Abstract
OBJECTIVE
To estimate the effect of hormone therapy on risk of stress and urge urinary incontinence.
METHODS
The Heart Estrogen/progestin Replacement Study was a randomized, placebo-controlled, double-blinded trial to evaluate daily oral conjugated estrogen (0.625 mg) plus medroxyprogesterone acetate (2.5 mg) therapy for the prevention of heart disease events in women with established heart disease. The 1,208 participants in Heart Estrogen/progestin Replacement Study who reported no loss of urine in the previous 7 days at baseline are included in this analysis.
RESULTS
During 4.2 years of treatment, 64% of women randomly assigned to hormone therapy compared with 49% of those assigned to placebo reported weekly incontinence (P < .001). The higher risk of incontinence in the hormone group was evident at 4 months, persisted throughout the treatment period, and was independent of the age of the women. The odds ratios for weekly incontinence among women on hormone therapy compared with placebo were 1.5 for urge incontinence (95% confidence interval [CI] 1.2–1.8; P < .001) and 1.7 for stress incontinence (95% CI 1.5–2.1; P < .001). Four years of treatment with hormone therapy caused an excess risk of 12% for weekly urge incontinence and 16% for weekly stress incontinence; the corresponding numbers needed to harm were 8.6 (95% CI 5.8–16.6) and 6.2 (95% CI 4.6–9.4).
CONCLUSION
Estrogen plus progestin therapy increases risk of urge and stress incontinence within 4 months of beginning treatment. Precis: Estrogen plus progestin therapy increases risk of urge and stress incontinence within 4 months of beginning treatment.
LEVEL OF EVIDENCE: I
Nearly 40% of postmenopausal women have urinary incontinence.1 The presence of estrogen receptors throughout the urogenital tract indicates that estrogen may have physiologic effects on the continence mechanism. Estrogen increases urethral blood flow,2,3 α-adrenergic receptor sensitivity,4,5 and urethral closure pressure,6-8 and improves cellular maturation in the bladder, trigone, and urethra.9,10 These findings suggest that hormone therapy may improve urinary incontinence in postmenopausal women, and it has been widely used in clinical practice for this indication.
In postmenopausal women with incontinence, however, randomized controlled trials have shown that hormone therapy either has no effect11-15 or actually worsens preexisting incontinence.16,17 Among women without incontinence, the Nurses Health Study, a large prospective cohort study, found that use of estrogen plus progestin was associated with a 78% higher risk for developing weekly urinary incontinence.18 The Women's Health Initiative (WHI), a large randomized, controlled trial, found that treatment with oral estrogen plus progestin or estrogen alone increased the risk of incident urinary incontinence at 1 and 3 years.17
The Heart Estrogen/progestin Replacement Study (HERS) was a randomized, placebo-controlled, double-blind trial that evaluated 4 years of treatment with oral conjugated estrogen plus medroxyprogesterone acetate for the prevention of coronary disease events in women with established coronary disease.19 To estimate the effect of this oral hormone regimen on risk of urinary incontinence among women who denied episodes of incontinence in the prior week at baseline, we evaluated report of incontinence at 4 months after randomization and then annually for 4 years.
MATERIALS AND METHODS
The design, methods, baseline characteristics and primary findings of the HERS trial have been published.19 The research protocol was reviewed and approved by the University of California, San Francisco Institutional Review Board as well as those of all participating sites. Study participants were postmenopausal women aged younger than 80 years with coronary heart disease and an intact uterus. The participants were randomly allocated to receive oral daily conjugated estrogen (0.625 mg) and medroxy progesterone acetate (2.5 mg) in 1 pill or identical placebo. Subjects were randomly assigned at each of 20 clinical centers using randomly permuted blocks of 4.
At baseline, participants provided information on demographic and health variables and completed a questionnaire on incontinence. Incontinence questions began with “During the prior week, how many times on average…” and ended with “have you unintentionally leaked some urine with coughing, sneezing, straining, laughing or lifting?” (stress incontinence) or “have you unintentionally leaked some urine before you could get to the bathroom?” (urge incontinence). Only women who reported no episodes of incontinence within the last week at baseline were included in this analysis. At an initial 4-month visit and subsequent annual follow-up visits, women who reported 1 or more episodes of incontinence in the previous week were considered to have weekly incontinence.
Our primary analysis used the χ2 test to compare the proportions of women in the hormone and placebo groups reporting weekly incontinent episodes at least once during the first 4 years of follow-up. In addition, we calculated the cumulative proportions who had reported incontinence by each follow-up visit, and estimated excess risk by the between-treatment difference in the cumulative proportions reporting incontinence by the fourth annual visit. Confidence intervals for number needed to harm were estimated by the reciprocals of the normal approximation confidence limits for the excess risk.20 The effect of hormone therapy on incident urge or stress incontinence was analyzed by intention to treat, without regard to adherence to therapy.
We also used a continuation-ratio model21 to estimate the effects of treatment on risk of first reporting weekly incontinence at each successive visit at which incontinence was assessed. This is a form of survival analysis that takes account of censoring due to death and loss to follow-up, and estimates the relative odds of reporting weekly incontinence at a visit given that it had not been reported at a previous visit. We used this model to examine whether the effects of treatment differed over time, in our analysis stratified by age, and to carry out sensitivity analyses in which treatment effect estimates were adjusted for covariates known to be associated with incontinence. To summarize the severity of incontinence, we used the largest number of incontinent episodes each woman reported during the study visits, thus presenting her incontinence at its worst.
RESULTS
Between January 1993 and September 1994, 2,763 women were enrolled in the HERS. At the beginning of the trial, 1,228 participants reported no weekly stress or urge incontinence episodes in the past week, and of these, 1,208 women provided urinary incontinence data for at least 1 follow-up visit. Five hundred ninety-seven (49%) were assigned to hormone therapy, and 611 (51%) were assigned to placebo.
These groups of continent women were comparable at baseline, with the exception that the women assigned to placebo were, on average, 1 year older and correspondingly further from menopause compared with women in the hormone group (Table 1).
Table 1.
Baseline Characteristics of Postmenopausal Women Without Incontinence in the Heart Estrogen/progestin Replacement Study
| Characteristics of Patients | Hormone Therapy (n = 597) | Placebo (n = 611) | P |
|---|---|---|---|
| Age | 66.0 ± 6.3 | 66.8 ± 6.5 | .02 |
| Race | .54 | ||
| White | 509 (85) | 533 (87) | |
| African American | 64 (11) | 54 (9) | |
| Years since menopause | 17.4 ± 8.0 | 18.7 ± 8.0 | .005 |
| Parous | 535 (90) | 553 (91) | .54 |
| Average body mass index (kg/m) | 28.0 ± 5.3 | 27.7 ± 5.4 | .31 |
| Body mass index > 30 (kg/m) | 186 (31) | 165 (27) | .11 |
| Poor overall health | 131 (22) | 132 (22) | .89 |
| Diabetes | 122 (20) | 113 (18) | .39 |
| Hypertension | 337 (56) | 345 (56) | .92 |
| Current Diuretic Use | 155 (26) | 151 (25) | .62 |
Values are mean (± standard deviation) or n (%).
In the hormone therapy group, 1 participant discontinued due to adverse reactions, 58 died, 5 failed to return, and 11 were lost to follow-up for other reasons. In the placebo group, 60 died, 5 failed to return, and 12 were lost to follow-up for other reasons. The compliance rates were 80% in the placebo and 68% in the hormone groups.
During 4 years of treatment, weekly incontinence was reported at least once during follow-up by 382 women (64%) taking hormone therapy and 302 women (49%) taking placebo (P ≤ .001). Weekly urge incontinence was reported by 48% of the hormone therapy group and 36% of the placebo group (P < .001). Weekly stress incontinence was reported by 54% of the hormone therapy group and 38% of the placebo group (P < .001). The difference between treatment groups was evident by the 4-month visit and persisted throughout the treatment period (Figure 1).
Fig. 1.
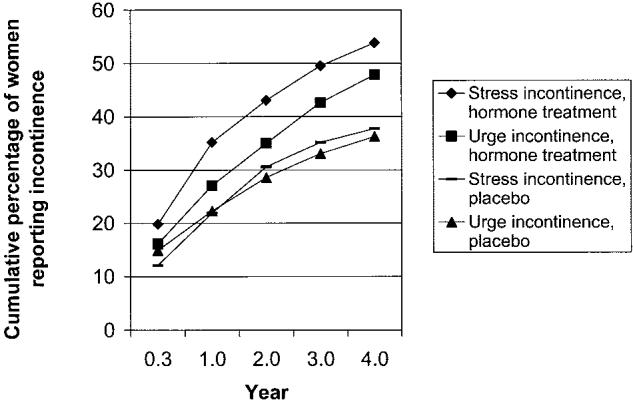
Cumulative percentage of women reporting incident weekly urinary incontinence at each visit.
In the continuation-ratio model, women assigned to hormone therapy had 50% higher odds of reporting urge incontinence compared with those assigned to placebo (P = .001) and 70% higher odds of reporting stress incontinence (P < .001) (Table 2). In supplementary analyses we found that the adverse effect on urge incontinence seemed to increase with time (P = .03 for linear trend in the treatment effect). The effects of treatment were unchanged in multivariate continuation-ratio models controlling for age, years since menopause, race, parity, diabetes, and body mass index.
Table 2.
Risk of Developing Urinary Incontinence Among Postmenopausal Hormone Users Compared With Nonusers
| Odds Ratio* | 95% Confidence Interval* | P* | |
|---|---|---|---|
| Weekly incontinence | 1.6 | 1.3,1.9 | < .001 |
| Urge incontinence | 1.5 | 1.2,1.8 | < .001 |
| Stress incontinence | 1.7 | 1.5,2.1 | < .001 |
From continuation-ratio analyses with treatment as the predictor and new report of weekly incontinence as the outcome.
In women under 60 years of age (n = 184) the effect of hormone therapy on risk of incontinence was minimally and not significantly elevated (odds ratio [OR] 1.31; 95% confidence interval [CI] 0.85–2.04; P = .23). Of the women age younger than 60 years and assigned to placebo (n = 88), 48% reported weekly incontinence of any type, 32% reported weekly urge incontinence, and 37% reported weekly stress incontinence during the 4 years. The respective proportions of women assigned to hormone therapy who reported incontinence (n = 96) were 59%, 40%, and 48%.
The cumulative percentage of women reporting incontinence by type and treatment group is presented in Figure 1. Excess risk after 4 years of treatment with hormone therapy was 15% for weekly incontinence of any type, 12% for weekly urge incontinence, and 16% for weekly stress incontinence. Numbers needed to harm were 6.9, 8.6, and 5.9 respectively (Table 3).
Table 3.
Risks, Excess Risks, and Numbers Needed to Harm After 4 Years of Hormone Treatment
| Cumulative 4-Year Risk (%) |
||||
|---|---|---|---|---|
| Placebo | Hormone Treatment | Excess Risk (%) | Numbers Needed to Harm | |
| Weekly incontinence | 49 | 64 | 15 | 6.9 (5.0–11.1) |
| Urge incontinence | 36 | 48 | 12 | 8.6 (5.8–16.6) |
| Stress incontinence | 38 | 54 | 16 | 6.2 (4.6–9.4) |
Of the women who reported urinary incontinence of any type, 23% reported 4 or more episodes per week at least once during follow-up. Eighteen percent and 22% reported 4 or more episodes of urge and stress incontinence, respectively.
DISCUSSION
Use of oral estrogen and progestin caused both urge and stress urinary incontinence in this randomized clinical trial of postmenopausal women with heart disease. Women on hormone therapy had 50% higher odds of urge incontinence and 70% higher odds of stress incontinence. An increased risk of urinary incontinence among hormone users became apparent within 4 months of starting therapy, and persisted for the duration of oral hormone use. Treatment of 8 women with hormone therapy for 4 years caused 1 additional woman to report weekly urge incontinence, and treatment of 6 women caused 1 additional woman to report weekly stress incontinence. Approximately one fourth of the women with incontinence reported 4 or more leaking episodes per week.
Large randomized trials of hormone therapy in women with incontinence at baseline have found that standard doses of oral estrogen, with or without progestin, worsen urinary incontinence. The WHI (12,386 women with baseline incontinence) and the HERS (1,535 women with baseline incontinence) demonstrated significant worsening of both stress and urge incontinence; this worsening was measured by increased frequency in both trials, and by worsening amount of urine lost and worsening limitations in daily activities related to urinary incontinence in the WHI.16,17
It is unclear why hormone therapy increases the risk of developing incontinence and worsens the symptoms of existing incontinence. Alpha- and β-estrogen receptors have been identified throughout the urogenital tract, including the bladder mucosa, trigone, urethra, and vaginal mucosa.22-24 These receptors are also present in the structures that support the pelvic organs: uterosacral ligaments, levator ani muscles, and pubocervical fascia.22-24 This suggests that estrogen may have a role in both structure and function of the urinary continence mechanism.
A number of studies have shown that estrogen therapy results in a reduction of the total collagen concentration, a decrease in the cross-linking of collagen, and an increase in markers of collagen turnover in the periurethral tissues of incontinent women.25-28 Animal studies have also found that estrogen administration decreases the number of collagen fibers and increases the number of smooth muscle fibers in the urethra and bladder wall.29-31 These histologic changes are associated with higher bladder contractility and resting tension.31 Estrogen treatment also increases periurethral vascularity in monkeys32 and rats.33 Although this increased vascularization has been interpreted as a positive effect of estrogen, it may be that vascular and loose connective tissue is replacing collagen, resulting in poor urethral support. A combination of higher pressures in the bladder and weaker supportive architecture of the urethra may promote incontinence.
Our results are based on treatment with standard-dose oral conjugated equine estrogen combined with medroxyprogesterone acetate and might not be generalizable to other hormone treatment regimens. For example, the association of hormone therapy and incontinence may be due to the progestin component of the regimen. Although evidence suggests that urinary symptoms increase during the progesterone-dominant phase of the menstrual cycle, the mechanism is less clear.34 However, the generalizability of our findings to other hormone regimens is supported by the results of the Nurse's Health Study and the WHI. The Nurse's Health Study found an elevated risk of incident incontinence for users of transdermal estrogen, estrogen alone, and estrogen plus progestin. The WHI found an elevated risk of incident incontinence in users of oral estrogen alone and oral estrogen plus progestin.
This report focuses on women who did not report incontinent episodes in the last week at baseline, and thus almost surely includes some women with prevalent but intermittent incontinence. As a result, the postbaseline episodes of leakage analyzed in this report most likely represent some prevalent as well as incident incontinence. In contrast, the WHI defined incident incontinence only in women who reported having never leaked urine at baseline. In our study, 22.6% of women assigned to placebo reported stress and 18.1% reported incident urge incontinence at the first annual visit as compared with 11.4% and 13.6% of the women in the WHI.17 Although the incontinence analyzed in this report cannot be strictly interpreted as incident incontinence, this does not threaten the validity of the between-group comparison. The evidence we present for the negative effect of hormone therapy on the risk of incontinence remains persuasive. It is also consistent with the effect of hormone therapy on incontinence risk found among the complementary group of HERS women who did report incontinence during the last week at baseline.16
Because the clinical indications for hormone therapy use are now largely limited to perimenopausal symptoms, we evaluated its effect in the youngest women in the HERS, those aged younger than 60 years. The difference between hormone treatment and placebo was not significant in this smaller sample, but both the relative and absolute increases in risk of incontinence were similar in magnitude to the increases observed in the entire cohort.
In conclusion, oral estrogen plus progestin therapy increased the risk for stress and urge urinary incontinence in postmenopausal women. Women who are using or considering hormone therapy should be informed about this increased risk.
Footnotes
Supported by Wyeth-Ayerst Research, Radnor, Pennsylvania. Dr. Subak was a Women's Reproductive Health Research Scholar supported by the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland (K12 HD01262-02). Dr. Brown is supported by a NIDDK K-24 Mid-career Investigator Award in Patient Oriented Research.
Financial Disclosure
The Heart and Estrogen/Progestin Replacement Study (HERS) was funded by a contract from Wyeth Ayerst Research. The HERS investigators received research support from the contract, including salaries, during the trial. The HERS investigators on this manuscript include Dr. Hulley, Dr. Grady, Dr. Vittinghoff, and Ms. Lin. The HERS investigators were prohibited from owning any stock in the company, and none of the authors of this paper were paid for talks or consulting by the company during the HERS trial. The University of California, San Francisco, was the coordinating center for the trial and was responsible for data analysis and publications. This manuscript and all manuscripts from the HERS trial are written solely by the authors appearing on the manuscript.
REFERENCES
- 1.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 2.Jarmy-Di Bella ZI, Girao MJ, Sartori MF, Di Bella Junior V, Lederman HM, Baracat EC, et al. Power Doppler of the urethra in continent or incontinent, pre- and postmenopausal women. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:148–154. doi: 10.1007/s001920070042. [DOI] [PubMed] [Google Scholar]
- 3.Girao MJ, Jarmy-Di Bella ZI, Sartori MG, Baracat EC, Lima GR. Doppler velocimetry parameters of periurethral vessels in postmenopausal incontinent women receiving estrogen replacement. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:241–246. doi: 10.1007/s001920170046. [DOI] [PubMed] [Google Scholar]
- 4.Beisland HO, Fossberg E, Moer A, Sander S. Urethral sphincteric insufficiency in postmenopausal females: treatment with phenylpropanolamine and estriol separately and in combination. A urodynamic and clinical evaluation. Urol Int. 1984;39:211–6. doi: 10.1159/000280978. [DOI] [PubMed] [Google Scholar]
- 5.Ek A, Andersson KE, Gullberg B, Ulmsten U. Effects of oestradiol and combined norephedrin and oestradiol treatment on female stress incontinence. Zentralbl Gynakol. 1980;102:839–44. [PubMed] [Google Scholar]
- 6.Elia G, Bergman A. Estrogen effects on the urethra: beneficial effects in women with genuine stress incontinence. Obstet Gynecol Surv. 1993;48:509–17. doi: 10.1097/00006254-199307000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Fantl JA, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. First report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1994;83:12–8. [PubMed] [Google Scholar]
- 8.Sacco F, Rigon G, Carbone A, Sacchini D. Transvaginal estrogen therapy in urinary stress incontinence [in Italian] Minerva Ginecol. 1990;42:539–44. [PubMed] [Google Scholar]
- 9.Bergman A, Karram MM, Bhatia NN. Changes in urethral cytology following estrogen administration. Gynecol Obstet Invest. 1990;29:211–3. doi: 10.1159/000293384. [DOI] [PubMed] [Google Scholar]
- 10.van der Linden MC, Gerretsen G, Brandhorst MS, Ooms EC, Kremer CM, Doesburg WH. The effect of estriol on the cytology of urethra and vagina in postmenopausal women with genito-urinary symptoms. Eur J Obstet Gynecol Reprod Biol. 1993;51:29–33. doi: 10.1016/0028-2243(93)90187-h. [DOI] [PubMed] [Google Scholar]
- 11.Cardozo L, Rekers H, Tapp A, Barnick C, Shepherd A, Schussler B, et al. Oestriol in the treatment of postmenopausal urgency: a multicentre study. Maturitas. 1993;18:47–53. doi: 10.1016/0378-5122(93)90028-g. [DOI] [PubMed] [Google Scholar]
- 12.Fantl JA, Bump RC, Robinson D, McClish DK, Wyman JF. Efficacy of estrogen supplementation in the treatment of urinary incontinence. The Continence Program for Women Research Group. Obstet Gynecol. 1996;88:745–9. doi: 10.1016/0029-7844(96)00281-5. [DOI] [PubMed] [Google Scholar]
- 13.Jackson S, Shepherd A, Brookes S, Abrams P. The effect of oestrogen supplementation on post-menopausal urinary stress incontinence: a double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106:711–8. doi: 10.1111/j.1471-0528.1999.tb08372.x. [DOI] [PubMed] [Google Scholar]
- 14.Ouslander JG, Greendale GA, Uman G, Lee C, Paul W, Schnelle J. Effects of oral estrogen and progestin on the lower urinary tract among female nursing home residents. J Am Geriatr Soc. 2001;49:803–807. doi: 10.1046/j.1532-5415.2001.49160.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson PD, Faragher B, Butler B, Bu'Lock D, Robinson EL, Brown AD. Treatment with oral piperazine oestrone sulphate for genuine stress incontinence in postmenopausal women. Br J Obstet Gynaecol. 1987;94:568–74. doi: 10.1111/j.1471-0528.1987.tb03152.x. [DOI] [PubMed] [Google Scholar]
- 16.Grady D, Brown JS, Vittinghoff E, Applegate W, Varner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116–20. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 17.Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935–48. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 18.Grodstein F, Lifford K, Resnick NM, Curhan GC. Postmenopausal hormone therapy and risk of developing urinary incontinence. Obstet Gynecol. 2004;103:254–60. doi: 10.1097/01.AOG.0000107290.33034.6f. [DOI] [PubMed] [Google Scholar]
- 19.Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley SB. Heart and Estrogen/progestin Replacement Study (HERS): design, methods, and baseline characteristics. Control Clin Trials. 1998;19:314–35. doi: 10.1016/s0197-2456(98)00010-5. [DOI] [PubMed] [Google Scholar]
- 20.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. Int J Epidemiol. 1997;26:1323–33. doi: 10.1093/ije/26.6.1323. [DOI] [PubMed] [Google Scholar]
- 22.Blakeman PJ, Hilton P, Bulmer JN. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int. 2000;86:32–8. doi: 10.1046/j.1464-410x.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein IT. The pelvic floor muscles: muscle thickness in healthy and urinary-incontinent women measured by perineal ultrasonography with reference to the effect of pelvic floor training. Estrogen receptor studies. Neurourol Urodyn. 1997;16:237–75. doi: 10.1002/(sici)1520-6777(1997)16:4<237::aid-nau2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Smith P, Heimer G, Norgren A, Ulmsten U. Localization of steroid hormone receptors in the pelvic muscles. Eur J Obstet Gynecol Reprod Biol. 1993;50:83–5. doi: 10.1016/0028-2243(93)90169-d. [DOI] [PubMed] [Google Scholar]
- 25.Rud T. The effects of estrogens and gestagens on the urethral pressure profile in urinary continent and stress incontinent women. Acta Obstet Gynecol Scand. 1980;59:265–70. doi: 10.3109/00016348009155409. [DOI] [PubMed] [Google Scholar]
- 26.Falconer C, Ekman-Ordeberg G, Malmstrom A, Ulmsten U. Clinical outcome and changes in connective tissue metabolism after intravaginal slingplasty in stress incontinent women. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:133–7. doi: 10.1007/BF01894201. [DOI] [PubMed] [Google Scholar]
- 27.Falconer C, Ekman-Ordeberg G, Blomgren B, Johansson O, Ulmsten U, Westergren-Thorsson G, et al. Paraurethral connective tissue in stress-incontinent women after menopause. Acta Obstet Gynecol Scand. 1998;77:95–100. doi: 10.1034/j.1600-0412.1998.770120.x. [DOI] [PubMed] [Google Scholar]
- 28.Jackson S, James M, Abrams P. The effect of oestradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG. 2002;109:339–44. doi: 10.1111/j.1471-0528.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 29.Sartori MG, Girao MJ, de Jesus Simoes M, Sartori JP, Baracat EC, Rodrigues de Lima G, et al. Quantitative evaluation of collagen and muscle fibers in the lower urinary tract of castrated and under-hormone replacement female rats. Clin Exp Obstet Gynecol. 2001;28:92–6. [PubMed] [Google Scholar]
- 30.Fleischmann N, Christ G, Sclafani T, Melman A. The effect of ovariectomy and long-term estrogen replacement on bladder structure and function in the rat. J Urol. 2002;168:1265–8. doi: 10.1016/S0022-5347(05)64637-X. [DOI] [PubMed] [Google Scholar]
- 31.Aikawa K, Sugino T, Matsumoto S, Chichester P, Whitbeck C, Levin RM. The effect of ovariectomy and estradiol on rabbit bladder smooth muscle contraction and morphology. J Urol. 2003;170:634–7. doi: 10.1097/01.ju.0000068723.05004.ca. [DOI] [PubMed] [Google Scholar]
- 32.Robinson D, Rainer RO, Washburn SA, Clarkson TB. Effects of estrogen and progestin replacement on the urogenital tract of the ovariectomized cynomolgus monkey. Neurourol Urodyn. 1996;15:215–21. doi: 10.1002/(SICI)1520-6777(1996)15:3<215::AID-NAU6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 33.Endo RM, Girao MJ, Sartori MG, Simoes MJ, Baracat EC, Rodrigues de Lima G. Effect of estrogen-progestogen hormonal replacement therapy on periurethral and bladder vessels. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:120–23. doi: 10.1007/pl00004024. [DOI] [PubMed] [Google Scholar]
- 34.Hextall A, Bidmead J, Cardozo L, Hooper R. The impact of the menstrual cycle on urinary symptoms and the results of urodynamic investigation. BJOG. 2001;108:1193–6. doi: 10.1111/j.1471-0528.2003.00280.x. [DOI] [PubMed] [Google Scholar]


