Abstract
OBJECTIVE
To estimate the effect of 2 years of treatment with ultralow-dose transdermal estradiol (E2) on incontinence in postmenopausal women.
METHODS
Ultra Low Dose Transdermal estRogen Assessment (ULTRA) was a multicenter, randomized, double-blinded, placebo-controlled trial of unopposed ultralow-dose (0.014 mg/d) transdermal E2 for prevention of osteoporosis in 417 postmenopausal women aged 60 to 80 years. Frequency of incontinence episodes was assessed at baseline and after 4 months and 2 years of treatment using a self-reported questionnaire. We used an intention-to-treat analysis to compare change in incontinence frequency, improved (decreased 2 or more episodes per week), unchanged (increased or decreased no more than 1 episode per week), or worsened (increased 2 or more episodes per week) between the E2 and placebo groups among women with and without at least weekly incontinence at baseline.
RESULTS
At baseline, the prevalence of at least weekly incontinence was similar between E2 and placebo groups (43%). After 2 years, there was no difference between groups in the proportions of women with incontinence at baseline whose incontinence improved, worsened, or was unchanged. The odds ratio for worsening incontinence in the E2 compared with placebo group was 1.35 (95% confidence interval 0.75–2.42. In women without incontinence at baseline, the odds of developing at least weekly incontinence after 2 years in the E2 compared with placebo group was not significant (odds ratio 1.2, 95% confidence interval 0.7–2.2).
CONCLUSION
Two years of treatment with unopposed ultralow-dose transdermal E2 did not substantially change the frequency of incontinence symptoms or alter the risk of developing at least weekly incontinence.
LEVEL OF EVIDENCE: I
The presence of α and β estrogen receptors throughout the urogenital tract suggests that estrogen has a role in the continence mechanism. In observational studies and nonrandomized trials, estrogen therapy increases urethral closure pressure,1-3 urethral blood flow,4 α-adrenergic receptor sensitivity5,6 improves cellular maturation in women with urogenital atrophy7-9 and reduces the frequency of incontinence episodes.10 Based on these findings, estrogen has been used to treat urinary incontinence in postmenopausal women.
The presumed clinical benefit of systemic estrogen as a treatment for incontinence has been challenged by results of randomized controlled trials demonstrating that oral estrogen and estrogen plus progestin either have no effect or worsen incontinence among postmenopausal women.11-18 Currently, there are no controlled trials examining the effects of transdermal estrogen or lower-than-standard doses of systemic estrogen on urinary incontinence.
The Ultra Low Dose Transdermal estRogen Assessment (ULTRA) trial was a multicenter, randomized, double-blind, placebo-controlled trial that investigated effects on bone density and the safety of unopposed transdermal estradiol (E2) administered to postmenopausal women at a dose approximately 75% lower than usually prescribed. The objective of this analysis is to report the effect of 2 years of treatment with ultralow-dose transdermal E2 on urinary incontinence in postmenopausal women.
METHODS
The ULTRA trial was a randomized, double-blind, placebo-controlled trial to assess the effectiveness of 14 μg of transdermal E2 per day (one fourth of the 50-μg standard transdermal E2 dose) for osteoporosis prevention in postmenopausal women.19 The trial enrolled 417 women at 9 clinical centers in the United States between February 2000 and November 2002. Figure 1 details recruitment and retention in the trial. The ULTRA trial was coordinated at the University of California, San Francisco and funded by Berlex Laboratories (Montville, NJ), the maker of the patch used in this study. The institutional review boards at each clinical site and the coordinating center approved the ULTRA study protocol and all participants gave written informed consent. The institutional review board at the University of California, Davis approved the protocol for this planned secondary analysis of the ULTRA study.
Fig. 1.
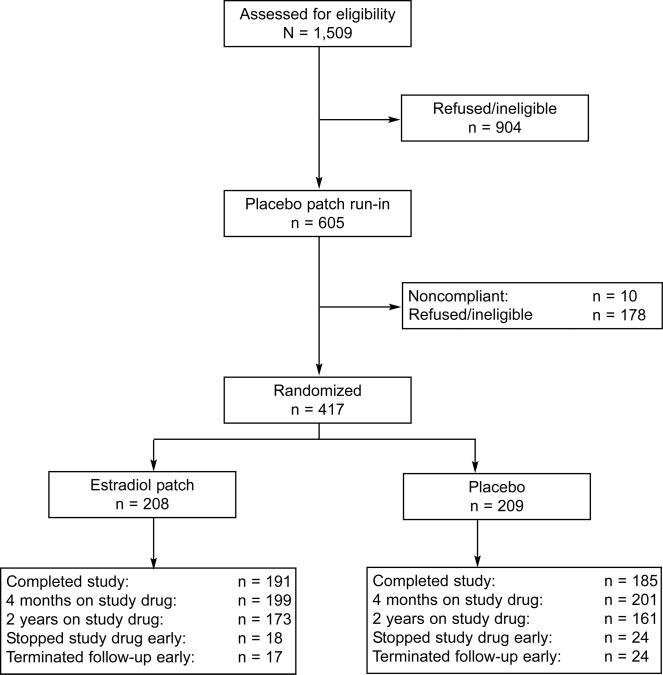
Flow chart detailing recruitment and retention of participants in the Ultra Low dose Transdermal estrogen Assessment (ULTRA) trial.
The participants in ULTRA were women aged 60 to 80 years who had a uterus and were at least 5 years beyond menopause. All were required to have a normal bone mineral density for age (z score not below −2.0 at the lumbar spine). Women were excluded if they had taken estrogen or progestin within 3 months of randomization or had any of the following: unexplained uterine bleeding, endometrial hyperplasia or an endometrium 5 mm or more in double-wall thickness, abnormal mammogram suggestive of breast cancer, a history of metabolic bone disease, cancer, coronary disease, cerebrovascular disease, uncontrolled hypertension, uncontrolled thyroid disease, liver disease, fasting triglycerides more than 300 mg/dL, or fasting glucose more than 180 mg/dL.
Upon entry, all women were randomly assigned to E2 or placebo by a computer-generated randomization scheme stratified by clinical center in blocks of 4. Treatment numbers were printed on labels adhered to the identical-looking study medications. Allocation was concealed because these numbers were assigned sequentially to women by order of arrival at the different clinical centers. Treatment was a weekly 3.25-cm3 area E2 patch releasing 0.014 mg of E2 per day or an identical placebo patch. Participants, investigators, and outcome assessors were blinded to treatment assignment and no unblinding occurred during the trial.
At baseline, participants completed a questionnaire assessing demographics, health habits, health history, and medication use. Physical examination included breast and pelvic examination and body mass measurements. Serum estrone (competitive radioimmunoassay, Diagnostic Systems Laboratories, Inc, Webster, TX) and E2 (double-antibody sequential radioimmunoassay, Diagnostics Products Corporation, Los Angeles, CA) were measured. Adherence to treatment was measured by patch counts every 4 months. Any surgeries that occurred during the course of the study were reported as adverse events.
At baseline, 4 months, and 2 years, participants completed a questionnaire that assessed incontinence. An episode of incontinence was categorized as stress incontinence if participants reported leakage “while coughing, sneezing, straining, laughing, or lifting” and as urge incontinence if reported to occur with “an urge to urinate and could not get to the toilet fast enough.” Participants were asked to record the number of stress incontinence episodes and the number of urge incontinence episodes in the prior week. The frequency of any incontinence was determined by summing urge and stress episodes. Changes in frequency of incontinence from baseline to 4 months and 2 years were classified as “worsened” if incontinence episodes increased by 2 or more per week, “unchanged” if frequency changed by no more than 1 episode per week and “improved” if incontinence episodes decreased by 2 or more per week. This definition has been used previously12 and is based on face validity, that is, 2 episodes can be considered clinically important but still outside the realm of reporting variability.
We estimated that at the 4-month visit, we had 80% power in 2-tailed tests with α of 5% to detect an odds ratio for worsening of 1.9 for any incontinence, 2.0 for stress incontinence, and 2.3 for urge incontinence. These computations make use of the information on the proportions improved, with no change, and worsened in the placebo group. Results for other time points were comparable.
Differences in baseline characteristics between the placebo and E2 groups were assessed using t-tests, Wilcoxon's rank sum, χ2, and Fisher exact tests as appropriate. The ULTRA study retained women in the trial after discontinuation of treatment to the extent that they were willing and obtained a measurement at the end of study for these women. For the women unwilling to continue in follow-up, a measurement was obtained at the point of discontinuation of treatment. However, the 41 women who did not have one or the other type of measurement were omitted from the analysis of 2-year endpoint. Among women with at least weekly incontinence at baseline, we compared the percent in the E2 and placebo groups that improved, was unchanged, and worsened after 4 months and 2 years of treatment. To better characterize the precision of negative findings and to account for the ordinal nature of this outcome, we used proportional odds models,20 adjusting for clinical site. Among women who did not report weekly incontinence at baseline, we used a logistic model to compare the proportion of women in the 2 treatment groups who reported developing at least weekly incontinence at 4 months and 2 years of treatment, adjusting for clinical site. Similar analyses were used to determine separately the effects of treatment on stress and on urge incontinence.
RESULTS
At the end of the 2-year study period, 376 (90%) women completed the study (Fig. 1). Overall, 83% of the women in the E2 group and 77% in the placebo group continued to use their assigned patch; 84% in both groups used at least 75% of their patches.
Baseline characteristics of the 417 women are presented in Table 1. There were no significant differences between E2 and placebo groups in demographics, reproductive characteristics, reported health history, smoking, or endogenous estrogen levels. The participants ranged in age from 60 to 80 years with a mean age of 67 ± 5 years.
Table 1.
Baseline Characteristics of 417 Ultra Low Dose Transdermal estRogen Assessment Participants by Treatment Group
| Estradiol n = 20 | Placebo n = 20 | P* | |
|---|---|---|---|
| Age | 66.8 ± 5.1 | 66.7 ± 4.8 | .96 |
| Years since menopause | 37.0 ± 5.1 | 37.4 ± 4.7 | .57 |
| White race | 92.8 | 91.9 | .45 |
| Body mass index | 28.3 ± 5.3 | 28.0 ± 5.3 | .60 |
| Parity | 3.2 ± 1.7 | 3.3 ± 1.8 | .70 |
| Medical conditions | |||
| Diabetes | 3.4 | 1.0 | .09 |
| Hypertension | 16.4 | 14.8 | .65 |
| Current smoker | 7.7 | 6.2 | .55 |
| Estradiol level in pg/mL [median (interquartile range)] | 4.8 (2.7–8.0) | 4.7 (2.7–8.3) | .62 |
| Weekly or more frequent incontinence | |||
| Any type | 41.3 | 44.0 | .62 |
| Stress | 25.0 | 27.5 | .53 |
| Urge | 26.0 | 25.8 | 1.00 |
| Frequency of incontinence, episodes/wk | |||
| None | 58.6 | 56.5 | .80 |
| 1 | 16.8 | 16.9 | |
| 2–6 | 18.8 | 18.4 | |
| ≥ 7 | 5.8 | 8.2 | |
| Incontinence type, mean episodes/wk | |||
| Any | 1.9 ± 6.9 | 1.7 ± 4.3 | .57 |
| Stress | 1.3 ± 6.0 | 1.1 ± 3.6 | .47 |
| Urge | 0.6 ± 1.8 | 0.7 ± 1.7 | .84 |
SD, standard deviation.
Values are mean ± standard deviation or %, except where otherwise specified.
The P values for categorical variables were calculated using generalized Cochran-Mantel-Haenszel tests, stratified by clinic site. The P values for continuous variables are from analysis of variance or rank analysis of variance, adjusted for site.
Prevalence, frequency and type of incontinence were also similar between the E2 and placebo groups at baseline (Table 1). Overall, 178 women reported weekly incontinence (43%), with 7% reporting 7 or more episodes of leakage per week; 239 (57%) women reported no incontinence. Equal proportions of women in the treatment groups reported at least weekly stress (26%) and urge (26%) symptoms, and approximately 18% reported both types. None of the participants underwent incontinence surgery during the study.
Table 2 presents the percent of participants with at least weekly incontinence at baseline that improved, was unchanged, or worsened after 4 months and 2 years of treatment with ultralow-dose E2 or placebo. At 4 months, the proportion of women improved was slightly higher in the placebo group (35.2% compared with 25%), and the proportion of women worsened was slightly higher in the E2 group (23.8% compared with 19.3), but the differences between the groups were not statistically significant. After 2 years of treatment, these small differences between the groups were reduced. The effects of treatment did not vary by baseline serum E2 levels (data not shown).
Table 2.
Percent of Women Improved, Unchanged, or Worsened Compared With Baseline Frequency of Urinary Incontinence by Treatment Group and Type of Incontinence at 4 Months and 2 Years of Treatment
| Incontinence Type | Visit | Treatment | Improved* | Unchanged* | Worsened* | OR (95% CI)† | P† |
|---|---|---|---|---|---|---|---|
| Any | |||||||
| 4 mo | Estradiol | 25.0 | 51.2 | 23.8 | 1.57 (0.88–2.81) | .13 | |
| Placebo | 35.2 | 45.5 | 19.3 | ||||
| 2 y | Estradiol | 27.4 | 56.0 | 16.7 | 1.35 (0.75–2.42) | .32 | |
| Placebo | 38.2 | 44.9 | 16.9 | ||||
| Stress | |||||||
| 4 mo | Estradiol | 14.3 | 65.5 | 20.2 | 2.05 (1.09–3.85) | .03 | |
| Placebo | 27.3 | 59.1 | 13.6 | ||||
| 2 y | Estradiol | 17.9 | 72.6 | 9.5 | 1.52 (0.79–2.93) | .21 | |
| Placebo | 29.2 | 61.8 | 9.0 | ||||
| Urge | |||||||
| 4 mo | Estradiol | 11.9 | 85.7 | 2.4 | 0.60 (0.27–1.34) | .22 | |
| Placebo | 12.5 | 77.3 | 10.2 | ||||
| 2 y | Estradiol | 13.1 | 73.8 | 13.1 | 0.95 (0.50–1.82) | .88 | |
| Placebo | 16.9 | 65.2 | 18.0 |
OR, odds ratio; CI, confidence interval.
Values are %.
Improved means that the number of incontinence episodes per week decreased by 2 or more, unchanged means that the number of incontinence episodes per week increased or decreased no more than 1, and worsened means that the number of incontinence episodes per week increased by 2 or more.
OR for worsening incontinence and P value from proportional odds model with ordinal outcome, adjusting for clinical site.
Table 2 also presents changes in stress and urge incontinence symptoms and odds ratios for these changes by treatment group at 4 months and 2 years. A higher proportion of women in the E2 group reported worsened (20.2% compared with 13.6%), whereas a lower proportion reported improved (14.3% compared with 27.3%) stress incontinence at 4 months compared with placebo (P = .03). This difference was small (average increase in frequency of 1.4 episodes/week) and did not persist after 2 years of treatment. There was no difference between groups in the proportions of women whose urge incontinence improved, worsened, or was unchanged.
Among the 239 women who did not report incontinence at baseline, 39.0% in the treatment compared with 36.8% in the placebo group developed incontinence during the 2 years of follow-up (P = .74). The odds ratio for new onset of incontinence among E2-treated compared with placebo-treated women was 1.2 (95% confidence interval 0.7–2.2). Of the women who developed incontinence at 4 months, there was no difference between the treatment groups in the proportions who improved (4.9%), were unchanged (88.5%), or worsened (6.6%) at year 2 (P = .62).
DISCUSSION
Two years of treatment with unopposed ultralow-dose transdermal E2 had no substantial effect on urinary incontinence symptoms in older postmenopausal women. Women with at least weekly incontinence at baseline had no significant worsening with treatment and women without urinary incontinence at baseline were not more likely to develop at least weekly incontinence. Women who were treated with E2 had slightly worsening stress incontinence compared with those treated with placebo at 4 months, but this difference was small, not observed for urge incontinence, and did not persist at 2 years of treatment.
Participants were observed during 2 years of treatment with the ultralow-dose E2 patch, providing information about use of this medication over an extended period. Compliance with the medication was high and follow-up was excellent.
In many observational studies estrogen use is associated with incontinence,21 but determining whether incontinence preceded or followed the initiation of estrogen in these retrospective or cross-sectional studies is difficult. The large, prospective Nurses Health Study demonstrated that postmenopausal women had up to a 68% increased risk of developing new incontinence after initiation of estrogen. This risk did not vary significantly by dose, type of estrogen, or route of administration.22
The best evidence for the clinical effects of estrogen on incontinence is from 9 randomized, placebo-controlled trials. Oral estrogen,11-23 oral estrogen plus progestin,11-13,15 and subcutaneous estrogen implants24 had no effect or worsened incontinence. Most of the trials that showed no significant effect of estrogen on incontinence were small and may not have observed an effect of estrogen due to limited statistical power. The 2 largest trials however, both showed worsening of incontinence after treatment with estrogen.11-12 In the Women's Health Initiative (WHI) randomized trial, women who reported incontinence at baseline assigned to conjugated oral estrogens with and without medroxyprogesterone experienced an increase in frequency and bothersomeness of incontinence symptoms compared with women assigned to placebo.11 Similarly in the Heart and Estrogen/progestin Replacement Study, conjugated oral estrogens with medroxyprogesterone worsened both stress and urge incontinence.12 Among women without incontinence at baseline in the WHI, conjugated oral estrogens with and without medroxyprogesterone increased the risk of developing stress incontinence approximately 2-fold.11 The effects of estrogen might vary with the preparation. Clinical trials of E2,14-24 estriol,18 and estrone17 showed no effect, whereas conjugated estrogens seem to worsen incontinence or increase the risk of developing incontinence.11-13,15,23 However, many of the trials of E2, estriol, and estrone were too small to demonstrate small effects on incontinence.
Alpha and β estrogen receptors have been identified in the bladder mucosa, trigone, urethra, vaginal mucosa, the uterosacral ligaments, levator ani muscles, and pubocervical fascia, suggesting a role for estrogen in the continence mechanism.25-27 Oral estrogen seems to reduce collagen concentration, decrease the cross-linking of collagen,28 and increase the levels of collagen turnover in periurethral tissues,29-31 which could lead to weakened urethral support and incontinence. In a randomized clinical trial of levormeloxifene, a selective estrogen receptor modulator with estrogenic effects on the genital tract,32 treatment was associated with a 5-fold increased incidence of incontinence compared with placebo (17% compared with 4%).33 On the other hand, raloxifene, a selective estrogen receptor modulator with a neutral effect on the genital tract,34 seems to have no effect on incontinence.23,35
Although the number of women in the ULTRA trial is larger than all previous trials except Heart and Estrogen/Progestin Replacement Study10 and the WHI,9 the power of our study is still limited. Our results suggest that ultralow-dose transdermal E2 has no clinically significant effect on the symptoms of at least weekly incontinence. However, we could be missing as much as a 20% improvement or a 2-fold worsening of incontinence. We presume that at least 1 episode of incontinence reported in the prior week represents an average incontinence frequency of at least weekly incontinence. It is possible that women with only 1 incontinence episode per year, but this episode just happened to occur in the week before completing the questionnaire, would have been misclassified as having weekly incontinence. This may have affected our progression and remission rates. However, because the participants were randomly assigned, this misclassification would likely occur equally between placebo and treatment groups and would not affect the conclusions of our study.
Because this is a secondary analysis of ULTRA, our results are based on responses to a few questionnaire items assessing incontinence frequency and type at scheduled visits. Although self-report reflects the symptoms rather than the diagnosis of stress and urge incontinence, the experience of incontinence is of more direct clinical and public health importance than the presence or absence of urodynamic abnormalities. Finally, our study is based on the responses of healthy, mostly white, older postmenopausal women with normal bone density for age. There is no apparent reason why our findings should not generalize to many other older postmenopausal women. However, they may not be generalizable to women who are in the early postmenopausal period.
Our study suggests that ultralow-dose unopposed transdermal E2 has no substantial clinical effect on stress or urge incontinence symptoms in older postmenopausal women and should not be used to treat incontinence. The use of ultralow-dose transdermal E2 for prevention of osteoporosis is not associated with a significant risk of worsening or developing incontinence.
Footnotes
Supported by a grant from Berlex Laboratories Inc., Montville, NJ, producer of Menostar, the ultralow-dose transdermal E2 preparation used in this study and by grant IND No. 98188 from the U.S. Food and Drug Administration.
Financial Disclosure
Dr. Pinkerton is on the Berlex speaker's bureau.
REFERENCES
- 1.Fantl JA, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. First report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1994;83:12–8. [PubMed] [Google Scholar]
- 2.Elia G, Bergman A. Estrogen effects on the urethra: beneficial effects in women with genuine stress incontinence. Obstet Gynecol Surv. 1993;48:509–17. doi: 10.1097/00006254-199307000-00028. [DOI] [PubMed] [Google Scholar]
- 3.Sacco F, Rigon G, Carbone A, Sacchini D. Transvaginal estrogen therapy in urinary stress incontinence [in Italian] Minerva Ginecol. 1990;42:539–44. [PubMed] [Google Scholar]
- 4.Jarmy-Di Bella ZI, Girao MJ, Sartori MF, Di Bella V, Junior, Lederman HM, Baracat EC, et al. Power Doppler of the urethra in continent or incontinent, pre- and postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:148–54. doi: 10.1007/s001920070042. [DOI] [PubMed] [Google Scholar]
- 5.Beisland HO, Fossberg E, Moer A, Sander S. Urethral sphincteric insufficiency in postmenopausal females: treatment with phenylpropanolamine and estriol separately and in combination. A urodynamic and clinical evaluation. Urol Int. 1984;39:211–6. doi: 10.1159/000280978. [DOI] [PubMed] [Google Scholar]
- 6.Ek A, Andersson KE, Gullberg B, Ulmsten U. Effects of oestradiol and combined norephedrine and oestradiol treatment on female stress incontinence. Zentralbl Gynakol. 1980;102:839–44. [PubMed] [Google Scholar]
- 7.Bergman A, Karram MM, Bhatia NN. Changes in urethral cytology following estrogen administration. Gynecol Obstet Invest. 1990;29:211–3. doi: 10.1159/000293384. [DOI] [PubMed] [Google Scholar]
- 8.van der Linden MC, Gerretsen G, Brandhorst MS, Ooms EC, Kremer CM, Doesburg WH. The effect of estriol on the cytology of urethra and vagina in postmenopausal women with genito-urinary symptoms. Eur J Obstet Gynecol Reprod Biol. 1993;51:29–33. doi: 10.1016/0028-2243(93)90187-h. [DOI] [PubMed] [Google Scholar]
- 9.Marx P, Schade G, Wilbourn S, Blank S, Moyer DL, Nett R. Low-dose (0.3 mg) synthetic conjugated estrogens A is effective for managing atrophic vaginitis. Maturitas. 2004;47:47–54. doi: 10.1016/s0378-5122(03)00240-8. [DOI] [PubMed] [Google Scholar]
- 10.Moehrer B, Hextall A, Jackson S. Oestrogens for urinary incontinence in women. Cochrane Database Syst Rev. 2003:CD001405. doi: 10.1002/14651858.CD001405. [DOI] [PubMed] [Google Scholar]
- 11.Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935–48. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 12.Grady D, Brown JS, Vittinghoff E, Applegate W, Varner E, Snyder T, HERS Research Group Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116–20. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 13.Fantl JA, Bump RC, Robinson D, McClish DK, Wyman JF. Efficacy of estrogen supplementation in the treatment of urinary incontinence. The Continence Program for Women Research Group. Obstet Gynecol. 1996;88:745–9. doi: 10.1016/0029-7844(96)00281-5. [DOI] [PubMed] [Google Scholar]
- 14.Jackson S, Shepherd A, Brookes S, Abrams P. The effect of oestrogen supplementation on post-menopausal urinary stress incontinence: a double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:711–8. doi: 10.1111/j.1471-0528.1999.tb08372.x. [DOI] [PubMed] [Google Scholar]
- 15.Ouslander JG, Greendale GA, Uman G, Lee C, Paul W, Schnelle J. Effects of oral estrogen and progestin on the lower urinary tract among female nursing home residents. J Am Geriatr Soc. 2001;49:803–7. doi: 10.1046/j.1532-5415.2001.49160.x. [DOI] [PubMed] [Google Scholar]
- 16.Mattsson LA, Silfverstolpe G, Samsioe G. Fatty acid composition of serum lecithin and cholesterol ester in the normal menstrual cycle. Horm Metab Res. 1985;17:414–7. doi: 10.1055/s-2007-1013561. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PD, Faragher B, Butler B, Bu'Lock D, Robinson EL, Brown AD. Treatment with oral piperazine oestrone sulphate for genuine stress incontinence in postmenopausal women. Br J Obstet Gynaecol. 1987;94:568–74. doi: 10.1111/j.1471-0528.1987.tb03152.x. [DOI] [PubMed] [Google Scholar]
- 18.Cardozo L, Rekers H, Tapp A, Barnick C, Shepherd A, Schussler B, et al. Oestriol in the treatment of postmenopausal urgency: a multicentre study. Maturitas. 1993;18:47–53. doi: 10.1016/0378-5122(93)90028-g. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger B, Ensrud KE, Wallace R, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol. 2004;104:443–51. doi: 10.1097/01.AOG.0000137833.43248.79. [DOI] [PubMed] [Google Scholar]
- 20.McCullagh P. Regression models for ordinal data. (Series B).J R Stat Soc. 1980;42:109–42. [Google Scholar]
- 21.Thom DH, Brown JS. Reproductive and hormonal risk factors for urinary incontinence in later life: a review of the clinical and epidemiologic literature. J Am Geriatr Soc. 1998;46:1411–7. doi: 10.1111/j.1532-5415.1998.tb06009.x. [DOI] [PubMed] [Google Scholar]
- 22.Grodstein F, Lifford K, Resnick NM, Curhan GC. Postmenopausal hormone therapy and risk of developing urinary incontinence. Obstet Gynecol. 2004;103:254–60. doi: 10.1097/01.AOG.0000107290.33034.6f. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein SR, Johnson S, Watts NB, Ciaccia AV, Elmerick D, Muram D. Incidence of urinary incontinence in postmenopausal women treated with raloxifene or estrogen. Menopause. 2005;12:160–4. doi: 10.1097/00042192-200512020-00010. [DOI] [PubMed] [Google Scholar]
- 24.Rufford J, Hextall A, Cardozo L, Khullar V. A double-blind placebo-controlled trial on the effects of 25 mg estradiol implants on the urge syndrome in postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:78–83. doi: 10.1007/s00192-003-1054-3. [DOI] [PubMed] [Google Scholar]
- 25.Blakeman PJ, Hilton P, Bulmer JN. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int. 2000;86:32–8. doi: 10.1046/j.1464-410x.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith P, Heimer G, Norgren A, Ulmsten U. The round ligament: a target organ for steroid hormones. Gynecol Endocrinol. 1993;7:97–100. doi: 10.3109/09513599309152487. [DOI] [PubMed] [Google Scholar]
- 27.Smith P, Heimer G, Norgren A, Ulmsten U. Localization of steroid hormone receptors in the pelvic muscles. Eur J Obstet Gynecol Reprod Biol. 1993;50:83–5. doi: 10.1016/0028-2243(93)90169-d. [DOI] [PubMed] [Google Scholar]
- 28.Keane DP, Sims TJ, Abrams P, Bailey AJ. Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol. 1997;104:994–8. doi: 10.1111/j.1471-0528.1997.tb12055.x. [DOI] [PubMed] [Google Scholar]
- 29.Falconer C, Ekman-Ordeberg G, Ulmsten U, WestergrenThorsson G, Barchan K, Malmstrom A. Changes in paraurethral connective tissue at menopause are counteracted by estrogen. Maturitas. 1996;24:197–204. doi: 10.1016/s0378-5122(96)82010-x. [DOI] [PubMed] [Google Scholar]
- 30.Falconer C, Ekman-Ordeberg G, Blomgren B, Johansson O, Ulmsten U, Westergren-Thorsson G, et al. Paraurethral connective tissue in stress-incontinent women after menopause. Acta Obstet Gynecol Scand. 1998;77:95–100. doi: 10.1034/j.1600-0412.1998.770120.x. [DOI] [PubMed] [Google Scholar]
- 31.Jackson S, James M, Abrams P. The effect of oestradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG. 2002;109:339–44. doi: 10.1111/j.1471-0528.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 32.Warming L, Christoffersen C, Riis BJ, Stakkestad JA, Delmas PD, Christiansen C. Adverse effects of a SERM (Levormeloxifene). Safety parameters and bone mineral density 12 months after treatment withdrawal. Maturitas. 2003;44:189–99. doi: 10.1016/s0378-5122(02)00342-0. [DOI] [PubMed] [Google Scholar]
- 33.Hendrix SL, McNeeley SG. Effect of selective estrogen receptor modulators on reproductive tissues other than endometrium. Ann N Y Acad Sci. 2001;949:243–50. doi: 10.1111/j.1749-6632.2001.tb04028.x. [DOI] [PubMed] [Google Scholar]
- 34.Cohen FJ, Watts S, Shah A, Akers R, Plouffe L., Jr Uterine effects of 3-year raloxifene therapy in postmenopausal women younger than age 60. Obstet Gynecol. 2000;95:104–10. doi: 10.1016/s0029-7844(99)00554-2. [DOI] [PubMed] [Google Scholar]
- 35.Waetjen LE, Brown JS, Modelska K, Blackwell T, Vittinghoff E, Cummings SR, MORE Study Group Effect of raloxifene on urinary incontinence: a randomized controlled trial. Obstet Gynecol. 2004;103:261–6. doi: 10.1097/01.AOG.0000109429.67671.d1. [DOI] [PubMed] [Google Scholar]


