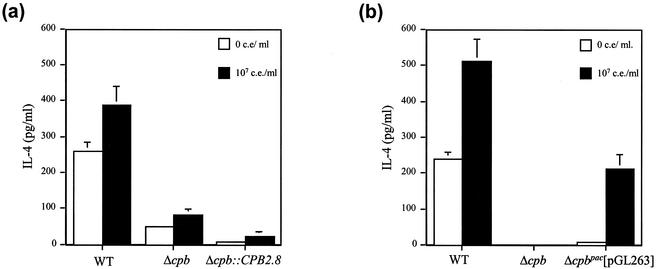
The production of IgE is IL-4 dependent, so popliteal lymph node cells were harvested from mice and the production of IL-4 following stimulation with Leishmania antigen was determined. Popliteal lymph node cells from mice infected with Δcpb and Δcpb::CPB2.8 parasites produced significantly less (P < 0.01) IL-4 than popliteal lymph node cells from wild-type parasite-infected mice. Reinsertion of CPB2.8 into Δcpb parasites did not result in increased IL-4 production (Fig. 5a). Conversely popliteal lymph nodes from Δcpbpac[pGL263] mutant-infected mice produced significant quantities of IL-4 following stimulation with Leishmania antigen (Fig. 5b). This was significantly greater than the quantity produced by mice infected with Δcpb parasites (P < 0.002) but less than that produced by mice infected with wild-type organisms (P < 0.005). Similar results were obtained with splenocyte cultures (results not shown).
FIG. 5.
Lymphocyte IL-4 responses of mice infected with L. mexicana mutants. IL-4 production was measured after stimulation of isolated splenocytes with 107 cell equivalents (c.e.) of soluble Leishmania antigen/ml. Error bars, standard errors of the means (n = 5). (a) Δcpb::CPB2.8 mutant. (b) Δcpbpac[pGL263] mutant.
DISCUSSION
The aim of this study was to investigate the significance of the multiplicity of the CPB isoenzymes of L. mexicana in the parasite's interaction with its mammalian host. To do this we compared lesion development and the immune response in mice infected with clonal populations of Δcpb null mutant parasites reexpressing different CPB genes. We first developed parasite lines where single CPB genes CPB2 and CPB2.8 were reintegrated in the CPB locus of the Δcpb mutant (6). CPB2 and CPB2.8 were expressed in the two lines in the metacyclic and amastigote stages, respectively, as occurs in wild-type L. mexicana. However, wild-type L. mexicana expresses multiple CPB isoenzymes in the amastigote stage, where it is probable that the enzymes interact and complement each other's activities (5, 14), and so we also reexpressed multiple CPB genes in the Δcpb mutant so that we could investigate whether the multiplicity of the CPB isoenzymes was important for the parasite's virulence. As reintegration of the complete array into the genome was not possible, we chose to reexpress the CPB genes from an episomal cosmid vector. The only intact genes on this cosmid were the CPB genes, so the effects of gene expression from the cosmid could be attributed directly to CPB and not other proteins. Gelatin SDS-PAGE and fluorogenic peptidyl substrate assays confirmed that these multiple CPB genes carried on the cosmid were expressed in the Δcpbpac[pGL263] mutant, although the expression levels were lower than those for wild-type parasites (Fig. 2).
The finding that Δcpb mutants reexpressing CPB2, an isoenzyme expressed primarily in the metacyclic form, failed to promote lesion development was not unexpected. While it is conceivable that expression of metacyclic stage-specific CPB2 may support initial infection, the low levels of CPB activity expressed following transformation into the amastigote stage (6) would afford these Δcpb::CPB2 mutants no advantage over Δcpb parasites. It is interesting, however, that the parasites with amastigote-specific CPB2.8 induced increased initial lesion growth compared with Δcpb parasites, even though this was not sustained. Presumably, for prolonged virulence in mice the parasites require higher levels of expression of CPB than was mediated by the single gene or, alternatively, expression of other CPB isoenzymes with different substrate specificities (6, 14). Both of these possibilities are consistent with the results obtained with the mutant expressing multiple CPB genes, the Δcpbpac[pGL263] mutant, which produced lesions that were significantly larger throughout the course of the experiment than those induced by the Δcpb mutant. These data suggest that the expression of multiple CPB isoenzymes greatly facilitates virulence. However, they do not indicate whether the effect is mediated simply by enhanced CPB activity or requires several isoenzymes with subtly different substrate specificities (14). Previous studies where single CPB genes were reexpressed from episomes which were present in multiple copies in the parasite but did not restore virulence (8) could indicate that higher expression alone is insufficient to induce higher infectivity in vivo. Nevertheless this question requires further investigation.
The mean lesion size produced by the Δcpbpac[pGL263] mutant was only one-half that produced by using equivalent numbers of wild-type parasites. This could have been due to differing expression of isoenzymes from the cosmid compared with that for the natural CPB array or different overall CPB activity. The regulation of gene expression from the cosmid is likely to differ from the regulation of chromosomally located-gene expression, and indeed the gelatin SDS-PAGE data (Fig. 2) suggest that the two parasite lines are not equivalent and express different numbers or different arrays of isoenzymes. Moreover, some parasites in the population in the lesions may have lost the cosmid, which clearly would make them less virulent. Further studies, involving reexpression of all different combinations of CPB genes, will be necessary to understand whether all of the isoenzymes are required for full effect and whether any in particular are playing the major role in the polarization of the immune response.
Infection with L. mexicana in the BALB/c mouse is associated with a polarized Th2 response, and endogenous IL-4 has been demonstrated to be necessary for early lesion development (3, 22). Moreover, susceptibility of BALB/c mice to Leishmania major has been shown to be the result of IL-4 production by Vβ4 Vα8CD4+ T cells in response to a single T-cell epitope derived from the parasite LACK antigen (the Leishmania homologue of receptors for activated C kinase) (11). However, recent studies have demonstrated that LACK-tolerant BALB/c mice remain susceptible to L. mexicana despite resistance to L. major (26). As L. mexicana expresses LACK and as LACK is recognized by BALB/c CD4+ T cells that produce IL-4, these observations clearly demonstrate that there are alternative or additional mechanisms for inducing the susceptibility of mice to L. mexicana. Our data suggest that the CPB enzymes may be crucially involved. Significantly, L. mexicana Δcpb mutants have reduced infectivity for BALB/c mice and induce a polarized type 1 response (2). Of relevance to this is the report that during wild-type infections CPB occurs in large quantities in the extracellular milieu (9). Subsequently we have demonstrated that recombinant, enzymatically active CPB2.8 is a potent stimulator of IL-4 and IgE production in BALB/c mice (15), like the DerP1 antigen, which is also a cysteine protease (23). Our present findings, that reexpression of multiple CPB genes, but not single genes, in Δcpb mutants restored a significantly increased IgE production as well as significantly elevated lymph node and splenocyte IL-4 production, are consistent with this hypothesis of a key role for CPB in the host-L. mexicana interactions and particularly in the modulation of the immune response.
The findings of this study confirm that the level of CPB expression or the diversity in CPB isoenzymes or both are important for parasite virulence. Moreover, this study has demonstrated a role for the multiple CPB isoenzymes in the modulation of the immune response to L. mexicana infection, although the mechanisms underlying this process await further characterization.
Acknowledgments
This investigation received financial support from the UNDP/World Bank/WHO special program for research and training in tropical diseases and the Wellcome Trust. J. C. Mottram is an MRC Senior Research Fellow.
Editor: J. M. Mansfield
REFERENCES
- 1.Alexander, J., and J. M. Blackwell. 1986. The immunological significance of genetically determined cross reactivity between taxonomically distinct Leishmania species, p.185-191. In J.-A. Rioux (ed.), Leishmania. Taxonomie et phylogenese. Applications eco-epidemiologiques. Institut Méditerranéen d’Etudes Épidémiologiques et Ecologiques, Montpellier, France.
- 2.Alexander, J., G. H. Coombs, and J. C. Mottram. 1998. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a T helper 1 (TH1) response. J. Immunol. 161:6794-6801. [PubMed] [Google Scholar]
- 3.Alexander, J., F. Brombacher, H. A. McGachy, A. N. J. McKenzie, W. Walker, and K. C. Carter. 2002. An essential role for IL-13 in maintaining a non-healing response following Leishmania mexicana infection. Eur. J. Immunol. 32:2923-2933. [DOI] [PubMed] [Google Scholar]
- 4.Bart, G., G. H. Coombs, and J. C. Mottram. 1995. Isolation of lmcpc, a gene encoding a Leishmania mexicana cathepsin B-like cysteine proteinase. Mol. Biochem. Parasitol. 73:271-274. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, D. R., L. Tetley, G. H. Coombs, and J. C. Mottram. 2000. Processing and trafficking of cysteine proteases in Leishmania mexicana. J. Cell Sci. 113:4035-4041. [DOI] [PubMed] [Google Scholar]
- 6.Brooks, D. R., H. Denise, G. D. Westrop, G. H. Coombs, and J. C. Mottram. 2001. The stage-regulated expression of Leishmania mexicana CPB cysteine proteases is mediated by an intercistronic sequence element. J. Biol. Chem. 276:47081-47089. [DOI] [PubMed] [Google Scholar]
- 7.Descoteaux, A., L. A. Garraway, K. A. Ryan, L. K. Garrity, S. J. Turco, and S. M. Beverley. 1994. Identification of genes by functional complementation in protozoan parasite Leishmania. Methods Mol. Genet. 3:22-48. [Google Scholar]
- 8.Frame, M. J., J. C. Mottram, and G. H. Coombs. 2000. Analysis of the roles of cysteine proteinases of Leishmania mexicana in the host-parasite interaction. Parasitology 121:367-377. [DOI] [PubMed] [Google Scholar]
- 9.Ilg, T., M. Fuchs, V. Gnau, M. Wolfram, D. Harbecke, and P. Overath. 1994. Distribution of parasite cysteine proteinases in lesions of mice infected with Leishmania mexicana amastigotes. Mol. Biochem. Parasitol. 67:193-203. [DOI] [PubMed] [Google Scholar]
- 10.Klemba, M., and D. E. Goldberg. 2002. Biological roles of proteases in parasitic protozoa. Annu. Rev. Biochem. 71:275-305. [DOI] [PubMed] [Google Scholar]
- 11.Launois, P., I. Maillard, S. Pingel, K. G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, H. R. MacDonald, and J. A. Louis. 1997. IL-4 rapidly produced by V beta 4 V alpha 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity 6:541-549. [DOI] [PubMed] [Google Scholar]
- 12.Mottram, J. C., C. D. Robertson, G. H. Coombs, and J. D. Barry. 1992. A developmentally regulated cysteine proteinase gene of Leishmania mexicana. Mol. Microbiol. 6:1925-1932. [DOI] [PubMed] [Google Scholar]
- 13.Mottram, J. C., A. E. Souza, J. E. Hutchison, R. Carter, M. J. Frame, and G. H. Coombs. 1996. Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc. Natl. Acad. Sci. USA 93:6008-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mottram, J. C., M. J. Frame, D. R. Brooks, L. Tetley, J. E. Hutchison, A. E. Souza, and G. H. Coombs. 1997. The multiple cpb cysteine proteinase genes of Leishmania mexicana encode isoenzymes which differ in their stage-regulation and substrate preferences. J. Biol. Chem. 272:14285-14293. [DOI] [PubMed] [Google Scholar]
- 15.Pollock, K. G. J., K. S. McNeil, J. C. Mottram, R. E. Lyons, J. M. Brewer, P. Scott, G. H. Coombs, and J. Alexander. 2002. The Leishmania mexicana cysteine protease, CPB2.8, induces potent Th2 responses. J. Immunol. 170:1746-1753. [DOI] [PubMed] [Google Scholar]
- 16.Robertson, C. D., and G. H. Coombs. 1990. Characterization of 3 groups of cysteine proteinases in the amastigotes of Leishmania mexicana mexicana. Mol. Biochem. Parasitol. 42:269-276. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal, P. J. 1999. Proteases of protozoan parasites. Adv. Parasitol. 43:105-159. [DOI] [PubMed] [Google Scholar]
- 18.Ryan, K. A., S. Dasgupta, and S. M. Beverley. 1993. Shuttle cosmid vectors for the trypanosomatid parasite Leishmania. Gene 131:145-150. [DOI] [PubMed] [Google Scholar]
- 19.Sajid, M., and J. H. McKerrow. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120:1-21. [DOI] [PubMed] [Google Scholar]
- 20.Satoskar, A., H. Bluethmann, and J. Alexander. 1995. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect. Immun. 63:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoskar, A., F. Brombacher, W. J. Dai, I. McInnes, F. Y. Liew, J. Alexander, and W. Walker. 1997. SCID mice reconstituted with IL-4-deficient lymphocytes, but not immunocompetent lymphocytes, are resistant to cutaneous leishmaniasis. J. Immunol. 159:5005-5013. [PubMed] [Google Scholar]
- 22.Satoskar, A., H. H. Al-Quassi, and J. Alexander. 1998. Sex-determined resistance against Leishmania mexicana is associated with the preferential induction of a Th1-like response and IFN-gamma production by female but not male DBA/2 mice. Immunol. Cell Biol. 76:159-166. [DOI] [PubMed] [Google Scholar]
- 23.Schulz, O., P. Laing, H. F. Sewell, and F. Shakib. 1995. DerpI, a major allergen of the house dust mite, proteolytically cleaves the low-affinity receptor for human IgE (CD23). Eur. J. Immunol. 25:3191-3194. [DOI] [PubMed] [Google Scholar]
- 24.Scott, P., E. Pearce, P. Natovitz, and A. Sher. 1987. Vaccination against cutaneous leishmaniasis in a murine model. Induction of protective immunity with a soluble extract of promastigotes. J. Immunol. 139:221-227. [PubMed] [Google Scholar]
- 25.Souza, A. E., S. Waugh, G. H. Coombs, and J. C. Mottram. 1992. Characterization of a multicopy gene for a major stage-specific cysteine proteinase of Leishmania mexicana. FEBS Lett. 311:124-127. [DOI] [PubMed] [Google Scholar]
- 26.Torrentera, F. A., N. Glaichenhaus, J. D. Laman, and Y. Carlier. 2001. T-cell responses to immunodominant LACK antigen do not play a critical role in determining susceptibility of BALB/c mice to Leishmania mexicana. Infect. Immun. 69:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]